Surgical management of neuroendocrine tumor-associated liver metastases: a review
Background
Liver metastases are commonly encountered in intestinal and pancreatic neuroendocrine tumors (NETs). Around 12–34% of patients presented with distant metastases at initial diagnosis (1-5), and another 38% after the initial diagnosis (2). Majority of them presented with liver and/or lymph node metastases. Interestingly, various studies found that the incidence of gastroenteropancreatic NET has been rising in the past decades around the world (3,6), though partly maybe contributed by the changes in the classification and registration of the disease entity. Nonetheless, what was previously considered as a rare disease is now beginning to accumulate more evidence, giving a clearer picture about the optimal treatment strategy.
Known by its indolent clinical course, it was once controversial to offer surgery to patients with asymptomatic NET liver metastases (NELM) (7). A mean overall survival up to 8.1 years from symptom onset has been reported, with a range up to 41 years (8). Over the years, growing evidence showed that the overall survival is improved after resection with curative intent, or other modalities such as ablation and liver transplantation (LT). In 2016, the European Neuroendocrine Tumor Society updated their guidelines on NELM and provided a comprehensive overview of the disease. This article aims to review the current evidence on the management strategies of NELM, mainly focusing on the potential challenges and controversies from a surgeon’s point of view.
Liver resection
As mentioned in the latest update of the ENET guidelines (9), surgery with curative intent should always be considered first in grade 1 (G1) and grade 2 (G2) NET, even in the presence of liver and/or lymph node metastases. Although there is no randomized controlled trial to support such practice (10), various reports already showed prolonged survival following resection of the primary tumors and liver metastases with a 5-year survival rate ranging from 58% to 70% and a 10-year survival rate of 35% (11-14). Liver resection is now used as the benchmark against all other treatments for resectable NELM.
In a systematic review on the surgical outcomes by Saxena et al. (15), 29 clinical studies on liver resection with or without concomitant ablation for NELM were included. It showed that the 5-year median overall survival (OS) was 70.5% (range, 31–100%) and 10-year median OS was 42% (range, 0–100%). The overall perioperative mortality rate was 0–9% (median 0%) and morbidity rate ranged from 3% to 45% (median 23%). These figures were comparable to the liver resections for hepatocellular carcinoma, if not better (16-18). Given the relatively preserved liver function in most of the patients suffering from NELM, liver resection should be safe and feasible in experienced hands.
Despite the prolonged overall survival, many studies showed a high rate of recurrence. From the review by Saxena et al. (15), the 5-year median progression-free survival (PFS) was 29% (range, 6–66%). Nearly all patients experienced recurrence by 10 years after resection, with a 10-year median PFS of 1% only (range, 0–11%). In a retrospective study from Zhang et al. (19), 46.4% of patients (223 out of 481 patients) were found to have recurrences after 5 years. 70.9% of those recurrences (158 patients) occurred early (defined as recurrence occurring within 3 years after curative-intent resection). It was shown that there is no difference in PFS between early and late recurrence as long as curative treatment was given, although the overall survival was not mentioned in this group of patients.
Resection also offers excellent results in terms of symptomatic relief, especially for hormone-related symptoms, with a response rate up to 90–100% in certain reports (13,20,21). However symptoms are bound to recur with high rate of tumor recurrence, be it hormone-related or symptoms related to tumor bulk. The 5-year symptom-free survival ranges from 15% to 46%, reflecting the high rate of tumor recurrence (15).
In order to guide management, three types of metastases pattern have been described (22):
- Type I: single metastasis;
- Type II: isolated metastatic bulk with smaller deposits;
- Type III: disseminated metastases.
It was suggested that type I would be more suitable for resection, whereas types II and III would be more suitable for ablation and other non-surgical therapies. This can only serve as a rough guide for clinical decision, as sometimes type II tumors may still be amenable to combined surgery with ablation. In case of inadequate liver reserve, surgery may not be feasible in even type I tumors.
A number of prognostic factors were identified to predict the outcomes of resection. Due to the heterogeneity in patient inclusion criteria and treatment strategies, it is difficult to identify the exact risk factors affecting the OS and PFS. From a meta-analysis by Yuan et al. (23), factors favoring survival include resection with curative intent and functional tumors. Several clinical studies also reported that the presence of extrahepatic disease, Ki67 expression and the level of differentiation of the tumor affect survival (24-26). Ruzzenente et al. (25) has recently developed a simple nomogram with number of liver metastases, maximum tumor size and Ki67 expression to predict the overall survival at 5 and 10 years. This may be useful clinically to aid decision-making, but further validation is still needed.
It was still controversial as to whether the site of primary tumor affects prognosis. In a large population series by Fairweather et al. (24) of over 600 patients with NELM, it was found that primary small bowel tumors was one of the significant independent prognosticators for overall survival (hazard ratio 0.5, P<0.001). However in another study by Spolverato et al. (26), there was no significant difference in 5-year OS and 5-year PFS between pancreatic NET and non-pancreatic NET after propensity score matching.
Resection versus non-surgical therapies
Cochrane review was previously performed by Gurusamy in 2009, and concluded that there was no good quality evidence to show that surgery is superior to other non-surgical treatment (10). A summary of studies comparing surgery to other non-surgical treatments was listed in Table 1. All of the studies showed a significantly better survival for surgery group.
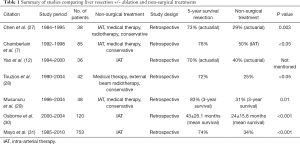
Full table
In the more recent meta-analysis by Yuan et al. (23), the efficacy of liver resection versus nonsurgical regimens was investigated with the results of seven clinical studies. It was found that liver resection was significantly associated with a higher rate of symptomatic relief, longer median survival time and 5-year survival. However one should note that there was statistically significant heterogeneity in the studies included, and none of the studies is randomized controlled trial.
One of the largest series so far comparing surgery with intra-arterial therapy (IAT) was by Mayo et al. Propensity score matching analysis was used. Median survival was significantly longer in surgery group (84 vs. 38.9 months; P=0.05). Patients with more than 25% liver tumor burden and asymptomatic disease did not derive as much benefit from surgery (median survival 16.7 vs. 18.5 months; P=0.78).
Role of debulking surgery
Even if curative surgery could not be carried out, debulking surgery could still be performed to alleviate symptoms. When >80–90% of the tumor load could be resected, there may be associated survival benefit as shown in some studies (13,32). Some studies even allow a larger proportion of residual tumour up to 30%, while still able to demonstrate survival benefit (33,34). In a retrospective study by Osborne et al. (30), debulking surgery was performed in 23 out of 191 patients (12%). In combination with ablation intra-operatively, there was significant survival benefit when comparing debulking surgery with embolization (P=0.03), although the benefit was not as great when compared with curative surgery.
Resection of grade 3 (G3) tumors
Due to the very different behavior of high-grade NET, the ENETS deliberately published a separated guideline for high-grade tumors and neuroendocrine carcinoma. In the ENETS guidelines (35), surgical resection and ablation of metastases were not recommended. Chemotherapy was suggested as first line therapy. Other non-surgical therapies including peptide receptor radionuclide therapy (PRRT) and radiotherapy could also be considered in individual patients.
However there are new reports showing that there may be potential benefits in surgery in selected patients. Galleberg et al. (36) reported 5-year OS of 43% and 5-year PFS of 13% in 32 patients with curative-intent resections for high-grade NELM. Patients with relatively lower Ki67 expression (21–54%) and those who received adjuvant chemotherapy were found to have a better OS but there was no benefit in PFS. In another retrospective study by Du et al. (37), resection was performed in seven patients with G3 tumors. Three-year OS was 42.8%, while another nine patients treated with non-surgical therapies all died in 3 years (P=0.049). Further data is needed to determine the best treatment strategy in these patients.
Resection of primary tumors
In patients with unresectable liver metastases, one would assume that resection for the primary tumor would not provide any benefit. Both the ENETS (9) and NCCN guidelines (38) suggested that resection of primary tumors should not be considered if the metastasis is not resectable. However another school of thought was that primary tumor resection can help prevent subsequent complications like obstruction and malnutrition (39).
In a study by Bettini et al. (40), it was shown that the only benefit of resecting the primary tumors was to prevent any symptoms arising from the tumor bulk such as biliary obstruction or symptoms from functional tumors. There was no difference in PFS between two groups. However, Bertani et al. has published several studies showing that there is a survival benefit in resecting the primary for metastatic disease (41-43). The author proposed that even in the absence of complication or symptoms, the primary tumor should still be removed as it may enhance the efficacy of systemic therapy. His findings were further supported by Citterio et al. in a recent retrospective study (44). However as none of the studies above is randomized, the potential bias arisen from patient selection should not be neglected. Patients who underwent operation were probably having a smaller tumor load and better performance status. In patients requiring pancreaticoduodenectomy, there is another potential complication of sepsis if IAT is to be considered after the operation.
Local ablative therapies
In patients who have multifocal disease, suboptimal liver function or poorer performance status, ablative therapies such as radiofrequency ablation (RFA) can be considered. It could be used as both a primary treatment or as combined treatment with resection in order to maximize tumor clearance. Mazzaglia et al. (45) reported outcomes for 63 patients who underwent RFA for NELM and demonstrated a median survival of 11 years, with only a 6.3% recurrence rate. Most of the other studies included RFA as an adjunct to resection, which showed a clear survival benefit in oppose to non-surgical treatments (Table 1). Taner et al. (46) reported an overall survival of 80% and 59% at 5 and 10 years respectively when RFA was used as combined treatment. RFA is particularly useful in recurrent disease, when resection becomes limited after multiple operations (19).
The limitation of effectiveness of ablation is mainly by the size of the tumour. It would be difficult to achieve complete ablation once the tumour diameter exceeds 3 cm, whereas lesions over 5 cm should deem unsuitable. If there are large number of bilobar lesions, ablation alone is contraindicated (45,47).
LT
LT as a treatment for NELM is still highly controversial. Due to the lack of long-term results and prospective trials, the selection criteria are still poorly defined. Some of the guidelines such as Milan-NET criteria (48) or the ENETS guidelines (9) may provide some reference. Table 2 summarizes the results from various studies. There was no randomized study comparing LT versus other treatment modalities (55,56). According to the review by Fan et al. (57), the 5-year OS was similar between transplanted and non-transplanted patients, but the 5-year DFS was improved for post-LT patients (50% vs. 34%). When interpreting the results, one must bear in mind that LT is usually only performed when all other treatment modalities have been exhausted. It would be better to measure survival after diagnosis of NELM rather than survival after LT, and any pre-LT treatment should be taken into account.
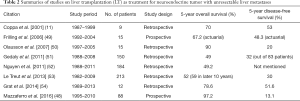
Full table
Some of the more commonly used selection criteria includes younger age (<45–55 years old), low Ki67 expression (<10%), primary tumors solely draining into portal system, lower hepatic tumor load (<50%) and absence of extrahepatic disease (11,54,58,59). With these criteria, the 5-year OS was up to 70–90% and the 5-year PFS was as high as 80% (60). Some suggested a delay in LT for over 6 months may help to select a group of patient with better prognosis (58). This was also recommended in Milan-NET criteria, ENETS guidelines and the Organ Procurement and Trans-plantation Network in the United States (61). This may imply these patients simply would have a better survival because of their stable disease. Whether LT should be offered to patients with stable disease, or those with progressive disease despite other treatment, is still a matter of debate.
Role of neoadjuvant or adjuvant treatment
Neoadjuvant and adjuvant treatment for down-staging or preventing recurrence have been introduced in single case reports and smaller series. Therapies such as PRRT are reported to be useful in down-staging unresectable NELM (62-65). The combination of PRRT with radiosensitizing chemotherapy has also been considered as promising to improve resectability of NELM (66). In another retrospective cohort of 52 patients (67), it was found that the PFS at 5 years were similar in patients who received adjuvant chemotherapy with streptozotocin-5-FU when compared with observation group. Since there was no randomized controlled trial performed, the results may be confounded as those who are with advanced disease are more likely to be selected for neoadjuvant or adjuvant therapy (19). Concrete evidence is still lacking in this topic and these therapeutic strategies should be used with caution (68).
Conclusions
Surgical resection remains to be the mainstay of treatment for NELM. Radical resection of both the primary and NELM should be contemplated whenever possible. If the tumor load could be reduced by 80–90%, surgical debulking with or without the combination of ablation should still be considered. LT is possible in patients with unresectable NELM, but only applicable in a highly selected group. Otherwise in patients with bilobar involvement or disseminated disease, intra-arterial therapies and medical therapies still play an important role.
Currently there are still a lot of unknowns in the management of NELM, as it is particularly difficult to conduct large scale randomized controlled trial in a relatively rare disease. Since complete resection is still the only hope of cure so far, ways to downstage or downsize tumor should be explored in patients with NELM. Although there are proven efficacy in multiple systemic treatments (somatostatin analogues, PRRT, chemotherapy, targeted therapy), there are few studies which explored the potential in neoadjuvant setting. There is also no clear answer to the management of recurrent disease and the optimal treatment after debulking surgery. In order to come up with the best treatment strategy, collaboration between different specialties such as oncologists, radiologists and surgeons is of paramount importance.
Acknowledgements
None.
Footnote
Conflicts of Interest: The authors have no conflicts of interest to declare.
References
- Fraenkel M, Faggiano A, Valk GD. Epidemiology of Neuroendocrine Tumors. Front Horm Res 2015;44:1-23. [Crossref] [PubMed]
- Hallet J, Law CH, Cukier M, et al. Exploring the rising incidence of neuroendocrine tumors: a population-based analysis of epidemiology, metastatic presentation, and outcomes. Cancer 2015;121:589-97. [Crossref] [PubMed]
- Ito T, Igarashi H, Nakamura K, et al. Epidemiological trends of pancreatic and gastrointestinal neuroendocrine tumors in Japan: a nationwide survey analysis. J Gastroenterol 2015;50:58-64. [Crossref] [PubMed]
- Lawrence B, Gustafsson BI, Chan A, et al. The epidemiology of gastroenteropancreatic neuroendocrine tumors. Endocrinol Metab Clin North Am 2011;40:1-18. vii. [Crossref] [PubMed]
- Sandvik OM, Soreide K, Gudlaugsson E, et al. Epidemiology and classification of gastroenteropancreatic neuroendocrine neoplasms using current coding criteria. Br J Surg 2016;103:226-32. [Crossref] [PubMed]
- Fraenkel M, Kim M, Faggiano A, et al. Incidence of gastroenteropancreatic neuroendocrine tumours: a systematic review of the literature. Endocr Relat Cancer 2014;21:R153-63. [Crossref] [PubMed]
- Chamberlain RS, Canes D, Brown KT, et al. Hepatic neuroendocrine metastases: does intervention alter outcomes? J Am Coll Surg 2000;190:432-45. [Crossref] [PubMed]
- Moertel CG. Karnofsky memorial lecture. An odyssey in the land of small tumors. J Clin Oncol 1987;5:1502-22. [Crossref] [PubMed]
- Pavel M, O'Toole D, Costa F, et al. ENETS Consensus Guidelines Update for the Management of Distant Metastatic Disease of Intestinal, Pancreatic, Bronchial Neuroendocrine Neoplasms (NEN) and NEN of Unknown Primary Site. Neuroendocrinology 2016;103:172-85. [Crossref] [PubMed]
- Gurusamy KS, Ramamoorthy R, Sharma D, et al. Liver resection versus other treatments for neuroendocrine tumours in patients with resectable liver metastases. Cochrane Database Syst Rev 2009.CD007060. [PubMed]
- Coppa J, Pulvirenti A, Schiavo M, et al. Resection versus transplantation for liver metastases from neuroendocrine tumors. Transplant Proc 2001;33:1537-9. [Crossref] [PubMed]
- Yao KA, Talamonti MS, Nemcek A, et al. Indications and results of liver resection and hepatic chemoembolization for metastatic gastrointestinal neuroendocrine tumors. Surgery 2001;130:677-82; discussion 682-5. [Crossref] [PubMed]
- Sarmiento JM, Heywood G, Rubin J, et al. Surgical treatment of neuroendocrine metastases to the liver. J Am Coll Surg 2003;197:29-37. [Crossref] [PubMed]
- Partelli S, Inama M, Rinke A, et al. Long-Term Outcomes of Surgical Management of Pancreatic Neuroendocrine Tumors with Synchronous Liver Metastases. Neuroendocrinology 2015;102:68-76. [Crossref] [PubMed]
- Saxena A, Chua TC, Perera M, et al. Surgical resection of hepatic metastases from neuroendocrine neoplasms: a systematic review. Surg Oncol 2012;21:e131-41. [Crossref] [PubMed]
- Fan ST, Lai EC, Lo CM, et al. Hospital mortality of major hepatectomy for hepatocellular carcinoma associated with cirrhosis. Arch Surg 1995;130:198-203. [Crossref] [PubMed]
- Fan ST, Lo CM, Liu CL, et al. Hepatectomy for hepatocellular carcinoma: toward zero hospital deaths. Ann Surg 1999;229:322. [Crossref] [PubMed]
- Poon RT, Fan ST, Lo CM, et al. Improving perioperative outcome expands the role of hepatectomy in management of benign and malignant hepatobiliary diseases: analysis of 1222 consecutive patients from a prospective database. Ann Surg 2004;240:698. [PubMed]
- Zhang XF, Beal EW, Chakedis J, et al. Early Recurrence of Neuroendocrine Liver Metastasis After Curative Hepatectomy: Risk Factors, Prognosis, and Treatment. J Gastrointest Surg 2017;21:1821-30. [Crossref] [PubMed]
- Cho CS, Labow DM, Tang L, et al. Histologic grade is correlated with outcome after resection of hepatic neuroendocrine neoplasms. Cancer 2008;113:126-34. [Crossref] [PubMed]
- Hibi T, Sano T, Sakamoto Y, et al. Surgery for hepatic neuroendocrine tumors: a single institutional experience in Japan. Jpn J Clin Oncol 2007;37:102-7. [Crossref] [PubMed]
- Frilling A, Clift AK. Therapeutic strategies for neuroendocrine liver metastases. Cancer 2015;121:1172-86. [Crossref] [PubMed]
- Yuan CH, Wang J, Xiu DR, et al. Meta-analysis of Liver Resection Versus Nonsurgical Treatments for Pancreatic Neuroendocrine Tumors with Liver Metastases. Ann Surg Oncol 2016;23:244-9. [Crossref] [PubMed]
- Fairweather M, Swanson R, Wang J, et al. Management of neuroendocrine tumor liver metastases: long-term outcomes and prognostic factors from a large prospective database. Ann Surg Oncol 2017;24:2319-25. [Crossref] [PubMed]
- Ruzzenente A, Bagante F, Bertuzzo F, et al. A novel nomogram to predict the prognosis of patients undergoing liver resection for neuroendocrine liver metastasis: an analysis of the italian neuroendocrine liver metastasis database. J Gastrointest Surg 2017;21:41-8. [Crossref] [PubMed]
- Spolverato G, Bagante F, Aldrighetti L, et al. Neuroendocrine Liver Metastasis: Prognostic Implications of Primary Tumor Site on Patients Undergoing Curative Intent Liver Surgery. J Gastrointest Surg 2017;21:2039-47. [Crossref] [PubMed]
- Chen H, Hardacre JM, Uzar A, et al. Isolated liver metastases from neuroendocrine tumors: does resection prolong survival? J Am Coll Surg 1998;187:88-92; discussion 92-3. [Crossref] [PubMed]
- Touzios JG, Kiely JM, Pitt SC, et al. Neuroendocrine Hepatic Metastases. Ann Surg 2005;241:776-83; discussion 783-5. [Crossref] [PubMed]
- Musunuru S, Chen H, Rajpal S, et al. Metastatic neuroendocrine hepatic tumors: Resection improves survival. Arch Surg 2006;141:1000-4. [Crossref] [PubMed]
- Osborne DA, Zervos EE, Strosberg J, et al. Improved outcome with cytoreduction versus embolization for symptomatic hepatic metastases of carcinoid and neuroendocrine tumors. Ann Surg Oncol 2006;13:572-81. [Crossref] [PubMed]
- Mayo SC, de Jong MC, Bloomston M, et al. Surgery versus intra-arterial therapy for neuroendocrine liver metastasis: a multicenter international analysis. Ann Surg Oncol 2011;18:3657-65. [Crossref] [PubMed]
- Que FG, Nagorney DM, Batts KP, et al. Hepatic resection for metastatic neuroendocrine carcinomas. Am J Surg 1995;169:36-42; discussion 42-3. [Crossref] [PubMed]
- Graff-Baker AN, Sauer DA, Pommier SJ, et al. Expanded criteria for carcinoid liver debulking: Maintaining survival and increasing the number of eligible patients. Surgery 2014;156:1369-76; discussion 1376-7. [Crossref] [PubMed]
- Maxwell JE, Sherman SK, O'Dorisio TM, et al. Liver-directed surgery of neuroendocrine metastases: What is the optimal strategy? Surgery 2016;159:320-33. [Crossref] [PubMed]
- Garcia-Carbonero R, Sorbye H, Baudin E, et al. ENETS consensus guidelines for high-grade gastroenteropancreatic neuroendocrine tumors and neuroendocrine carcinomas. Neuroendocrinology 2016;103:186-94. [Crossref] [PubMed]
- Galleberg RB, Knigge U, Janson ET, et al. Results after surgical treatment of liver metastases in patients with high-grade gastroenteropancreatic neuroendocrine carcinomas. Eur J Surg Oncol 2017;43:1682-9. [Crossref] [PubMed]
- Du S, Wang Z, Sang X, et al. Surgical resection improves the outcome of the patients with neuroendocrine tumor liver metastases: large data from Asia. Medicine (Baltimore) 2015;94:e388. [Crossref] [PubMed]
- Kulke MH, Benson AB, Bergsland E, et al. National Comprehensive Cancer Network Guidelines in Oncology: Neuroendocrine Tumors (Version 3.2017). 2017. Available online: (Accessed September 21, 2017).https://www.nccn.org/professionals/physician_gls/pdf/neuroendocrine.pdf
- Boudreaux JP, Putty B, Frey DJ, et al. Surgical Treatment of Advanced-Stage Carcinoid Tumors. Ann Surg 2005;241:839-45. [Crossref] [PubMed]
- Bettini R, Mantovani W, Boninsegna L, et al. Primary tumour resection in metastatic nonfunctioning pancreatic endocrine carcinomas. Dig Liver Dis 2009;41:49-55. [Crossref] [PubMed]
- Bertani E, Falconi M, Grana C, et al. Small intestinal neuroendocrine tumors with liver metastases and resection of the primary: Prognostic factors for decision making. Int J Surg 2015;20:58-64. [Crossref] [PubMed]
- Bertani E, Fazio N, Botteri E, et al. Resection of the primary pancreatic neuroendocrine tumor in patients with unresectable liver metastases: possible indications for a multimodal approach. Surgery 2014;155:607-14. [Crossref] [PubMed]
- Bertani E, Fazio N, Radice D, et al. Assessing the role of primary tumour resection in patients with synchronous unresectable liver metastases from pancreatic neuroendocrine tumour of the body and tail. A propensity score survival evaluation. Eur J Surg Oncol 2017;43:372-9. [Crossref] [PubMed]
- Citterio D, Pusceddu S, Facciorusso A, et al. Primary tumour resection may improve survival in functional well-differentiated neuroendocrine tumours metastatic to the liver. Eur J Surg Oncol 2017;43:380-7. [Crossref] [PubMed]
- Mazzaglia PJ, Berber E, Milas M, et al. Laparoscopic radiofrequency ablation of neuroendocrine liver metastases: a 10-year experience evaluating predictors of survival. Surgery 2007;142:10-9. [Crossref] [PubMed]
- Taner T, Atwell TD, Zhang L, et al. Adjunctive radiofrequency ablation of metastatic neuroendocrine cancer to the liver complements surgical resection. HPB (Oxford) 2013;15:190-5. [Crossref] [PubMed]
- Macedo D, Amaral T, Fernandes I, et al. The Treatment of Liver Metastases in Patients with Neuroendocrine Tumors in 2012. ISRN Hepatol 2013;2013:702167. [PubMed]
- Mazzaferro V, Sposito C, Coppa J, et al. The Long-Term Benefit of Liver Transplantation for Hepatic Metastases From Neuroendocrine Tumors. Am J Transplant 2016;16:2892-902. [Crossref] [PubMed]
- Frilling A, Malago M, Weber F, et al. Liver transplantation for patients with metastatic endocrine tumors: single-center experience with 15 patients. Liver Transpl 2006;12:1089-96. [Crossref] [PubMed]
- Olausson M, Friman S, Herlenius G, et al. Orthotopic liver or multivisceral transplantation as treatment of metastatic neuroendocrine tumors. Liver Transpl 2007;13:327-33. [Crossref] [PubMed]
- Gedaly R, Daily MF, Davenport D, et al. Liver transplantation for the treatment of liver metastases from neuroendocrine tumors: An analysis of the unos database. Arch Surg 2011;146:953-8. [Crossref] [PubMed]
- Nguyen NT, Harring TR, Goss JA, et al. Neuroendocrine Liver Metastases and Orthotopic Liver Transplantation: The US Experience. Int J Hepatol 2011;2011:742890. [PubMed]
- Le Treut YP, Gregoire E, Klempnauer J, et al. Liver transplantation for neuroendocrine tumors in Europe-results and trends in patient selection: a 213-case European liver transplant registry study. Ann Surg 2013;257:807-15. [Crossref] [PubMed]
- Grąt M, Remiszewski P, Smoter P, et al. Outcomes following liver transplantation for metastatic neuroendocrine tumors. Transplant Proc 2014;46:2766-9. [Crossref] [PubMed]
- Moris D, Tsilimigras DI, Ntanasis-Stathopoulos I, et al. Liver transplantation in patients with liver metastases from neuroendocrine tumors: A systematic review. Surgery 2017;162:525-36. [Crossref] [PubMed]
- Rossi RE, Burroughs AK, Caplin ME. Liver Transplantation for Unresectable Neuroendocrine Tumor Liver Metastases. Ann Surg Oncol 2014;21:2398-405. [Crossref] [PubMed]
- Fan ST, Le Treut YP, Mazzaferro V, et al. Liver transplantation for neuroendocrine tumour liver metastases. HPB (Oxford) 2015;17:23-8. [Crossref] [PubMed]
- Mazzaferro V, Pulvirenti A, Coppa J. Neuroendocrine tumors metastatic to the liver: how to select patients for liver transplantation? J Hepatol 2007;47:460-6. [Crossref] [PubMed]
- Clift AK, Frilling A. Management of patients with hepatic metastases from neuroendocrine tumors. Ann Saudi Med 2014;34:279-90. [Crossref] [PubMed]
- Alagusundaramoorthy SS, Gedaly R. Role of surgery and transplantation in the treatment of hepatic metastases from neuroendocrine tumor. World J Gastroenterol 2014;20:14348-58. [Crossref] [PubMed]
- The Organ Procurement and Transplantation Network (OPTN): Guidance on MELD PELD exception review. 2015. Available online: http://optn.transplant.hrsa.gov/resources/by-organ/liver-intestine/guidance-on-meld-peld-exception-review/#PSC
- Stoeltzing O, Loss M, Huber E, et al. Staged surgery with neoadjuvant 90Y-DOTATOC therapy for down-sizing synchronous bilobular hepatic metastases from a neuroendocrine pancreatic tumor. Langenbecks Arch Surg 2010;395:185-92. [Crossref] [PubMed]
- Sowa-Staszczak A, Pach D, Chrzan R, et al. Peptide receptor radionuclide therapy as a potential tool for neoadjuvant therapy in patients with inoperable neuroendocrine tumours (NETs). Eur J Nucl Med Mol Imaging 2011;38:1669-74. [Crossref] [PubMed]
- van Vliet EI, van Eijck CH, de Krijger RR, et al. Neoadjuvant Treatment of Nonfunctioning Pancreatic Neuroendocrine Tumors with [177Lu-DOTA0,Tyr3]Octreotate. J Nucl Med 2015;56:1647-53. [Crossref] [PubMed]
- La Salvia A, Partelli S, Tampellini M, et al. Management of hepatic metastases of well/moderately differentiated neuroendocrine tumors of the digestive tract. J Cancer Metasta Treat 2016;2:294-303. [Crossref]
- Kong G, Johnston V, Ramdave S, et al. High-administered activity In-111 octreotide therapy with concomitant radiosensitizing 5FU chemotherapy for treatment of neuroendocrine tumors: preliminary experience. Cancer Biother Radiopharm 2009;24:527-33. [Crossref] [PubMed]
- Maire F, Hammel P, Kianmanesh R, et al. Is adjuvant therapy with streptozotocin and 5-fluorouracil useful after resection of liver metastases from digestive endocrine tumors? Surgery 2009;145:69-75. [Crossref] [PubMed]
- Frilling A, Modlin IM, Kidd M, et al. Recommendations for management of patients with neuroendocrine liver metastases. Lancet Oncol 2014;15:e8-21. [Crossref] [PubMed]


