Cooled tip radiofrequency ablation of benign thyroid nodules: preliminary experience with two different devices
Introduction
Thyroid nodules are a common finding in general population. They can be approximatively discovered in 20–76% by ultrasound (US) scan, and in 3–7% by palpation (1).
Most nodules are asymptomatic and benign, and can be managed by observation only. However, even benign nodules may cause problems, such as subjective symptoms or cosmetic concerns (2).
Surgery and radioiodine therapy are still considered the mainstay of treatment, but both of these options have drawbacks. In particular, surgery carries a 2–10% complication rate and requires general anesthesia and hospitalization. Furthermore, it is costly, and may cause problems such as scar formation and iatrogenic hypothyroidism (3).
In recent years, nonsurgical minimally invasive techniques have been developed: percutaneous ethanol injection (PEI), laser ablation (LA), radiofrequency ablation (RFA), microwave ablation (MWA) and high-intensity focused ultrasound (HIFU) (4-8).
In particular, RFA is a minimally invasive treatment that had been used for benign and malignant tumors in liver, kidney and lung, resulting in long-term tissue necrosis (9-11).
Since 2006, many studies have evaluated feasibility, safety and efficacy of RFA in benign thyroid nodules, reporting good results in reducing both the volume of the nodules and the associated compressive symptoms/cosmetic concerns. More recent studies have demonstrated the absence of regrowth at the mid-term follow-up controls (39.4±21.7 months) in 76% of cases (12).
In 2015, a strong consensus regarding RFA treatment of selected types of thyroid nodules was reached, and stated that this treatment should be applied to “large (volume >20 mL), nonfunctioning, benign thyroid nodules in patients presenting with local symptoms or cosmetic complaints when surgery is contraindicated or declined”.
Nevertheless, only partial agreement has been reached for smaller (volume <20 mL) nonfunctioning, benign thyroid nodules that cause early local discomfort and show a significant growth over time (13).
In the present study, we report our experience and our preliminary results about RFA of benign thyroid nodules performed with two different devices, comparing our results.
Methods
This study was approved by our Internal Review Board.
From June 2015 to June 2017 a total of 27 nodules in 24 patients were treated (9 males; 15 females; mean age 57.9 years; range, 34–83 years). In three patients two different nodules were treated in the same session, because the second nodule was closer to the main one and both could be reached by a single percutaneous access. In order to avoid the bias caused by the influence of the treatment of one nodule on the other one, in our results we considered the two contiguous nodules as a single entity.
Patients were divided in two groups, according to the system used:
- Group A: monopolar, straight-type, internally cooled 18 gauge needle, with 7–10 mm active tip; AMICA, HS Hospital Service, Aprilia, Italy: from June 2015 to September 2016;
- Group B: monopolar, straight-type, internally cooled 17 gauge needle, with 7–10–20 mm active tip; COVIDIEN, Mansfield, MA, USA: from October 2016 to June 2017.
Equipment
Arietta V70 (Hitachi Aloka Medical, Tokyo, Japan) was used as US-guidance during the procedure, for the initial diagnostic evaluation and for the follow-up.
All patients were studied with US before the treatment by one of the two radiologists enrolled in the study, with 25 and 22 years of thyroid US experience, respectively. The procedure was successively performed by one of them.
Pre-ablation assessment
Clinical history, symptoms and thyroid-focused laboratory data were registered for each patient. In particular, the symptom and cosmetic scores were evaluated as described in a previous consensus statement (14): all patients were asked to rate pressure symptoms on a 10 cm (grade 0–10) visual analogue scale (VAS) at enrollment and during follow-up. The cosmetic score was evaluated by the clinician as follows: (I) no palpable mass; (II) no cosmetic problem but palpable mass; (III) a cosmetic problem on swallowing only; (IV) easily visible mass.
The following nodules characteristics were assessed for each patient:
Number:
- Single nodule;
- Dominant nodule in goiter.
Composition:
- Solid: solid portion >80%;
- Cystic: cystic portion >80%;
- Mixed: not meeting the criteria for solid or cystic.
Vascularization:
- 0: spotty perinodular vascularization;
- 1: perinodular vascularization with few intranodular vascular spots;
- 2: both perinodular and intra-nodular vascularization.
Size:
- Volume: calculated through the formula V = πabc/6 (where V is the volume, a is the largest dimeter, b and c the two perpendicular diameters). Blinded results, in particular the average of the two measurements, were calculated before, during and after the procedure.
A US-guided fine needle aspiration cytology (FNAC) was performed within 36 months before the RFA to confirm the benignity of the nodules. Fiber laryngoscopy was not considered as a mandatory pre-ablation examination.
Inclusion criteria
- Symptomatic nodules (symptom score from 2 to 10): compressive symptoms, neck discomfort, foreign body sensation, throat irritation, dyspnea;
- Asymptomatic nodules (symptom score from 0 to 1) with:
- Tracheal deviation/compression;
- And/or enlargement >20% of the volume in the last year.
- Refusal or contraindications to surgery.
Cosmetic concerns alone were not considered an inclusion criterion because of the lack of refund from our National Health Service (15).
Exclusion criteria
- Nodules different from “Tir-1c” or “Tir-2” according to SIAPEC classification (16);
- Absence of a dominant thyroid nodule at US;
- Coagulation disorders.
Procedure
The following intraprocedural data were recorded for each patient: thin needle aspiration pre-RFA, number of skin punctures, active tip used, needle visibility, power output, total ablation time. All the procedures were performed on an outpatient basis.
Laboratory tests, including complete blood count and blood coagulation tests (prothrombin time, activated partial thromboplastin time) were performed and the values were within normal range in all patients. Informed consent was obtained from each patient.
The patients were put in a supine position with hyperextended neck. Two ground pads were positioned on the patient’s thighs before the procedure.
Sedation was obtained in accordance with the confidence of the interventional radiologist about the use of sedation drugs. We used a bland systemic sedoanalgesia (20 mg of sublingual morphin sulfate and 1–2 mg intravenous midazolam) together with local anesthesia (10–20 mg/mL of lidocaine hydrochloride) of the subcutaneous tissues. In the last five patients treated we also injected a mixture of anesthetics and physiological saline (mepivacaine 10 mL + bupivacaine 10 mL + physiological saline 10 mL) into the thyroid capsule, thus creating a “liquid isolating region”, in order to protect the surrounding structures from thermal injury and to reduce intra-procedural pain.
For mixed or mainly cystic nodules, fluid was aspirated under US-guidance with a 22 gauge needle just before ablation.
The US visibility of the needle was judged by the operator and ranked on a scale from 1 to 4 (1: insufficient; 2: sufficient; 3: good; 4: excellent) (Figure 1).
The needle was positioned under US-guidance through a <2 mm incision of the anterior neck tissues. The incision was made in a central position of the nodule along the cranio-caudal axis in order to reach all of its parts with a single access, if possible.
In all cases a trans-isthmic approach together with the “moving-shot” technique was used (2). This technique allows creating multiple units of ablation of the nodule. The tip of the functioning needle is positioned into a specific nodule unit, until a growth in the electric impedance on the RF generator is recorded. When this happens, and usually corresponds to the moment when the typical US hyperechoic changes in the treated area appear, the needle is placed into the next unit to treat. The procedure is stopped when the entire area of the nodule becomes a transient hyperechoic zone.
In Figures 2-4 a scheme of our treatment modality is reported, “from riskier to safer”, in order to reduce the risk of double skin punctures and the risk of suboptimal visualization of the needle during RFA, especially in the most dangerous regions to treat.
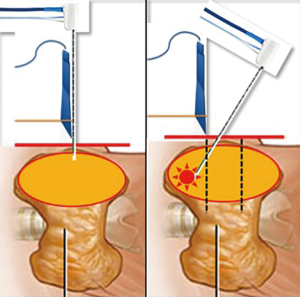
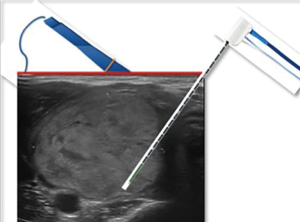
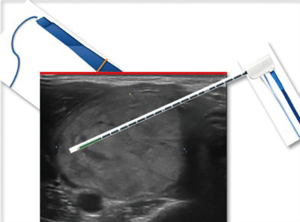
After the procedure only cold ice was applied at the entry site. No neck compression was used. The patient was observed for 3–5 hours before discharge.
Post-ablation assessment
Our study protocol plans to perform a clinical and US evaluation at 1-, 3-, 6- and 12-month. However, all the results were evaluated only at 1 month follow-up as it was the only one post-ablation control reached by all the patients.
At the 1 month follow-up the complications occurred, changes in volume of the treated nodules and any variation in the symptom and cosmetic score were registered.
Complications were classified according to Common Terminology Criteria for Adverse Events (17). Safety was evaluated on the basis of the complications recorded immediately after the procedure and during the follow-up (18). Major complications were defined as complications that, if untreated, might threaten the patient’s life, lead to substantial morbidity and disability, or result in hospital admission or substantially lengthen the patient’s hospital stay (19-20). Minor complications included typical post-ablation syndrome (fever <38 °C, pain, nausea and vomiting) if present for more than 4 days after the ablation procedure. The post-ablation syndrome lasting less than 4 days without any treatment is considered a side effect.
The volume of the nodules, symptom score and cosmetic score were evaluated as described in the pre-procedural assessment.
Statistical analysis
All calculations have been performed in R (R Foundation for Statistical Computing, Vienna, Austria). To evaluate the difference between the two needles in terms of efficacy, we used a linear model with the initial volume of the nodule and the assigned group (A or B) as predictors. We modeled the difference between the two groups as an interaction term. This was used to test whether the slopes in the two groups were significantly different. For all clinical scores, we performed a Mann-Whitney test on the differences between pre and post treatment for the two groups. To assess whether an increased risk of complications could arise in one of the two groups, we performed a logistic regression. Differences were considered significant when P<0.05.
Results
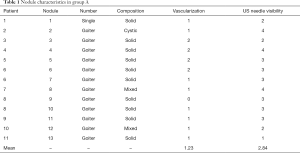
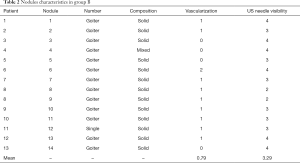
The characteristics of the nodules of the two groups are shown in the Tables 1,2. All the results reported were obtained at 1-month follow-up.
Full table
Full table
Group A includes 11 patients (male:female ratio 5:6; mean age: 56.9 years; range, 44–75 years). In two patients two contiguous nodules were treated in the same procedure, therefore a total of 13 nodules were treated in the group A.
Group B includes 13 patients (male:female ratio 4:9; mean age: 58.7 years; range, 34–83 years). In one patient two contiguous nodules were treated in the same procedure, therefore a total of 14 nodules were treated in the group B.
Pre-ablation assessment
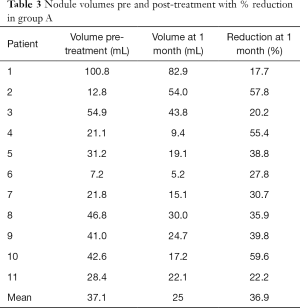
All the data collected before the treatments are more extensively reported in Tables 1-6.
Full table
Full table

Full table

Full table
Symptom score and cosmetic score
Group A
Eight patients (72.3%) complained of symptomatic nodules (from grade 2 to grade 8). The other three patients (27.7%) had asymptomatic nodules. Before the treatment, the mean symptom score in group A was 3.1.
Ten patients (90.9%) suffered from visible nodules (grade 3–4 in cosmetic score), while the left one (9.1%) had a grade 1 nodule. Before the treatment, the mean cosmetic score in group A was 3.7.
Group B
Eleven patients (84.6%) complained of symptomatic nodules. The other two patients (15.4%) had asymptomatic nodules. Before the treatment, the mean symptom score in group B was 4.
All the patients (100%) suffered from visible nodules (grades 3–4 in cosmetic score). Before the treatment, the mean cosmetic score in group B was 3.9.
Nodules characteristics
Group A
Twelve out of 13 (92.3%) nodules were part of a goiter.
Composition was mainly solid (76.9%), with 2 mixed (15.4%) and 1 cystic (7.7%) nodules.
Vascularization was principally intermediate (61.5%), the other cases being high (30.8%) or low (7.7%).
The mean volume of the nodules treated at baseline was 37.1 mL (range, 7.2–100.8 mL).
Group B
Thirteen out of 14 (92.9%) nodules were part of a goiter.
Composition was mainly solid (92.9%), with only 1 mixed nodule (7.1%).
Vascularization was principally intermediate (64.3%), the other cases being high (7.1%) or low (28.6%).
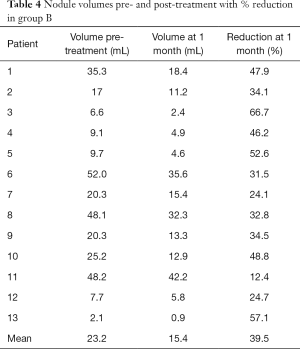
The mean volume of the nodules treated at baseline was 23.2 mL (range, 2.1–52 mL).
Volume variation
Nodule volume at baseline did not significantly differ between the two groups (P=0.129).
Procedure
All the intraprocedural data are more extensively reported in Table 7.

Full table
Group A
Five patients (45.5%) received a double skin puncture due to the dimensions of the nodule.
Mean ablation time was 10.1 minutes. Mean power output was 38.2 Watts.
The 10 mm active tip was used in nine patients (72.7%); 7 mm active tip in the remaining three patients (27.3%).
Pre-procedural thin needle aspiration was performed in three patients (27.3%).
Group B
Three patients (23.1%) received a double skin puncture due to the dimensions of the nodule.
Mean ablation time was 10.8 minutes. Mean power output was 48.1 Watts.
The 10 mm active tip was used in eight patients (61.5%); 7 mm active tip was used in three patients (23.1%); 20 mm active tip in the remaining two patients (15.4%).
All the nodules were mainly solid, therefore pre-procedural needle aspiration was never performed.
Visibility
Mean US visibility of the needle during RFA corresponded to 2.84 in group A and to 3.29 in group B: no significant difference in visibility was observed by comparing the two needles (P=0.1448).
US changes
Group A
At 1 month follow-up the mean volume of the nodules treated was 25 mL (range, 5.2–82.9 mL). Comparing to the volume before the procedure, nodules of the group A showed a 36.9% mean volume reduction (range, 17.7–59.6%). The volume significantly decreased compared to baseline (P<0.0001).
Group B
At 1 month follow-up the mean volume of the nodules treated was 15.4 mL (range, 0.9–42.2 mL). Comparing to the volume before the procedure, nodules of the group B showed a 39.5% mean volume reduction (range, 12.4–66.7%) (Figures 5,6). The volume significantly decreased compared to baseline (P<0.0001).

However, the slope of the linear regression line did not significantly differ between the two groups (P=0.3137; Figure 7).

Clinical changes
No significant differences were observed by comparing the reduction of the symptom score and the cosmetic score between the two groups (Table 8).

Full table
Group A
An improvement in the symptom score was observed in all the symptomatic patients (8/11: 72.3%). In group A, the mean symptom score decreased from 3.1 to 1.4. None of the scores was found to be higher than 4 (Table 5).
A reduction of the cosmetic score in patients with a visible nodule (10/11: 90.9%) was observed in the 30% of cases; 40% kept on being assigned to grade 4 even if their nodule was less evident; 30% did not showed any kind of changes at inspection (Table 5).
Group B
An improvement in the symptom score was observed in all the symptomatic patients (11/13: 84.6%). In group B, the mean symptom score decreased from 4 to 1.6. None of the scores was found to be higher than 3 (Table 6).
A reduction of the cosmetic score in patients with a visible nodule (all the patients with 3 or 4 grade symptom score before the treatment) was observed in 15.4% of cases; 53.8% kept on being assigned grade 4 even if their nodule was less evident; 30.8% did not showed any kind of changes at inspection (Table 6).
Complications
All the complications, divided in major and minor complications, are reported in the Tables 9,10.

Full table
Full table
Group A
One major complication (9.1%) was observed and consisted in recurrent laryngeal nerve palsy. The patient complained of voice change and dyspnea the day after the procedure. Fiberoptic bronchoscopy revealed a previously unknown condition of tracheomalacia. Steroid therapy was started immediately and led to resolution of the dyspnea in about 24 hours; dysphonia resolved within 1 month.
One minor complication (9.1%) was registered: a 3-mm thickness subcapsular hematoma, resolved itself without any treatment.
Among the side effects, we observed one case of low-grade fever (<38 °C) the day after the procedure in the context of a mild post-ablation syndrome, that resolved within 24 hours.
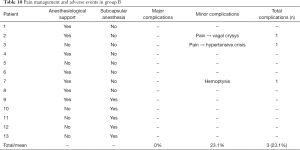
Group B
No major complications were observed.
Three minor complications were registered (23.1%), with two of them being related to pain during ablation. One patient suffered from a vasovagal reaction that resolved in few minutes after the administration of 1 mg intravenous atropine; another patient had a hypertensive crisis which subsided without any intervention, but required the procedure to be paused for few minutes.
In the last case, an episode of hemoptysis was observed immediately after the end of the procedure. Urgent bronchoscopy didn’t report iatrogenic lesion and revealed only a microscopic clot on the membranous pars of the trachea. Hemoptysis was therefore attributed to a mild thermal injury of the trachea. No other episodes of hemoptysis were registered until the discharge and during follow-up.
Among the side effects, in the group B we observed one case of low-grade fever (<38 °C) the day after the procedure in the context of a mild post-ablation syndrome, which resolved within 24 hours. Comparing both major and minor complications between the two groups, no significant difference was observed in the complication rate (18.2% vs. 23.1%, respectively; P=0.5049).
Conclusions
Single thyroid nodules or nodular goiter are two common clinical conditions, for which surgery has been the traditional treatment procedure (21). However, US-guided minimally invasive techniques have been developed in the last two decades for patients not suitable for thyroidectomy; these procedures carry on some advantages, like the absence of general anesthesia and scar formation, or the possibility of causing iatrogenic hypothyroidism. Furthermore, minimally invasive techniques are less costly respect to surgery (22).
Among these, PEI was the first treatment described in literature (4), and its indications are mainly related to cystic nodules. LA has shown good but slightly worse results, when compared to RFA (5). MWA is an emerging technique (7), but the needle’s diameter (14 gauge) represents a major limitation when working on the thyroid gland. HIFU (8) is a non-invasive procedure recently developed: more studies are necessary to evaluate its efficacy.
RFA is currently the most promising alternative to surgery in case of benign thyroid nodules. In a recent review, 989 benign thyroid nodules in 895 patients divided in 16 studies have been encountered (23): mean reduction volume after ranged from 50.7% to 93.5%. The Korean Society of Thyroid Radiology recently developed a consensus to conform RFA treatments (24), in particular regarding inclusion criteria and procedural techniques.
Our study represents a single-center experience in the treatment with RFA of benign thyroid nodules. To our knowledge, no previous studies have described the use and compared two different devices. The current series aims to evaluate if different needles are associated with differences in safety and efficacy of the treatment.
The two devices used were:
- Amica, HS Hospital Service, Aprilia, Italy (group A);
- Covidien, Mansfield, MA, USA (group B).
They had similar characteristics (both monopolar, straight-type, internally cooled needles), but differed for needle diameter (18 vs. 17 gauge, respectively) and, in our study, US visibility (2.84 in group A vs. 3.29 in group B).
The two groups presented a good matching in patients and nodules characteristics, so that a prospective comparative evaluation was performed:
- both groups show female prevalence (5:6 and 4:9, respectively) and similar mean age (56.9 and 58.7 years, respectively). Also symptom and cosmetic score before the treatment were similar (Tables 5,6);
- In both groups, nodules were mainly part of a goiter; composition was mainly solid and vascularization mostly intermediate (Tables 1,2). No significant difference was observed in the mean volume at baseline (Tables 3,4).
Our results confirmed safety and efficacy of percutaneous RFA.
RFA safety profile was confirmed by the presence of only one major complication (4.2% prevalence in total, while surgery carries a 2–10% complication rate) (3).
Effectiveness can be only partly confirmed because of our short follow-up: data reported in literature demonstrate how 1-month follow-up is a too short amount of time to evaluate the real reduction of the nodule treated (25,26); however, our mean volume reduction (38.3%) is in line with other results at 1 month follow-up visits in published literature (32.7–46.5%).
A good US visibility of the needle represents an important technical aspect that leads to a better working-condition for the operator and a safer procedure for the patient.
No significant differences in US visibility was observed (P=0.0787), between the two needles, even if the 17 gauge needle showed a slight better visibility for the operator (3.29 vs. 2.84). Even in terms of volume reduction and complication rates the two groups did not report any statistically significant difference (Table 8).
According to these data, our study confirms the efficacy of RFA in the treatment of benign thyroid nodules. No differences were registered between the uses of the two different systems.
In our opinion, a slightly thicker needle may reduce the complications due to the incorrect visualization of the tip during the procedure (damage to surrounding structures), without increasing of the complications related to the deployment of the device (mainly bleeding); in our experience, the only hematoma observed has been developed in one group A patient.
Our study has several limitations, the main ones being the little population enrolled and the short follow-up. Moreover, our series represent a retrospective observational experience. More patients and a longer follow-up are necessary to confirm our preliminary results.
Acknowledgements
None.
Footnote
Conflicts of Interest: The authors have no conflicts of interest to declare.
Ethical Statement: The institutional review board of the hospital approved the study (05_31_2015_M5.5) and was conducted in accordance with the Helsinki Declaration of 1964 (revised 2008). Informed consent was obtained from each patient.
References
- Gharib H, Papini E. Thyroid nodules: clinical importance, assessment, and treatment. Endocrinol Metab Clin North Am 2007;36:707-35. [Crossref] [PubMed]
- Jeong WK, Baek JH, Rhim H, et al. Radiofrequency ablation of benign thyroid nodules: safety and imaging follow-up in 236 patients. Eur Radiol 2008;18:1244-50. [Crossref] [PubMed]
- Bergenfelz A, Jansson S, Kristoffersson A, et al. Complications to thyroid surgery: results as reported in a database from a multicenter audit comprising 3,660 patients. Langenbecks Arch Surg 2008;393:667-73. [Crossref] [PubMed]
- Guglielmi R, Pacella CM, Bianchini A, et al. Percutaneous ethanol injection treatment in benign thyroid lesions: role and efficacy. Thyroid 2004;14:125-31. [Crossref] [PubMed]
- Papini E, Guglielmi R, Bizzarri G, et al. Treatment of benign cold thyroid nodules: a randomized clinical trial of percutaneous laser ablation versus levothyroxine therapy or follow-up. Thyroid 2007;17:229-35. [Crossref] [PubMed]
- De Bernardi IC, Floridi C, Muollo A, et al. Vascular and interventional radiology radiofrequency ablation of benign thyroid nodules and recurrent thyroid cancers: literature review. Radiol Med 2014;119:512-20. [Crossref] [PubMed]
- Morelli F, Sacrini A, Pompili G, et al. Microwave ablation for thyroid nodules: a new string to the bow for percutaneous treatments? Gland Surg 2016;5:553-8. [Crossref] [PubMed]
- Lang BH, Wu AL. High intensity focused ultrasound (HIFU) ablation of benign thyroid nodules - a systematic review. J Ther Ultrasound 2017;5:11. [Crossref] [PubMed]
- Zhang B, Moser M, Zhang E, et al. Radiofrequency ablation technique in the treatment of liver tumours: review and future issues. J Med Eng Technol 2013;37:150-9. [Crossref] [PubMed]
- Wagstaff P, Ingels A, Zondervan P, et al. Thermal ablation in renal cell carcinoma management: a comprehensive review. Curr Opin Urol 2014;24:474-82. [Crossref] [PubMed]
- Alexander ES, Dupuy DE. Lung cancer ablation: technologies and techniques. Semin Intervent Radiol 2013;30:141-50. [Crossref] [PubMed]
- Sim JS, Baek JH, Lee J, et al. Radiofrequency ablation of benign thyroid nodules: depicting early sign of regrowth by calculating vital volume. Int J Hyperthermia 2017;33:905-10. [PubMed]
- Garberoglio R, Aliberti C, Appetecchia M, et al. Radiofrequency ablation for thyroid nodules: which indications? The first Italian opinion statement. J Ultrasound 2015;18:423-30. [Crossref] [PubMed]
- Na DG, Lee JH, Jung SL, et al. Radiofrequency ablation of benign thyroid nodules and recurrent thyroid cancers: consensus statement and recommendations. Korean J Radiol 2012;13:117-25. [Crossref] [PubMed]
- D.Lgs. 30 dicembre 1992, n. 502. Art. 1. Available online: http://www.stranieriinitalia.it/briguglio/immigrazione-e-asilo/2008/aprile/d-lgs-502-1992.pdf
- Nardi F, Basolo F, Crescenzi A, et al. Italian consensus for the classification and reporting of thyroid cytology. J Endocrinol Invest 2014;37:593-9. [Crossref] [PubMed]
- The National Cancer Institute. Common Terminology Criteria for Adverse Events (CTCAE) Version 4.0, Bethesda. Approved after October 1, 2009.
- Rhim H, Dodd GD 3rd, Chintapalli KN, et al. Radiofrequency thermal ablation of abdominal tumors: lessons learned from complications. Radiographics 2004;24:41-52. [Crossref] [PubMed]
- Goldberg SN, Grassi CJ, Cardella JF, et al. Image-guided tumor ablation: standardization of terminology and reporting criteria. J Vasc Interv Radiol 2009;20:S377-90. [Crossref] [PubMed]
- D'Onofrio M, Barbi E, Girelli R, et al. Radiofrequency ablation of locally advanced pancreatic adenocarcinoma: an overview. World J Gastroenterol 2010;16:3478-83. [Crossref] [PubMed]
- Röher HD, Goretzki PE. Management of goiter and thyroid nodules in an area of endemic goiter. Surg Clin North Am 1987;67:233-49. [Crossref] [PubMed]
- Bernardi S, Stacul F, Zecchin M, et al. Radiofrequency ablation for benign thyroid nodules. J Endocrinol Invest 2016;39:1003-13. [Crossref] [PubMed]
- Radzina M, Cantisani V, Rauda M, et al. Update on the role of ultrasound guided radiofrequency ablation for thyroid nodule treatment. Int J Surg 2017;41 Suppl 1:S82-93. [Crossref] [PubMed]
- Na DG, Lee JH, Jung SL, et al. Radiofrequency ablation of benign thyroid nodules and recurrent thyroid cancers: consensus statement and recommendations. Korean J Radiol 2012;13:117-25. [Crossref] [PubMed]
- Deandrea M, Limone P, Basso E, et al. US-guided percutaneous radiofrequency thermal ablation for the treatment of solid benign hyperfunctioning or compressive thyroid nodules. Ultrasound Med Biol 2008;34:784-91. [Crossref] [PubMed]
- Kim YS, Rhim H, Tae K, et al. Radiofrequency ablation of benign cold thyroid nodules: initial clinical experience. Thyroid 2006;16:361-7. [Crossref] [PubMed]