Factors leading to pancreatic resection in patients with pancreatic cancer: a national perspective
Introduction
Pancreatic cancer is the fourth leading cause of cancer related death in the United States today (1). With 2015 estimated incidence and mortality rates of 48,960 and 40,560 respectively and a reported 5-year survival rate of 7.2% after diagnosis, pancreatic malignancy remains one of the most challenging and lethal cancers (2). Resection, though technically complex, remains the only option for potential cure (3). Though post resection mortality continues to decrease, morbidity remains high with reported rates from 30–50% (4-9).
Potential cure and/or long-term survival correlate with stage and potential resectability. Determination of resectability is contingent upon its relationship to nearby vascular structures and evidence of metastasis (10). Although it is important to evaluate tumor characteristics to determine resectability and to predict outcomes and survival, it is equally important to consider patient and hospital-specific factors (11). These can, not only contribute to post-resection survival, but may also play a role in patients’ ability to achieve surgical resection (3,12,13).
Using the Nationwide Inpatient Sample (NIS) database, we aim determine if patient-specific characteristics (patient demographics, economic factors, payers of health services and comorbidities) and hospital-specific factors (surgeon volume, hospital volume, hospital teaching status) were associated with pancreatic resection for pancreatic cancer.
Methods
The study is a cross-sectional analysis using the NIS database for the years 2003–2009. NIS is part of the Healthcare Cost and Utilization Project (HCUP), sponsored by the Agency for Healthcare Research and Quality (AHRQ). This is the largest all-payer inpatient care database that is publicly available in the United States. It contains data from approximately 8 million hospital stays from about 1,000 hospitals sampled to approximate a 20% stratified sample of U.S. community hospitals (14). The NIS database consists of publicly available de-identified data that is exempt from the approval of the Institutional Review Board. International Classification of Disease, 9th Revision (ICD-9) was used in defining the diagnoses and procedures of interest.
The study population included adult (≥18 years) patients who were electively admitted with a primary diagnosis of pancreatic cancer (ICD-9: 157). Then based on the primary surgical procedure the study population was divided into those who underwent pancreatic resection (ICD-9: 52.5, 52.51, 52.52, 52.53, 52.59, 52.6, 52.7) or those who underwent abdominal procedures other than pancreatic resection.
The primary objective of this study was to assess patients’ characteristics and clinical factors association with pancreatic resection. Those factors included (I) patients’ demographics: age (<45, 45–65, >65 years), and gender; (II) economic factors: annual household income (≤ national median, > national median) (15), and main payer of health service (Medicare, Medicaid, private insurance, self-pay, no charge, other); (III) clinical factors: a modification of the Charlson Comorbidity Index Score (CCIS) (score: 0–1, ≥2) (16); and surgeon volume, classified based on the number of surgeries performed by each surgeon per year, subsequently, categorized based on the median into low-volume surgeons group (1–5 surgeries/year) and high-volume surgeons group (≥6 surgeries/year); (IV) hospital characteristics: hospital volume, classified based on the number of surgeries performed in each hospital per year, subsequently, categorized based on the median into low-volume hospitals group (1–17 surgeries/year) and high-volume surgeons group (≥18 surgeries/year), and hospital teaching status (non-teaching, teaching).
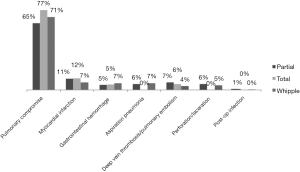
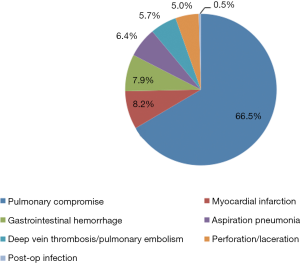
The secondary objective was to assess post-pancreatectomy outcomes in relation to surgeon volume in the sub-population of patients who underwent pancreatic resection. Those outcomes included: (I) postoperative complications, classified as the presence or absence of one or more of pulmonary, cardiovascular, gastrointestinal, infectious, or procedural complications (Figure 1) (17); (II) in-hospital death; (III) length of stay (LOS), classified based on the 75th percentile into short stay (≤14 days) and long stay (>14 days); and (IV) cost of health services per case, adjusted for the inflation rate to reflect 2015 U.S. dollar values, and classified based on the 75th percentile into low cost (≤$41,564.67) and high cost (>$41,564.67).
Other secondary independent factors that were assessed for their confounding included: race (white, black, Hispanic, Asian/Pacific Islander, Native American, other) and type of pancreatectomy (partial, total, and Whipple procedure). All variable were checked for completeness, subjects with missing values were eliminated. However, surgeon volume was only available for 5,438 subjects, those without surgeon volume were not dropped from the whole study; instead models that included surgeon volume as the main factor were performed in a smaller sample size and annotated accordingly in the tables of this manuscript.
Statistical analysis used weighted data reflecting the national estimate. The records’ weights are available in the NIS data and calculated based on the stratification variables that were used in sampling methodology. These variables are hospital geographic region, urban/rural location, teaching status, bed-size, and ownership (14).
Cross-tabulation and Chi-square test were used to examine the association between each of the independent factors and the outcomes of interest. Factors with significant association were considered confounders and were included in multivariate logistic regression models. Multivariate logistic regression models were used to calculate the odds ratio (OR) and 95% confidence interval (CI). Significance level was set as (α =0.05). All data analyses were performed using SAS 9.3 for Windows (SAS Institute Inc., Cary, NC, USA).
Results
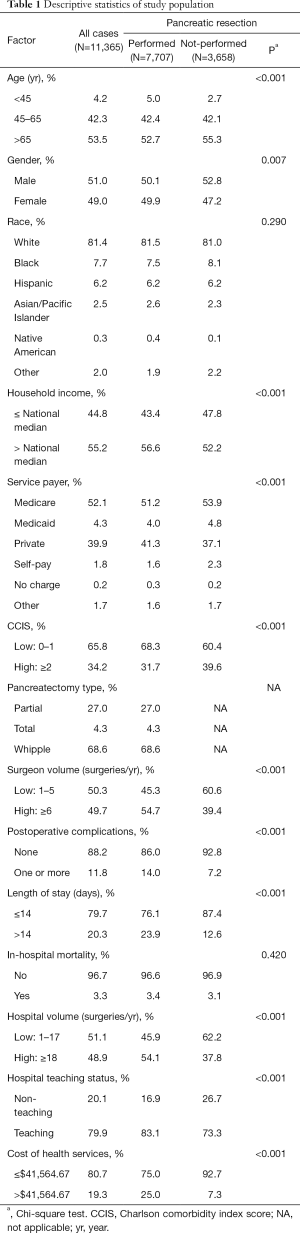
A total of 11,365 discharge records were included (Table 1). Pancreatic resection was performed in 7,707 (68.0%) subjects, while the remaining 3,658 (32.0%) underwent procedures other than pancreatectomy (Table 2). The mean age of the study population was 65.5±0.1 years old. Whites made up 81.4% of the study sample and the majority had Medicare as health insurance (52.1%). Female and male each represented approximately half of the study population. In regard to the type of pancreatectomy, Whipple procedure was the most performed operation (68.6%), while partial and total pancreatectomies formed 27.0% and 4.3% respectively. Postoperative complications were reported in 1,344 (11.8%) patients (Figures 1,2), and 378 (3.3%) died during their hospital stay. The average LOS was 11.2±0.2 days, and the average cost of health services per case was $32,510.00±843.41.
Full table
Full table
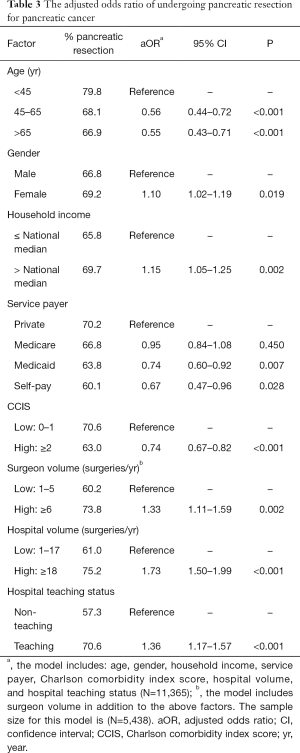
Younger patients (<60 years) and females with pancreatic malignancy were more likely to undergo pancreatic resection (P<0.05 each) (Table 3). Contrarily, pancreatic cancer patients with household income below the national median and those with Medicaid coverage were less likely to have pancreatectomy (P<0.01 each). Patients with two or more comorbidities were also less likely to undergo pancreatectomy (OR: 0.74, 95% CI: 0.67–0.82, P<0.001) (Table 3).
Full table
High-volume surgeons performed more pancreatic resections for pancreatic cancer compared to low-volume surgeons (OR: 1.33, 95% CI: 1.11–1.59, P=0.002). Similarly, pancreatic resections were more prevalent in high-volume hospitals than low-volume hospitals (OR: 1.73, 95% CI: 1.50–1.99, P<0.001). Furthermore, teaching hospitals had a higher rate of pancreatic cancer resection than non-teaching hospitals (OR: 1.36, 95% CI: 1.17–1.57, P<0.001).
In the subpopulation of patients who underwent pancreatectomy, high-volume surgeons were more likely to perform a Whipple procedure than low-volume surgeons (OR: 1.37, 95% CI: 1.05–1.80, P=0.021). Patients who underwent pancreatic surgery by high-volume surgeons had a lower postoperative complications risk (10.4% vs. 18.1%, P<0.001), and lower mortality risk (2.1% vs. 4.8%, P=0.008) (Table 4). Additionally, surgeries performed by high-volume surgeons associated with a shorter hospital stay and lower healthcare cost (P<0.05 for all).

Full table
Discussion
With reported mortality rates upwards of 7% in some series, a plethora of studies have evaluated patient-specific factors that affect outcomes after pancreatic resection (4,5,7). Demographics, socioeconomic status (SES) and other patient-specific factors such as comorbidities play a significant role in post-resection outcomes. Ragulin-Coyne et al. (4) developed a risk score estimating in-hospital mortality after pancreatectomy. Age >80, having multiple comorbidities, and having had multiple operations for pancreatitis were factors most predictive of mortality after pancreatectomy. These factors, along with resection for non-benign disease, have been attributed to increased postoperative complication rates (7).
SES is associated with outcome differentials in pancreatic cancer patients (5,18). Lim et al. (11) in a review of 396 patients undergoing resection for pancreatic cancer demonstrated a difference in post resection survival for individuals above the median household income level (11). Similarly, our results demonstrated an advantage towards high SES. There is very limited data in the literature addressing this important issue. We conducted our study using the large NIS database to further investigate the association between SES and pancreatic resection. We found individuals above the national median household income were more likely to achieve pancreatic resection when compared to those below the national median.
For the first time, our analysis significantly demonstrates patient age (<60), female sex, and household income above the national median as patient-specific factors most associated with undergoing pancreatic resection. Patients with Medicaid coverage and a Charlson comorbidity score ≥2 were least likely to undergo pancreatic resection.
Zell et al. (19), in their review of 17,326 patients with pancreatic cancer from the California Cancer Registry in 2007, addressed pancreatic resection. By dividing patients into quintiles based on their SES, they were able to demonstrate increasing quintiles were associated with several treatment modalities such as surgery, chemotherapy and radiation (19). Cress et al. (20) in a comparable review also concluded that patients of low SES are less likely to undergo pancreatic resection, which may negatively affect survival.
Case volume as it relates to outcomes has been considered in the literature since the 1970s (21). Many studies have indicated a relationship between surgeon and/or hospital volume and mortality, complication rate, LOS, hospital costs, and resection rates in pancreatic surgery (12,22-29). Our analysis shows high-volume surgeons performed more pancreatectomies for pancreatic cancer compared to low-volume surgeons. More specifically, pancreaticoduodenectomy was more likely to be performed by high-volume surgeons when compared to low-volume surgeons. Pancreatectomy for pancreatic cancer was more prevalent and more likely to be performed in high-volume hospitals.
In several large series reviews, high volume surgeons have been shown to improve outcomes in complex procedures (30,31). Eppsteiner et al. (23) reviewed 3,581 patients undergoing pancreatic resection from 1998–2005 using the NIS database. They determined that high-volume surgeons (≥5 surgeries/year) experienced a significant reduction in in-hospital mortality when compared to low-volume surgeons (2.6% vs. 6.7%). Similar findings were demonstrated in a database review of 91,241 patients by Nathan et al. (25). Surgeons performing a high volume of pancreatic resections were strongly associated with decreased in-hospital mortality, even after adjustment for hospital volume (25). Our findings were in line with the previously stated studies. High-volume surgeons had decreased post-resection complication and mortality. Moreover, shorter hospital stay and lower healthcare cost was associated with cases performed by high-volume surgeons.
Regarding hospital volume, we demonstrate that pancreatectomy is more prevalent and more likely to be performed in high-volume hospitals when compared to low-volume hospitals. This has also been described by Gooiker et al. (29) showing an increase in resection rates from 10.7% to 15.3% after regionalization of pancreatic resections in the Netherlands. They also display a small but significant increase in overall survival for high-volume hospitals compared to low/mid-volume centers. Others attribute superior outcomes in cancer surgery to hospital volume (12,22,26,27). Sosa et al. (32) conclude pancreatic cancer patients treated by resection or palliative procedure benefit from referral to high-volume center citing decreased in-hospital mortality rates, LOS, and hospital charges for services.
Our current study is limited by the administrative nature of the database and the cross-sectional design. Additionally, it is limited by having information on patients during their hospital stay only and there is a lack of follow-up for mortality or other complications that could develop after hospital discharge. The study has several strengths represented by the long study period, large sample size, and the application of a weighted analysis that reflects more accurate estimates at the national level. These national estimates warrant replication of this study be performed using different and more sophisticated resources.
With resection being the only option for potential cure and long-term survival in pancreatic cancer treatment, it is important we understand factors associated with pancreatic resection. For the first time, we have successfully described both patient-specific and hospital-specific factors that make patients more likely to undergo pancreatic resection for pancreatic cancer. We have demonstrated that patients of lower SES and patients with Medicaid health coverage are significantly less likely to undergo pancreatic resection for a diagnosis of pancreatic cancer. Factors increasing the likelihood of pancreatic resection include age <60, female gender, and higher household income. We have also shown that those patients cared for by high-volume surgeons were more likely to achieve resection for their disease, at a lower risk of postoperative complications, had lower mortality rates, experienced shorter hospital stays, and lower healthcare costs.
It is likely patients of low SES are receiving care at low volume hospitals by low volume surgeons where costs for health services are higher and disease-based outcomes are inferior. As a healthcare provider caring for pancreatic cancer patients, it is important to be aware of patient-specific and hospital specific factors associated with pancreatic resection. One cannot overlook the effect these factors have on patients’ ability to receive the best care and ultimately affect the course of their disease.
Acknowledgements
None.
Footnote
Conflicts of Interest: The authors have no conflicts of interest to declare.
References
- National Cancer Institute. Pancreatic Cancer Treatment (PDQ®)–Health Professional Version. 2015. Available online: http://www.cancer.gov/types/pancreatic/hp/pancreatic-treatment-pdq
- Howlader N, Noone AM, Krapcho M, et al. editors. Previous Version: SEER Cancer Statistics Review, 1975-2012. Bethesda: National Cancer Institute, 2015.
- Teh SH, Diggs BS, Deveney CW, et al. Patient and hospital characteristics on the variance of perioperative outcomes for pancreatic resection in the United States: a plea for outcome-based and not volume-based referral guidelines. Arch Surg 2009;144:713-21. [Crossref] [PubMed]
- Ragulin-Coyne E, Carroll JE, Smith JK, et al. Perioperative mortality after pancreatectomy: A risk score to aid decision-making. Surgery 2012;152:S120-7. [Crossref] [PubMed]
- Carroll JE, Smith JK, Simons JP, et al. Redefining mortality after pancreatic cancer resection. J Gastrointest Surg 2010;14:1701-8. [Crossref] [PubMed]
- Cameron JL, Riall TS, Coleman J, et al. One thousand consecutive pancreaticoduodenectomies. Ann Surg 2006;244:10-5. [Crossref] [PubMed]
- Simons JP, Shah SA, Ng SC, et al. National complication rates after pancreatectomy: beyond mere mortality. J Gastrointest Surg 2009;13:1798-805. [Crossref] [PubMed]
- Cameron JL, Cameron AM. Current Surgical Therapy. 11th Edition. Philadelphia: Elsevier Saunders, 2013.
- Winter JM, Cameron JL, Campbell KA, et al. 1423 pancreaticoduodenectomies for pancreatic cancer: a single-institution experience. J Gastrointest Surg 2006;10:1199-210; discussion 1210-1. [Crossref] [PubMed]
- Bilimoria KY, Bentrem DJ, Ko CY, et al. Validation of the 6th edition AJCC pancreatic cancer staging system: report from the national cancer database. Cancer 2007;110:738-44.
- Lim JE, Chien MW, Earle CC, et al. Prognostic factors following curative resection for pancreatic adenocarcinoma: a population-based, linked database analysis of 396 patients. Ann Surg 2003;237:74-85. [Crossref] [PubMed]
- McPhee JT, Hill JS, Whalen GF, et al. Perioperative mortality for pancreatectomy: A national perspective. Ann Surg 2007;246:246-53. [Crossref] [PubMed]
- Finlayson E, Fan Z, Birkmeyer JD, et al. Outcomes in octogenarians undergoing high-risk cancer operation: A national study. J Am Coll Surg 2007;205:729-34. [Crossref] [PubMed]
- Healthcare Cost and Utilization Project (HCUP). Overview of the National (Nationwide) Inpatient Sample (NIS). Available online: http://www.hcup-us.ahrq.gov/nisoverview.jsp
- Healthcare Cost and Utilization Project (HCUP). NIS description of data elements. Available online: http://hcup-us.ahrq.gov/db/nation/nis/nisdde.jsp
- Quan H, Sundararajan V, Halfon P, et al. Coding algorithms for defining comorbidities in ICD-9-CM and ICD-10 administrative data. Med Care 2005;43:1130-9. [Crossref] [PubMed]
- Murphy MM, Knaus WJ 2nd, Ng SC, et al. Total pancreatectomy: A national study. HPB (Oxford) 2009;11:476-82. [Crossref] [PubMed]
- Kuhn Y, Koscielny A, Glowka T, et al. Postresection survival outcomes of pancreatic cancer according to demographic factors and socio-economic status. Eur J Surg Oncol 2010;36:496-500. [Crossref] [PubMed]
- Zell JA, Rhee JM, Ziogas A, et al. Race, socioeconomic status, treatment, and survival time among pancreatic cancer cases in California. Cancer Epidemiol Biomarkers Prev 2007;16:546-52. [Crossref] [PubMed]
- Cress RD, Yin D, Clarke L, et al. Survival among patients with adenocarcinoma of the pancreas: a population based study (United States). Cancer Causes Control 2006;17:403-9. [Crossref] [PubMed]
- Luft HS, Bunker JP, Enthoven AC. Should operations be regionalized? The empirical relation between surgical volume and mortality. N Engl J Med 1979;301:1364-9. [Crossref] [PubMed]
- van Heek NT, Kuhlmann KF, Scholten RJ, et al. Hospital volume and mortality after pancreatic resection: a systematic review and an evaluation of intervention in the Netherlands. Ann Surg 2005;242:781-8; discussion 788-90. [Crossref] [PubMed]
- Eppsteiner RW, Csikesz NG, McPhee JT, et al. Surgeon volume impacts hospital mortality for pancreatic resection. Ann Surg 2009;249:635-40. [Crossref] [PubMed]
- Kelly JV, Hellinger FJ. Physician and hospital factors associated with mortality of surgical patients. Med Care 1986;24:785-800. [Crossref] [PubMed]
- Nathan H, Cameron JL, Choti MA, et al. The volume-outcomes effect in hepato-pancreato-biliary surgery: hospital versus surgeon contributions and specificity of the relationship. J Am Coll Surg 2009;208:528-38. [Crossref] [PubMed]
- Glasgow RE, Mulvihill SJ. Hospital volume influences outcome in patients undergoing pancreatic resection for cancer. West J Med 1996;165:294-300. [PubMed]
- Finlayson EV, Goodney PP, Birkmeyer JD. Hospital volume and operative mortality in cancer surgery: a national study. Arch Surg 2003;138:721-5; discussion 726. [Crossref] [PubMed]
- Finlayson EV, Birkmeyer JD. Effects of hospital volume on life expectancy after selected cancer operations in older adults: a decision analysis. J Am Coll Surg 2003;196:410-7. [Crossref] [PubMed]
- Gooiker GA, Lemmens VE, Besselink MG, et al. Impact of centralization of pancreatic cancer surgery on resection rates and survival. Brit J Surg 2014;101:1000-5. [Crossref] [PubMed]
- Hannan EL, Radzyner M, Rubin D, et al. The influence of hospital and surgeon volume on in-hospital mortality for colectomy, gastrectomy, and lung lobectomy in patients with cancer. Surgery 2002;131:6-15. [Crossref] [PubMed]
- Birkmeyer JD, Stukel TA, Siewers AE, et al. Surgeon volume and operative mortality in the United States. N Engl J Med 2003;349:2117-27. [Crossref] [PubMed]
- Sosa JA, Bowman HM, Gordon TA, et al. Importance of hospital volume in the overall management of pancreatic cancer. Ann Surg 1998;228:429-38. [Crossref] [PubMed]