Cardio-oncology: protecting the heart from curative breast cancer treatment
Introduction
As advances in screening and treatment have improved survival for patients with breast cancer (1), cardiovascular disease has emerged as a major cause of long-term non-cancer related morbidity and mortality (2,3). In the general population, cardiovascular disease is the leading cause of death in both women and men (4). However, patients with breast cancer have even higher rates of cardiovascular disease compared to age matched controls without cancer (2,5). This increased risk of cardiovascular disease likely reflects the role of shared risk factors for breast cancer and cardiovascular disease (6) as well as cancer therapy related cardiac effects (7-9). This review will describe (I) rates of cardiac disease with different breast cancer therapies; (II) risk factors and risk models for cardiac outcomes; (III) strategies for cardiotoxicity monitoring during and after cancer therapy and (IV) strategies to reduce the risk of cardiac disease with breast cancer therapy.
Section 1: what is the risk of cardiac disease with different breast cancer therapies?
Anthracycline-containing chemotherapeutic regimens
Anthracycline chemotherapeutic agents are effective cytotoxic antineoplastic agents and regimens containing doxorubicin or epirubicin are commonly used in the treatment of patients with breast cancer (10,11). Anthracycline agents cause oxidative and nitrosative stress, mitochondrial dysfunction and double stranded DNA breaks leading to cardiomyocyte apoptosis and fibrosis that manifests most commonly as asymptomatic reductions in left ventricular ejection fraction (LVEF) early after treatment that can progress to clinical heart failure (HF) with reduced ejection fraction (HFrEF) (12-14). The risk of cardiomyopathy and HF increases exponentially with increasing cumulative anthracycline dose (13,15). In a metastatic breast cancer population with rigorous assessment of LVEF using multigated acquisition (MUGA) scans, reductions in LVEF to less than normal or clinical HF occurred in 6%, 14%, 33%, 59% and 76% of patients at a cumulative doxorubicin dose of 250, 300, 400, 500 and 600 mg/m2 (13). In most early breast cancer trials using anthracyclines, the risk of clinical congestive HF over several years of follow up reported using the National Cancer Institute common terminology criteria for adverse events (CTCAE) has ranged from 0.5–1% (16-18), which is likely an underestimate of the true risk as most randomized trials of anthracycline regimens for early stage breast cancer have not included longitudinal LVEF assessment or detailed ascertainment of clinical HF events. In the 10 year follow-up of the BCIRG 001 trial (TAC, doxorubicin 300 mg/m2), the largest anthracycline trial to report longitudinal LVEF and clinical assessment in participants with early breast cancer, clinical HF rates continue to increase over time occurring in 3% of the population at 10 years. In the same cohort, 12% of the population had a reduction in LVEF to less than the lower limit of normal (19) (Table 1). In a substudy of the FASG 05 trial (FEC, epirubicin cumulative dose either 300 or 600 mg/m2) participants who were cancer free and agreed to cardiac assessment a median of 8.5 years after treatment, clinical HF rates were 2.3% and reductions in LVEF to less than the lower limit of normal occurred in 5% of study participants (20). Even these well-done studies potentially underestimate the true risk given the younger age of participants in clinical trials and only a minority of the cohort participated in the long-term follow up imaging studies. Prospective cohort studies with more rigorous collection of longitudinal echocardiographic data but smaller numbers of clinical events suggest that about 10–15% of women treated with an anthracycline without trastuzumab will have a reduction in LVEF to <50% (22,28); however, the long-term implications on risk of HF or other cardiac events requires further study. In an analysis of the Surveillance, Epidemiology, and End Results (SEER)-Medicare database of women 66 to 80 with early stage breast cancer and no history of HF, treatment with an anthracycline-containing chemotherapeutic regimen was associated with a 30% higher hazard of HF compared to women treated without anthracyclines (HR 1.3, 95% CI: 1.1–1.4) (7). Much of the research on cardiac effects of anthracyclines has focused on asymptomatic reductions in LVEF or HFrEF; however, smaller studies have shown changes in diastolic function with anthracycline therapy (29-31), suggesting that patients treated with anthracyclines may also be at increased risk for HFpEF. Further research into the association between anthracycline use and HFpEF is needed.
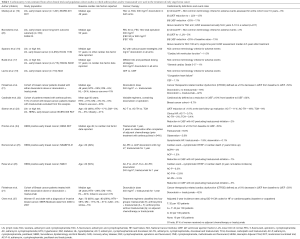
Full table
HER2 targeted therapies
The addition of monoclonal antibodies that block HER2/ErbB2 signaling improves survival outcomes in both the metastatic and adjuvant setting in patients with breast cancer that overexpresses HER2 (23,32-35). HER2/ErbB2 is also expressed in the myocardium and anti HER2 therapy disrupts cardiac homeostasis and myocardial repair and can result in LVEF reductions and clinical HF. The HER2/ErbB2 pathway is important for cardiomyocyte repair in response to anthracyclines and thus it is not surprisingly that concomitant anthracycline and trastuzumab therapy was associated with New York Heart Association Class III or IV (severe) HF in 16% of patients with metastatic breast cancer treated with the combination (32). Subsequent trials in early breast cancer showed lower rates of HF when anthracyclines and trastuzumab were given sequentially instead of concomitantly and with strict cardiac exclusion criteria mandating a normal LVEF after chemotherapy prior to starting trastuzumab therapy as well as frequent monitoring of LVEF every 3 months with standardized protocols for holding trastuzumab with LVEF reductions (23,34,35). In the BCIRG 006 trial, among women with HER2 positive early breast cancer randomized to treatment with doxorubicin and cyclophosphamide followed by docetaxel and 1 year of trastuzumab in those with a normal LVEF after anthracycline treatment, a total of 2% developed HF over a median follow up of 5 years. Although rates of clinical HF were relatively low, 18% of participants had a greater than 10% reduction in LVEF with incomplete recovery at 4 years. Mean LVEF was lower in the sequential anthracycline- trastuzumab arm than other arms of the study (Table 1). Longitudinal LVEF and clinical assessments from other large trials of sequential anthracyclines, taxanes and trastuzumab with strict criteria for withholding trastuzumab in participants with prior cardiac disease or with LVEF reductions after adjuvant chemotherapy and protocols for holding or discontinuing trastuzumab according to LVEF monitoring, confirm HFrEF in 1–4% and asymptomatic reductions in LVEF in 10–15% of participants (Table 1) (24,26,36). Recovery of LVEF to >50% is seen in more than half of those with a symptomatic or asymptomatic reduction in LVEF with sequential anthracycline and trastuzumab therapy; however, a significant number of women have persistent cardiomyopathy (25,26). Prospective cohort studies of unselected women with HER2 positive breast cancer undergoing treatment with doxorubicin and trastuzumab show rates of LVEF reductions to less than normal in upwards of 40% of women with only partial recovery of LVEF after treatment (21,28). Data from the SEER-Medicare registry suggest higher rates of clinical HF with trastuzumab therapy in women over the age of 65 years with rates of CHF of 29% among trastuzumab users and 19% among trastuzumab nonusers with breast cancer (37). In another SEER-Medicare analysis, among women 67 to 94 years of age, 3-year HF or cardiomyopathy incidence rates were 32.1 per 100 patients for those receiving trastuzumab without anthracyclines, 41.9 per 100 patients for those receiving both anthracyclines and trastuzumab and 18.1 per 100 patients in those receiving no adjuvant therapy; although only 2% of the cohort received trastuzumab (27). As the incidence of HF increases with older age it is not surprising that rates of HF are higher in observational studies that include a higher number of older patients than in clinical trial populations.
Radiation
Radiation therapy (RT) that includes the heart in the treatment field can cause a myriad of cardiac late effects. RT causes microvascular endothelial damage, capillary loss, inflammation and fibrosis and can affect all the layers of the heart (38). Late effects of RT include coronary artery disease (CAD), constrictive pericarditis, valvular stenosis or regurgitation, restriction, HFpEF and HFrEF (8,39-41). Multiple large cohort studies have demonstrated an increased risk of clinically meaningful cardiac events such as myocardial infarction (MI), HF or cardiac mortality with higher estimated mean heart dose, although mean heart dose has been estimated using various methods ranging from breast cancer laterality alone to sophisticated three dimensional dose distributions using computed tomography RT planning scans (39-43). Given that RT is associated with involvement of multiple cardiac substructures, it is not surprising that short and long-term cardiac mortality is higher in patients with prior RT undergoing percutaneous coronary intervention or cardiac surgery than in matched controls undergoing the same procedures without a history of RT. These data underscore the importance of efforts to prevent radiation-related heart disease (44-47). RT techniques continue to evolve with the goal of reducing the RT dose to normal structures with strategies such as computed tomography-based RT planning, intensity-modulated RT, lower total RT doses, reductions in RT field and deep-inspiration breath holding (38). As most of the data about long-term cardiac risks of RT are from studies of patients treated with older radiation techniques and the cardiac effects of radiation continue to increase decades after exposure, it is impossible to know to what extent modern RT has reduced the cardiac late effects of breast cancer RT.
Section 2: mitigating risk step 1—predicting risk prior to initiating therapy
Multiple studies have explored risk factors for the development of cardiotoxicity with breast cancer therapy and are categorized in this review as clinical risk factors, therapy related risk factors and genetic risk factors.
Clinical risk factors
Patients with reduced or low normal LVEF, known CAD, or atrial fibrillation prior to the initiation of anthracycline or trastuzumab treatment have been shown to be at increased risk of developing HF (7,25,26,37,48,49). In addition, traditional cardiac risk factors for CAD or HF such as hypertension, diabetes, tobacco use, hyperlipidemia, obesity and increasing age have been associated with an increased risk of cardiotoxicity with breast cancer therapy that includes anthracyclines, trastuzumab or radiation (5,7,22,25,26,37,41,48,49). Given these findings, screening and treatment of potentially modifiable risk factors to reduce the risk of cardiac events in patients with breast cancer is indicated (50).
Therapy risk factors
As described in Section 1, the risk of cardiomyopathy and HF increases exponentially with increasing cumulative anthracycline dose (13,15) and thus patients receiving higher doses of anthracyclines should be closely monitored for the development of asymptomatic reductions in LVEF or clinical HF. Both observational and clinical trial data shows that treatment with anthracyclines followed by trastuzumab is associated with higher risks of reduced LVEF and HF compared to either agent alone and these patients should also be considered high risk for the development of cardiotoxicity (14,23,27,51). Any combination of anthracyclines and radiation with the heart in the treatment field likely increases the risk of cardiotoxicity, although data for this is extrapolated from cohort studies of patients with Hodgkin lymphoma (52). Finally, multiple studies have shown that the risk of radiation induced cardiotoxicity increases with higher estimated dose to the heart and thus patients with the heart in the treatment field should be considered at risk for CAD, valvular disease, constrictive pericarditis and HFpEF and HFrEF (39-43).
Genetic risk factors
There has been interest in discovering germline genetic markers of increased risk for cancer therapy related cardiotoxicity. Most of the studies to date have focused on anthracycline cardiotoxicity with the majority of studies performed in childhood cancer survivors and most using a candidate gene or candidate single nucleotide polymorphism (SNP) approach (53-57). A recent genome-wide association study (GWAS) using three separate cohorts from randomized trials in breast cancer patients with validation and external validation suggested the rs28714259 SNP, which is located in an intergenic region in chromosome 15, was associated with an increased risk of HF (OR 1.9–4.2) (58). GWAS analysis of a pediatric population treated with anthracyclines suggested a SNP in RARG was associated with increased risk of HF (59); however, this SNP was associated with decreased rather than increased HF risk in an adult breast cancer population (58).
Several small studies have evaluated the association between candidate SNPs in the germline ERBB2 (Her2/Neu) gene and risk of trastuzumab cardiotoxicity with two studies showing an association with the proline allele of Pro1170 Ala (Rs1058808) and subsequent risk of trastuzumab cardiotoxicity (60,61). In the future, genetic information may be integrated with clinical and treatment related risk factors to personalize the treatment of patients with breast cancer; however, further studies evaluating the incremental value of genetic information above known clinical prognostic factors and additional validation studies in multiracial cohorts are necessary before genetic testing becomes a routine component of cardiotoxicity risk assessment.
Clinical prediction models (CPMs)
There are two reported CPMs for trastuzumab associated cardiotoxicity (Table 2) (25,48). The first trastuzumab CPM was developed using the National Surgical Adjuvant Breast and Bowel Project (NSABP) B-31 study cohort that included 947 patients with Node positive HER2 positive early breast cancer who were treated with doxorubicin (240 mg/m2) and cyclophosphamide followed by paclitaxel and trastuzumab for 1 year. In order to receive trastuzumab, participants had to be free from clinical HF events and meet LVEF criteria—LVEF could not have dropped more than 15% from baseline after doxorubicin and also had to be greater than the lower limit of normal (25). The NSABP B-31 prediction model includes two variables—baseline LVEF and age—and predicts the composite of cardiac death, NYHA Class III/IV HF or LVEF decrease >10% from baseline to <55% or >5% to less than the lower limit of institutional normal value after initiation of trastuzumab over 7 years of follow-up with fair discrimination (c-statistic 0.72, optimism corrected c-statistic 0.70) but has not been externally validated. Of note, this model excludes the 6% of patients that had reductions in LVEF or cardiac symptoms during or immediately after anthracycline treatment and thus did not receive trastuzumab and the number of trastuzumab related cardiac events was 37 (4% of the population). Another CPM for trastuzumab cardiotoxicity was derived using the SEER-Medicare database and includes 1,664 women over 66 years of age without a pre-existing diagnosis of HF or cardiomyopathy who had operable HER2 positive breast cancer and received trastuzumab (Table 2) (48). The SEER-Medicare model includes seven predictors—age, adjuvant chemotherapy (anthracycline based, non-anthracycline chemotherapy vs. none), history of CAD, atrial fibrillation/atrial flutter, diabetes, hypertension and renal failure and predicts the development of HF or cardiomyopathy over 3 years, which occurred in 19% of the cohort. All comorbidities and outcome variables were derived from International Classification of Disease, 9th edition (ICD-9) billing codes. The model was converted into a risk score (range 0–9) with risk of HF being 16% with risk score of <4 and 40% with risk score of ≥6; however, no discrimination or calibration were performed and the risk score has not been externally validated. The BETTER-CARE study included 165 patients with early breast cancer (both HER2 negative and positive disease), but excluded patients with known cardiac risk factors such as hypertension, diabetes, or kidney disease and used an outcome of questionable clinical significance—subclinical cardiotoxicity defined as LVEF change of 5% (Table 2) (62). There are no CPMs for anthracycline cardiotoxicity and external validation of the existing trastuzumab cardiotoxicity models is needed.
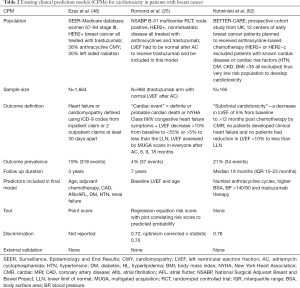
Full table
Section 3: mitigating risk step 2—monitoring during and after cancer therapy
Strategies to identify patients potentially at high risk for cardiotoxicity with breast cancer therapy may affect cancer treatment decisions and in some cases less cardiotoxic regimens may be selected. However, due to tumor biology, clinical stage or other factors affecting oncologic risk, many patients with risk factors for cardiotoxicity will need to be treated with anthracyclines, trastuzumab and/or radiation with the heart in the treatment field. In addition, patients without any known risk factors for the development of cardiotoxicity may develop cardiac effects after treatment. This section reviews the current evidence regarding screening for asymptomatic cardiotoxicity in patients with breast cancer receiving potentially cardiotoxic treatment.
Rationale for screening for asymptomatic cardiotoxicity
In the general population, individuals with a reduced LVEF but no previous or current signs or symptoms of HF have been shown to have a 5-fold increased risk of subsequent symptomatic HF and death as compared with those with normal LVEF (63). In addition, several randomized controlled trials have shown that treatment with an angiotensin-converting enzyme (ACE) inhibitor or angiotensin receptor blocker in patients with reduced LVEF but without HF symptoms reduces mortality, the development of HF symptoms, and prevents left ventricular remodeling (64-66). There is also randomized controlled trial data that beta-blocker therapy in addition to an ACE inhibitor can prevent HF events and reduce mortality in post MI patients with a reduced LVEF but no HF symptoms (67). Based on the compelling evidence for early intervention in patients with structural heart disease in order to prevent the development of HF, the American College of Cardiology/American Heart Association (ACC/AHA) categorize these individuals as having stage B HF (at considerable risk of HF events) and recommends consideration of ACE inhibitor and beta-blocker therapy to prevent progressive HF in select individuals with reduced LVEF (68,69). While much of the data in the general population comes for patients with ischemic cardiomyopathy or hypertensive nonischemic cardiomyopathy, studies of anthracycline-induced cardiotoxicity (22,70) and trastuzumab cardiotoxicity (71) also suggest that early treatment with neurohormonal antagonist therapy may prevent progressive HF, at least over the duration of these studies (median follow up 1.5–5 years). However, longer term follow-up of survivors of childhood cancer suggests that early improvements in LVEF with enalapril are followed by subsequent worsening of LV function and cardiac events in patients with early signs of “recovery”. Given this data in childhood survivors treated with anthracyclines, more studies of the long-term outcomes of neurohormonal therapy in breast cancer therapeutic cardiotoxicity is warranted (72).
Echocardiographic screening
The diagnosis of ACC/AHA stage B HF is often made by echocardiographic assessment. As described in Section 1, rates of asymptomatic LVEF reduction after anthracycline and/or trastuzumab therapy can be seen in 10–20% of the population and early initiation of neurohormonal therapy may prevent the development of HF and progressive LV dysfunction (73). For these reasons, the recent American Society of Clinical Oncology (ASCO) Practice Guidelines for the Prevention and Monitoring of Cardiac Dysfunction in Survivors of Adult Cancers suggest that an echocardiogram may be performed between 6 to 12 months after completion of cancer-directed therapy in asymptomatic patients considered to be at increased risk based on cardiac risk factors or therapy related risk factors (50). In patients with poor sonographic windows and poor echocardiographic quality, a cardiac magnetic resonance imaging (MRI) or MUGA scan can be considered with preference given to cardiac MRI. The ASCO guidelines also suggest that patients at increased risk for cardiac dysfunction can be monitored with periodic echocardiographic assessment during cancer therapy and patients with cardiac symptoms should be evaluated with careful history and physical exam as well as consideration of cardiac biomarkers such as troponin or natriuretic peptides as well as cardiac imaging, with preference for echocardiogram. Compared to MUGA scans, echocardiograms are not associated with radiation exposure and provide important information about valvular function, diastolic function, hemodynamic clues and pericardial disease in addition to LVEF assessment.
Serum biomarkers
The utility of serum biomarkers as a screening test for early signs of cardiotoxicity before there is a reduction in LVEF has been evaluated in several cohorts. In a large single-center cohort of 703 inpatients with various malignancies including patients with breast cancer who were treated with high dose chemotherapy with autologous stem cell rescue, troponin I (TnI) concentrations 12, 24, 36, 72 hours and 1 month after the chemotherapy infusion predicted subsequent reductions in LVEF and cardiac events including HF and sudden death with the highest risk patients being those with TnI >0.08 ng/mL in the first 72 hours after chemotherapy and at 1 month after chemotherapy (74). The same group further showed that treatment with ACE inhibitor in patients with elevated TnI after high dose chemotherapy can prevent reductions in LVEF and HF events (70). In a multicenter study of 81 patients with HER2 positive breast cancer treated with doxorubicin followed by trastuzumab, elevated high sensitivity TnI 2–3 months after doxorubicin initiation was associated with increased risk of cardiotoxicity, defined as a reduction in LVEF; however, the sensitivity of early elevations in TnI for subsequent cardiotoxicity was only 48% (75,76). Thus more research is needed to assess the sensitivity and predictive power of TnI or other serum biomarkers in screening for clinically relevant subclinical cardiotoxicity in patients treated with current breast cancer therapy regimens.
Strain
Similar to serum biomarkers, echocardiographic measures of myocardial deformation have been studied as potential early indicators of myocardial injury with the hypothesis that early detection of subclinical cardiotoxicity will allow for earlier treatment and thus a greater likelihood of preventing long-term HF events. Commonly evaluated measures of myocardial deformation include strain (the total deformation of the ventricular myocardium during a cardiac cycle expressed as a percentage) and strain rate (the rate of deformation) with measurements of each possible in the longitudinal, radial and circumferential directions in two-dimensional echocardiography. Of the available techniques, change in global longitudinal strain (GLS) using two-dimensional speckle-tracking echocardiography has been shown to have the most consistent association with subsequent reductions in LVEF (14,28,30,76-79). Multiple studies have shown that changes in strain or strain rate occur earlier than reductions in LVEF and thus may be sensitive early markers of cardiotoxicity (77). The ongoing Strain Surveillance during Chemotherapy for Improving Cardiovascular Outcomes (SUCCOUR) study is testing the hypothesis that a strain-based screening approach with recommended initiation of ACE inhibitor and beta blocker therapy in patients with reductions in strain of more than 11% will be associated with improved cardiovascular (reduced HF events, higher LVEF) and oncologic (fewer treatment interruptions) outcomes as compared with a screening algorithm employing LVEF changes alone as the threshold to initiate cardioprotective medical therapy.
Section 4: mitigating risk step 3—therapeutic strategies to reduce risk of cardiac events
Dexrazoxane
Dexrazoxane has been shown to substantially reduce the risk of cardiotoxicity of anthracycline chemotherapy in several randomized trials of patients with metastatic breast cancer (80-86). The mechanism of cardioprotection appears to involve the inhibition of DNA topoisomerase IIb-anthracycline mediated ds DNA breaks as well as the reduction of oxygen free radical formation in cardiomyocytes (87). Most of the patients in these studies received high cumulative anthracycline doses and most had metastatic disease. Dexrazoxane has not been studied in patients with early breast cancer.
Continuous infusion and liposomal formulations of anthracyclines have been compared in several small RCTs, usually in the setting of metastatic disease and generally in patients treated with high cumulative anthracycline doses (81). In these settings there appeared to be reduced cardiotoxicity with both of these strategies.
As with dexrazoxane, continuous infusion and liposomal formulations of anthracyclines have not been studied in the setting of early breast cancer.
Neurohormonal therapy
Given that ACE inhibitor and beta-blocker therapy is the cornerstone of treatment of stage B and stage C HF (68) and that animal models suggest these therapies can mitigate or reverse anthracycline cardiotoxicity (88), there have been multiple single center studies evaluating the role of neurohormonal therapy for prevention of chemotherapy related cardiotoxicity. In a seminal single center study out of Italy, patients receiving high dose chemotherapy with autologous stem cell rescue (25% with breast cancer), most of who had prior anthracycline exposure with mean doses of 300 mg/m2, were assessed with serum troponin values 12, 24, 36 and 72 hours after chemotherapy. Patients with elevated troponin values (TnI >0.07 ng/mL) were then randomized to enalapril starting 1 month after chemotherapy and continued for 1 year with the primary endpoint being a change in LVEF of >10% to less than the normal limit value. While no patients in the enalapril met the primary endpoint, 48% of those in the control group had a significant reduction in LVEF. In other observational analyses by the same group there was significant LVEF recovery after anthracycline-related reduction in LVEF with combined ACE inhibitor (enalapril) and beta-blocker (carvedilol) (22,89).
These and other observational and small single center data suggesting that early treatment with neurohormonal antagonist therapy can be beneficial in patients with early signs of cardiotoxicity, have led to the design of trials seeking to test the hypothesis that neurohormonal antagonist therapy given PRIOR to the initiation of cancer therapy may prevent cardiotoxicity. The prevention of cardiac dysfunction during adjuvant breast cancer therapy (PRADA) study randomized 130 patients with early breast cancer with planned treatment with adjuvant anthracycline therapy with epirubicin (FEC-5-fluorouracil, epirubicin and cylophospamide; 22% received subsequent trastuzumab) in a 2×2 factorial design to receive candesartan, metoprolol succinate or matching placebo prior to receiving anthracycline (90). The study was single center and the primary outcome measure was change in LVEF as measured by cardiac MRI at the completion of chemotherapy with a modest but statistically significant difference in LVEF decline with candesartan (P=0.026) but not metoprolol succinate (P=0.772). Absolute reductions in LVEF from baseline were low in all arms (−2.6% placebo, −1.6% metoprolol and −0.8% candesartan) and longer follow up of this cohort is ongoing. The Multidisciplinary approach to Novel Therapies in Cardio-Oncology Research (MANTICORE 101-Breast) trial randomized 94 patients with HER2-positive early breast cancer from two centers with planned treatment with trastuzumab and LVEF ≥50% to perindopril, bisoprolol or placebo prior to initiation of trastuzumab (77% of patients received a non-anthracycline-based chemotherapy regimen) (91). The study was stopped early due to DSMB assessment of futility in reaching the primary endpoint, which was change in left ventricular end diastolic volume index by cardiac MRI at the completion of trastuzumab therapy, which was similar among the three groups. A secondary endpoint, change in LVEF from baseline to completion of therapy was different between the three groups with LVEF reduced by 5% in the placebo group, 3% perindopril group and 1% bisoprolol group (P=0.001). There were also fewer interruptions of trastuzumab therapy in both of the treatment groups compared with the placebo group (P=0.02).
An ongoing multicenter randomized trial of carvedilol CR, lisinopril or placebo in 468 patients with HER2 positive breast cancer prior to trastuzumab initiation has completed enrollment and will be the largest trial to date to test the utility of these agents in preventing trastuzumab cardiotoxicity, trastuzumab treatment interruptions and cardiac events.
Statins
3-hydroxy-3-methylglutaryl-coenzyme A reductase inhibitors (statins) reduce oxidative/nitrosative stress in addition to lowering cholesterol. Animal model data (92), a small RCT (93) and observational data (94) suggests that statin therapy may reduce the risk of cardiotoxicity with anthracycline chemotherapy. The ongoing Preventing Anthracycline Cardiovascular Toxicity with Statins (PREVENT) trial is randomizing patients with breast cancer or lymphoma with planned anthracycline treatment to atorvastatin or placebo to be initiated prior to initiating chemotherapy with a primary outcomes of change in LVEF as assessed by cardiac MRI.
Healthy lifestyle behaviors
In the general population without cancer, healthy lifestyle behaviors such as regular exercise, healthy diet, not smoking and maintaining a healthy weight are associated with lower risk of cardiovascular events and cardiovascular mortality (95). In patients with breast cancer, self-reported exercise is associated with reduced risk of breast cancer mortality (96,97), total mortality (96,97) and reduced cardiovascular events (98). Given the increased risk of cardiovascular disease in patients with breast cancer, focusing on optimizing healthy lifestyle behaviors and cardiac risk factors is important to reducing cardiovascular risk and is endorsed by the ASCO guidelines (50).
Section 5: unanswered questions
While we know a lot about the cardiac effects of breast cancer therapy, there are many unanswered questions remaining. Some of these questions include:
- To what extent have modern RT techniques reduced the cardiac late effects of breast cancer RT?
- Are anthracycline and trastuzumab agents associated with an increased risk HFpEF?
- What is the prognosis and long-term implications of transient reductions in LVEF with subsequent recovery or partial recovery of LV function?
- Should patients be screened for subclinical cardiotoxicity with TnI and/or strain measurements during anthracycline or trastuzumab therapy? What are the appropriate cut-points and how should management change in response to these measures?
- Is there a role for primary prevention strategies for some or all patients treated with anthracyclines, trastuzumab or radiation? Which patients should be treated and with which agents?
Acknowledgements
None.
Footnote
Conflicts of Interest: The author has no conflicts of interest to declare.
References
- Siegel RL, Miller KD, Jemal A. Cancer Statistics, 2017. CA Cancer J Clin 2017;67:7-30. [Crossref] [PubMed]
- Bradshaw PT, Stevens J, Khankari N, et al. Cardiovascular Disease Mortality Among Breast Cancer Survivors. Epidemiology 2016;27:6-13. [Crossref] [PubMed]
- Hanrahan EO, Gonzalez-Angulo AM, Giordano SH, et al. Overall survival and cause-specific mortality of patients with stage T1a,bN0M0 breast carcinoma. J Clin Oncol 2007;25:4952-60. [Crossref] [PubMed]
- Benjamin EJ, Blaha MJ, Chiuve SE, et al. Heart Disease and Stroke Statistics-2017 Update: A Report From the American Heart Association. Circulation 2017;135:e146-603. [Crossref] [PubMed]
- Armenian SH, Xu L, Ky B, et al. Cardiovascular Disease Among Survivors of Adult-Onset Cancer: A Community-Based Retrospective Cohort Study. J Clin Oncol 2016;34:1122-30. [Crossref] [PubMed]
- Johnson CB, Davis MK, Law A, et al. Shared Risk Factors for Cardiovascular Disease and Cancer: Implications for Preventive Health and Clinical Care in Oncology Patients. Can J Cardiol 2016;32:900-7. [Crossref] [PubMed]
- Pinder MC, Duan Z, Goodwin JS, et al. Congestive heart failure in older women treated with adjuvant anthracycline chemotherapy for breast cancer. J Clin Oncol 2007;25:3808-15. [Crossref] [PubMed]
- Darby SC, Ewertz M, Hall P. Ischemic heart disease after breast cancer radiotherapy. N Engl J Med 2013;368:2527. [Crossref] [PubMed]
- Vaz-Luis I, Keating NL, Lin NU, et al. Duration and toxicity of adjuvant trastuzumab in older patients with early-stage breast cancer: a population-based study. J Clin Oncol 2014;32:927-34. [Crossref] [PubMed]
- Early Breast Cancer Trialists' Collaborative Group (EBCTCG), Peto R, Davies C, et al. Comparisons between different polychemotherapy regimens for early breast cancer: meta-analyses of long-term outcome among 100,000 women in 123 randomised trials. Lancet 2012;379:432-44. [Crossref] [PubMed]
- Blum JL, Flynn PJ, Yothers G, et al. Anthracyclines in Early Breast Cancer: The ABC Trials-USOR 06-090, NSABP B-46-I/USOR 07132, and NSABP B-49 (NRG Oncology). J Clin Oncol 2017;35:2647-55. [Crossref] [PubMed]
- Zhang S, Liu X, Bawa-Khalfe T, et al. Identification of the molecular basis of doxorubicin-induced cardiotoxicity. Nat Med 2012;18:1639-42. [Crossref] [PubMed]
- Swain SM, Whaley FS, Ewer MS. Congestive heart failure in patients treated with doxorubicin: a retrospective analysis of three trials. Cancer 2003;97:2869-79. [Crossref] [PubMed]
- Narayan HK, Finkelman B, French B, et al. Detailed Echocardiographic Phenotyping in Breast Cancer Patients: Associations With Ejection Fraction Decline, Recovery, and Heart Failure Symptoms Over 3 Years of Follow-Up. Circulation 2017;135:1397-412. [Crossref] [PubMed]
- Von Hoff DD, Layard MW, Basa P, et al. Risk factors for doxorubicin-induced congestive heart failure. Ann Intern Med 1979;91:710-7. [Crossref] [PubMed]
- Sparano JA, Wang M, Martino S, et al. Weekly paclitaxel in the adjuvant treatment of breast cancer. N Engl J Med 2008;358:1663-71. [Crossref] [PubMed]
- Budd GT, Barlow WE, Moore HC, et al. SWOG S0221: a phase III trial comparing chemotherapy schedules in high-risk early-stage breast cancer. J Clin Oncol 2015;33:58-64. [Crossref] [PubMed]
- Levine MN, Pritchard KI, Bramwell VH, et al. Randomized trial comparing cyclophosphamide, epirubicin, and fluorouracil with cyclophosphamide, methotrexate, and fluorouracil in premenopausal women with node-positive breast cancer: update of National Cancer Institute of Canada Clinical Trials Group Trial MA5. J Clin Oncol 2005;23:5166-70. [Crossref] [PubMed]
- Mackey JR, Martin M, Pienkowski T, et al. Adjuvant docetaxel, doxorubicin, and cyclophosphamide in node-positive breast cancer: 10-year follow-up of the phase 3 randomised BCIRG 001 trial. Lancet Oncol 2013;14:72-80. [Crossref] [PubMed]
- Bonneterre J, Roche H, Kerbrat P, et al. Long-term cardiac follow-up in relapse-free patients after six courses of fluorouracil, epirubicin, and cyclophosphamide, with either 50 or 100 mg of epirubicin, as adjuvant therapy for node-positive breast cancer: French adjuvant study group. J Clin Oncol 2004;22:3070-9. [Crossref] [PubMed]
- Finkelman BS, Putt M, Wang T, et al. Arginine-Nitric Oxide Metabolites and Cardiac Dysfunction in Patients With Breast Cancer. J Am Coll Cardiol 2017;70:152-62. [Crossref] [PubMed]
- Cardinale D, Colombo A, Bacchiani G, et al. Early detection of anthracycline cardiotoxicity and improvement with heart failure therapy. Circulation 2015;131:1981-8. [Crossref] [PubMed]
- Slamon D, Eiermann W, Robert N, et al. Adjuvant trastuzumab in HER2-positive breast cancer. N Engl J Med 2011;365:1273-83. [Crossref] [PubMed]
- Procter M, Suter TM, de Azambuja E, et al. Longer-term assessment of trastuzumab-related cardiac adverse events in the Herceptin Adjuvant (HERA) trial. J Clin Oncol 2010;28:3422-8. [Crossref] [PubMed]
- Romond EH, Jeong JH, Rastogi P, et al. Seven-year follow-up assessment of cardiac function in NSABP B-31, a randomized trial comparing doxorubicin and cyclophosphamide followed by paclitaxel (ACP) with ACP plus trastuzumab as adjuvant therapy for patients with node-positive, human epidermal growth factor receptor 2-positive breast cancer. J Clin Oncol 2012;30:3792-9. [Crossref] [PubMed]
- Perez EA, Suman VJ, Davidson NE, et al. Cardiac safety analysis of doxorubicin and cyclophosphamide followed by paclitaxel with or without trastuzumab in the North Central Cancer Treatment Group N9831 adjuvant breast cancer trial. J Clin Oncol 2008;26:1231-8. [Crossref] [PubMed]
- Chen J, Long JB, Hurria A, et al. Incidence of heart failure or cardiomyopathy after adjuvant trastuzumab therapy for breast cancer. J Am Coll Cardiol 2012;60:2504-12. [Crossref] [PubMed]
- Narayan HK, French B, Khan AM, et al. Noninvasive Measures of Ventricular-Arterial Coupling and Circumferential Strain Predict Cancer Therapeutics-Related Cardiac Dysfunction. JACC Cardiovasc Imaging 2016;9:1131-41. [Crossref] [PubMed]
- Serrano JM, Gonzalez I, Del Castillo S, et al. Diastolic Dysfunction Following Anthracycline-Based Chemotherapy in Breast Cancer Patients: Incidence and Predictors. Oncologist 2015;20:864-72. [Crossref] [PubMed]
- Ho E, Brown A, Barrett P, et al. Subclinical anthracycline- and trastuzumab-induced cardiotoxicity in the long-term follow-up of asymptomatic breast cancer survivors: a speckle tracking echocardiographic study. Heart 2010;96:701-7. [Crossref] [PubMed]
- Tassan-Mangina S, Codorean D, Metivier M, et al. Tissue Doppler imaging and conventional echocardiography after anthracycline treatment in adults: early and late alterations of left ventricular function during a prospective study. Eur J Echocardiogr 2006;7:141-6. [Crossref] [PubMed]
- Slamon DJ, Leyland-Jones B, Shak S, et al. Use of chemotherapy plus a monoclonal antibody against HER2 for metastatic breast cancer that overexpresses HER2. N Engl J Med 2001;344:783-92. [Crossref] [PubMed]
- Baselga J, Cortes J, Kim SB, et al. Pertuzumab plus trastuzumab plus docetaxel for metastatic breast cancer. N Engl J Med 2012;366:109-19. [Crossref] [PubMed]
- Cameron D, Piccart-Gebhart MJ, Gelber RD, et al. 11 years' follow-up of trastuzumab after adjuvant chemotherapy in HER2-positive early breast cancer: final analysis of the HERceptin Adjuvant (HERA) trial. Lancet 2017;389:1195-205. [Crossref] [PubMed]
- Romond EH, Perez EA, Bryant J, et al. Trastuzumab plus adjuvant chemotherapy for operable HER2-positive breast cancer. N Engl J Med 2005;353:1673-84. [Crossref] [PubMed]
- Tan-Chiu E, Yothers G, Romond E, et al. Assessment of cardiac dysfunction in a randomized trial comparing doxorubicin and cyclophosphamide followed by paclitaxel, with or without trastuzumab as adjuvant therapy in node-positive, human epidermal growth factor receptor 2-overexpressing breast cancer: NSABP B-31. J Clin Oncol 2005;23:7811-9. [Crossref] [PubMed]
- Chavez-MacGregor M, Zhang N, Buchholz TA, et al. Trastuzumab-related cardiotoxicity among older patients with breast cancer. J Clin Oncol 2013;31:4222-8. [Crossref] [PubMed]
- Stewart FA, Seemann I, Hoving S, et al. Understanding radiation-induced cardiovascular damage and strategies for intervention. Clin Oncol (R Coll Radiol) 2013;25:617-24. [Crossref] [PubMed]
- Saiki H, Petersen IA, Scott CG, et al. Risk of Heart Failure With Preserved Ejection Fraction in Older Women After Contemporary Radiotherapy for Breast Cancer. Circulation 2017;135:1388-96. [Crossref] [PubMed]
- McGale P, Darby SC, Hall P, et al. Incidence of heart disease in 35,000 women treated with radiotherapy for breast cancer in Denmark and Sweden. Radiother Oncol 2011;100:167-75. [Crossref] [PubMed]
- Hooning MJ, Botma A, Aleman BM, et al. Long-term risk of cardiovascular disease in 10-year survivors of breast cancer. J Natl Cancer Inst 2007;99:365-75. [Crossref] [PubMed]
- Darby SC, McGale P, Taylor CW, et al. Long-term mortality from heart disease and lung cancer after radiotherapy for early breast cancer: prospective cohort study of about 300,000 women in US SEER cancer registries. Lancet Oncol 2005;6:557-65. [Crossref] [PubMed]
- van den Bogaard VA, Ta BD, van der Schaaf A, et al. Validation and Modification of a Prediction Model for Acute Cardiac Events in Patients With Breast Cancer Treated With Radiotherapy Based on Three-Dimensional Dose Distributions to Cardiac Substructures. J Clin Oncol 2017;35:1171-8. [Crossref] [PubMed]
- Wu W, Masri A, Popovic ZB, et al. Long-term survival of patients with radiation heart disease undergoing cardiac surgery: a cohort study. Circulation 2013;127:1476-85. [Crossref] [PubMed]
- Reed GW, Masri A, Griffin BP, et al. Long-Term Mortality in Patients With Radiation-Associated Coronary Artery Disease Treated With Percutaneous Coronary Intervention. Circ Cardiovasc Interv 2016;9. [Crossref] [PubMed]
- Donnellan E, Masri A, Johnston DR, et al. Long-Term Outcomes of Patients With Mediastinal Radiation-Associated Severe Aortic Stenosis and Subsequent Surgical Aortic Valve Replacement: A Matched Cohort Study. J Am Heart Assoc 2017;6. [Crossref] [PubMed]
- Bertog SC, Thambidorai SK, Parakh K, et al. Constrictive pericarditis: etiology and cause-specific survival after pericardiectomy. J Am Coll Cardiol 2004;43:1445-52. [Crossref] [PubMed]
- Ezaz G, Long JB, Gross CP, et al. Risk prediction model for heart failure and cardiomyopathy after adjuvant trastuzumab therapy for breast cancer. J Am Heart Assoc 2014;3. [Crossref] [PubMed]
- Advani PP, Ballman KV, Dockter TJ, et al. Long-Term Cardiac Safety Analysis of NCCTG N9831 (Alliance) Adjuvant Trastuzumab Trial. J Clin Oncol 2016;34:581-7. [Crossref] [PubMed]
- Armenian SH, Lacchetti C, Lenihan D. Prevention and Monitoring of Cardiac Dysfunction in Survivors of Adult Cancers: American Society of Clinical Oncology Clinical Practice Guideline Summary. J Oncol Pract 2017;13:270-5. [Crossref] [PubMed]
- Bowles EJ, Wellman R, Feigelson HS, et al. Risk of heart failure in breast cancer patients after anthracycline and trastuzumab treatment: a retrospective cohort study. J Natl Cancer Inst 2012;104:1293-305. [Crossref] [PubMed]
- van Nimwegen FA, Schaapveld M, Janus CP, et al. Cardiovascular disease after Hodgkin lymphoma treatment: 40-year disease risk. JAMA Intern Med 2015;175:1007-17. [Crossref] [PubMed]
- Blanco JG, Leisenring WM, Gonzalez-Covarrubias VM, et al. Genetic polymorphisms in the carbonyl reductase 3 gene CBR3 and the NAD(P)H:quinone oxidoreductase 1 gene NQO1 in patients who developed anthracycline-related congestive heart failure after childhood cancer. Cancer 2008;112:2789-95. [Crossref] [PubMed]
- Blanco JG, Sun CL, Landier W, et al. Anthracycline-related cardiomyopathy after childhood cancer: role of polymorphisms in carbonyl reductase genes--a report from the Children's Oncology Group. J Clin Oncol 2012;30:1415-21. [Crossref] [PubMed]
- Wojnowski L, Kulle B, Schirmer M, et al. NAD(P)H oxidase and multidrug resistance protein genetic polymorphisms are associated with doxorubicin-induced cardiotoxicity. Circulation 2005;112:3754-62. [Crossref] [PubMed]
- Wang X, Liu W, Sun CL, et al. Hyaluronan synthase 3 variant and anthracycline-related cardiomyopathy: a report from the children's oncology group. J Clin Oncol 2014;32:647-53. [Crossref] [PubMed]
- Visscher H, Rassekh SR, Sandor GS, et al. Genetic variants in SLC22A17 and SLC22A7 are associated with anthracycline-induced cardiotoxicity in children. Pharmacogenomics 2015;16:1065-76. [Crossref] [PubMed]
- Schneider BP, Shen F, Gardner L, et al. Genome-Wide Association Study for Anthracycline-Induced Congestive Heart Failure. Clin Cancer Res 2017;23:43-51. [Crossref] [PubMed]
- Aminkeng F, Bhavsar AP, Visscher H, et al. A coding variant in RARG confers susceptibility to anthracycline-induced cardiotoxicity in childhood cancer. Nat Genet 2015;47:1079-84. [Crossref] [PubMed]
- Stanton SE, Ward MM, Christos P, et al. Pro1170 Ala polymorphism in HER2-neu is associated with risk of trastuzumab cardiotoxicity. BMC Cancer 2015;15:267. [Crossref] [PubMed]
- Boekhout AH, Gietema JA, Milojkovic Kerklaan B, et al. Angiotensin II-Receptor Inhibition With Candesartan to Prevent Trastuzumab-Related Cardiotoxic Effects in Patients With Early Breast Cancer: A Randomized Clinical Trial. JAMA Oncol 2016;2:1030-7. [Crossref] [PubMed]
- Kotwinski P, Smith G, Cooper J, et al. Body Surface Area and Baseline Blood Pressure Predict Subclinical Anthracycline Cardiotoxicity in Women Treated for Early Breast Cancer. PLoS One 2016;11. [Crossref] [PubMed]
- Wang TJ, Evans JC, Benjamin EJ, et al. Natural history of asymptomatic left ventricular systolic dysfunction in the community. Circulation 2003;108:977-82. [Crossref] [PubMed]
- SOLVD Investigators, Yusuf S, Pitt B, et al. Effect of enalapril on mortality and the development of heart failure in asymptomatic patients with reduced left ventricular ejection fractions. N Engl J Med 1992;327:685-91. [Crossref] [PubMed]
- Pfeffer MA, Braunwald E, Moye LA, et al. Effect of captopril on mortality and morbidity in patients with left ventricular dysfunction after myocardial infarction. Results of the survival and ventricular enlargement trial. The SAVE Investigators. N Engl J Med 1992;327:669-77. [Crossref] [PubMed]
- Pfeffer MA, McMurray JJ, Velazquez EJ, et al. Valsartan, captopril, or both in myocardial infarction complicated by heart failure, left ventricular dysfunction, or both. N Engl J Med 2003;349:1893-906. [Crossref] [PubMed]
- Dargie HJ. Effect of carvedilol on outcome after myocardial infarction in patients with left-ventricular dysfunction: the CAPRICORN randomised trial. Lancet 2001;357:1385-90. [Crossref] [PubMed]
- Yancy CW, Jessup M, Bozkurt B, et al. 2017 ACC/AHA/HFSA Focused Update of the 2013 ACCF/AHA Guideline for the Management of Heart Failure: A Report of the American College of Cardiology/American Heart Association Task Force on Clinical Practice Guidelines and the Heart Failure Society of America. J Card Fail 2017;23:628-51. [Crossref] [PubMed]
- Yancy CW, Jessup M, Bozkurt B, et al. 2013 ACCF/AHA guideline for the management of heart failure: executive summary: a report of the American College of Cardiology Foundation/American Heart Association Task Force on practice guidelines. Circulation 2013;128:1810-52. [Crossref] [PubMed]
- Cardinale D, Colombo A, Sandri MT, et al. Prevention of high-dose chemotherapy-induced cardiotoxicity in high-risk patients by angiotensin-converting enzyme inhibition. Circulation 2006;114:2474-81. [Crossref] [PubMed]
- Guarneri V, Lenihan DJ, Valero V, et al. Long-term cardiac tolerability of trastuzumab in metastatic breast cancer: the M.D. Anderson Cancer Center experience. J Clin Oncol 2006;24:4107-15. [Crossref] [PubMed]
- Lipshultz SE, Lipsitz SR, Sallan SE, et al. Long-term enalapril therapy for left ventricular dysfunction in doxorubicin-treated survivors of childhood cancer. J Clin Oncol 2002;20:4517-22. [Crossref] [PubMed]
- Goldberg LR, Jessup M. Stage B heart failure: management of asymptomatic left ventricular systolic dysfunction. Circulation 2006;113:2851-60. [Crossref] [PubMed]
- Cardinale D, Sandri MT, Colombo A, et al. Prognostic value of troponin I in cardiac risk stratification of cancer patients undergoing high-dose chemotherapy. Circulation 2004;109:2749-54. [Crossref] [PubMed]
- Ky B, Putt M, Sawaya H, et al. Early increases in multiple biomarkers predict subsequent cardiotoxicity in patients with breast cancer treated with doxorubicin, taxanes, and trastuzumab. J Am Coll Cardiol 2014;63:809-16. [Crossref] [PubMed]
- Sawaya H, Sebag IA, Plana JC, et al. Assessment of echocardiography and biomarkers for the extended prediction of cardiotoxicity in patients treated with anthracyclines, taxanes, and trastuzumab. Circ Cardiovasc Imaging 2012;5:596-603. [Crossref] [PubMed]
- Thavendiranathan P, Poulin F, Lim KD, et al. Use of myocardial strain imaging by echocardiography for the early detection of cardiotoxicity in patients during and after cancer chemotherapy: a systematic review. J Am Coll Cardiol 2014;63:2751-68. [Crossref] [PubMed]
- Sawaya H, Sebag IA, Plana JC, et al. Early detection and prediction of cardiotoxicity in chemotherapy-treated patients. Am J Cardiol 2011;107:1375-80. [Crossref] [PubMed]
- Negishi K, Negishi T, Haluska BA, et al. Use of speckle strain to assess left ventricular responses to cardiotoxic chemotherapy and cardioprotection. Eur Heart J Cardiovasc Imaging 2014;15:324-31. [Crossref] [PubMed]
- van Dalen EC, Caron HN, Dickinson HO, et al. Cardioprotective interventions for cancer patients receiving anthracyclines. Cochrane Database Syst Rev 2011. [PubMed]
- Smith LA, Cornelius VR, Plummer CJ, et al. Cardiotoxicity of anthracycline agents for the treatment of cancer: systematic review and meta-analysis of randomised controlled trials. BMC Cancer 2010;10:337. [Crossref] [PubMed]
- Marty M, Espie M, Llombart A, et al. Multicenter randomized phase III study of the cardioprotective effect of dexrazoxane (Cardioxane) in advanced/metastatic breast cancer patients treated with anthracycline-based chemotherapy. Ann Oncol 2006;17:614-22. [Crossref] [PubMed]
- Speyer JL, Green MD, Zeleniuch-Jacquotte A, et al. ICRF-187 permits longer treatment with doxorubicin in women with breast cancer. J Clin Oncol 1992;10:117-27. [Crossref] [PubMed]
- Swain SM, Whaley FS, Gerber MC, et al. Cardioprotection with dexrazoxane for doxorubicin-containing therapy in advanced breast cancer. J Clin Oncol 1997;15:1318-32. [Crossref] [PubMed]
- Venturini M, Michelotti A, Del Mastro L, et al. Multicenter randomized controlled clinical trial to evaluate cardioprotection of dexrazoxane versus no cardioprotection in women receiving epirubicin chemotherapy for advanced breast cancer. J Clin Oncol 1996;14:3112-20. [Crossref] [PubMed]
- Lopez M, Vici P, Di Lauro K, et al. Randomized prospective clinical trial of high-dose epirubicin and dexrazoxane in patients with advanced breast cancer and soft tissue sarcomas. J Clin Oncol 1998;16:86-92. [Crossref] [PubMed]
- Lyu YL, Kerrigan JE, Lin CP, et al. Topoisomerase IIbeta mediated DNA double-strand breaks: implications in doxorubicin cardiotoxicity and prevention by dexrazoxane. Cancer Res 2007;67:8839-46. [Crossref] [PubMed]
- Vaynblat M, Shah HR, Bhaskaran D, et al. Simultaneous angiotensin converting enzyme inhibition moderates ventricular dysfunction caused by doxorubicin. Eur J Heart Fail 2002;4:583-6. [Crossref] [PubMed]
- Cardinale D, Colombo A, Lamantia G, et al. Anthracycline-induced cardiomyopathy: clinical relevance and response to pharmacologic therapy. J Am Coll Cardiol 2010;55:213-20. [Crossref] [PubMed]
- Gulati G, Heck SL, Ree AH, et al. Prevention of cardiac dysfunction during adjuvant breast cancer therapy (PRADA): a 2 x 2 factorial, randomized, placebo-controlled, double-blind clinical trial of candesartan and metoprolol. Eur Heart J 2016;37:1671-80. [Crossref] [PubMed]
- Pituskin E, Mackey JR, Koshman S, et al. Multidisciplinary Approach to Novel Therapies in Cardio-Oncology Research (MANTICORE 101-Breast): A Randomized Trial for the Prevention of Trastuzumab-Associated Cardiotoxicity. J Clin Oncol 2017;35:870-7. [Crossref] [PubMed]
- Riad A, Bien S, Westermann D, et al. Pretreatment with statin attenuates the cardiotoxicity of Doxorubicin in mice. Cancer Res 2009;69:695-9. [Crossref] [PubMed]
- Acar Z, Kale A, Turgut M, et al. Efficiency of atorvastatin in the protection of anthracycline-induced cardiomyopathy. J Am Coll Cardiol 2011;58:988-9. [Crossref] [PubMed]
- Seicean S, Seicean A, Plana JC, et al. Effect of statin therapy on the risk for incident heart failure in patients with breast cancer receiving anthracycline chemotherapy: an observational clinical cohort study. J Am Coll Cardiol 2012;60:2384-90. [Crossref] [PubMed]
- Folsom AR, Yatsuya H, Nettleton JA, et al. Community prevalence of ideal cardiovascular health, by the American Heart Association definition, and relationship with cardiovascular disease incidence. J Am Coll Cardiol 2011;57:1690-6. [Crossref] [PubMed]
- Holmes MD, Chen WY, Feskanich D, et al. Physical activity and survival after breast cancer diagnosis. JAMA 2005;293:2479-86. [Crossref] [PubMed]
- Irwin ML, Smith AW, McTiernan A, et al. Influence of pre- and postdiagnosis physical activity on mortality in breast cancer survivors: the health, eating, activity, and lifestyle study. J Clin Oncol 2008;26:3958-64. [Crossref] [PubMed]
- Jones LW, Habel LA, Weltzien E, et al. Exercise and Risk of Cardiovascular Events in Women With Nonmetastatic Breast Cancer. J Clin Oncol 2016;34:2743-9. [Crossref] [PubMed]


