Percutaneous microwave thermosphere ablation of pancreatic tumours
Introduction
Pancreatic cancer, mostly represented by pancreatic adenocarcinoma, is the fourth cause of cancer-related deaths worldwide; 5-year survival rate in US and Western countries is about 8% (1). To date, surgery is still the only potentially curative treatment, but in most cases it can’t be offered as an option due to the advanced stage of disease at diagnosis (either locally advanced or metastatic disease) or to the patient’s comorbidities (2,3). General considerations, such as advanced age, comorbidities, may also contraindicate surgical approach (4). Given the inoperability of most pancreatic tumors and the minimal symptomatic improvement that chemotherapy alone can achieve in these patients, growing interest in ablative palliative therapies was observed in the last years (5,6). The numerous patients for whom the surgical approach is contraindicated can benefit from percutaneous ablative therapies on the basis of their mini-invasivity (7,8). Improvement of quality of life (QoL) and relief of pain may represent often the only indications for ablation (9,10). Microwave ablation (MWA) has been already described in the treatment of various neoplasms. In the last years a progressive and continuous evolution in energy delivery, application techniques, and therapeutic combinations was made in the field of ablative therapies, with a resulting improvement of their efficacy and safety (11). Nevertheless, the application of thermo-ablative techniques has been limited in some tumors by the risk of causing thermal injury to vital structures surrounding or encased by the tumor itself. This is particularly true for an organ such as the pancreas. In fact, the pancreatic gland is surrounded by delicate and critical organs like duodenum, common bile duct, upper mesenteric vessels, splenic vessels and spleen, portal vein, abdominal aorta and inferior vena cava, and transverse mesocolon with its vessels (12). This complex anatomy is responsible for the impossibility of including the whole cancer field in the ablation volume in patients with unresectable pancreatic cancer. Microwave energy has already shown to have several advantages when compared to other ablative modalities (13). Recently additional technologic developments and advances have led to a new MWA system with Thermosphere® technology (14). This system uses different kinds of intra-procedural controls that result in a more predictable spherical ablation volume, based on the position of the antenna, allowing the operator to safely keep the ablation zone’s margins completely within the tumor and away from surrounding vital structures (15). This advantage is particularly important to avoid iatrogenic damages in an organ like the pancreas, where it makes the procedure considerably safer (14,16). To date only one MWA system (EmprintTM ablation System/Covidien Ltd., USA) is capable of achieving a predictable spherical ablation volume. The purpose of the present study is to present our preliminary experience of percutaneous high energy MWA of unresectable pancreatic cancer and to evaluate feasibility and safety.
Methods
Five patients (4 men and 1 woman; mean age, 73.8 years) with pancreatic head cancer treated with MWA in the period August 2016–February 2017, were retrospectively reviewed. The percutaneous approach was used in all cases.
The inclusion criteria for percutaneous MW ablation treatment were an age of at least 18 years old, adenocarcinoma confirmed by a histologic report, presence of an unresectable tumor associated with a locally advanced disease stage (vascular and/or with nearby organ infiltration), lack of response to chemotherapy (stability or progression of disease), normal coagulation parameters, surgery refusal, comorbidities (severe cardiovascular and/or respiratory diseases), and percutaneous accessibility of the target lesion. The exclusion criteria were an age younger than 18 years old, pregnancy, abnormal blood coagulation tests, or impossibility of percutaneous access. In all cases, an interventional radiologist (G.C. or A.M.I., with 22 and 11 years of experience, respectively) evaluated the percutaneous accessibility of the tumors. In one patient, the treatment was performed with a previously placed internal/external biliary drain.
The initial diagnosis of pancreatic malignancy was based on classical imaging by computed tomography (CT) and ultrasonography (US) and a preoperative tissue biopsy. Each patient’s case was discussed in the contest of a multidisciplinary meeting, during which the indication for thermal ablation was given. Indications, benefits and risks of the procedure were explained and discussed with every patient and informed consent was obtained before treatment. Coagulation blood tests resulted within the reference values in all patients. Eventually ongoing anticoagulant and/or anti-platelet therapies were interrupted at least 7 days before the procedure, and low molecular weight heparin was initiated when necessary. Complete blood count, serum bilirubin and liver enzyme levels were assessed before and after treatment. A first-generation cephalosporin (cefazolin 2 gr b.i.d.) (Pfizer Srl, Milan, Italy) was administered at the beginning of every procedure as antibiotic prophylaxis. The study was approved by the Internal Review Board of our institution.
Baseline imaging
An abdominal CT examination (GE Lightspeed VCT 64), unenhanced and after intravenous contrast medium administration, was performed in every patient before the ablative treatment. Each CT scan was acquired with a thickness of 0.6 mm, a voltage of 120 kV, and a tube current of 250 mA. The contrast-enhanced scans were acquired after intravenous administration of 100 mL of iodinated contrast agent (Visipaque 320; GE Healthcare, Milwaukee, Wisconsin, USA) at an injection rate of 3 mL/s, followed by injection of 40 mL saline solution at a rate of 2 mL/s. Post-treatment imaging studies consisted of analogous abdominal CT performed at 1, 3, 6 and 12 months after the ablation procedure. Pre-treatment US was performed with Arietta V70 (Hitachi Aloka Medical, Tokyo, Japan), by the same operators who will perform the procedure.
Procedure
A 10-mL solution of lidocaine was injected to obtain local anesthesia in correspondence of the entrance site of the antenna. Anesthesiological specialized assistance was provided for every patient during the whole ablation session. Moderate sedation was achieved in each patient through intravenous injection of propofol (0.5–2.0 mg/kg/h), fentanyl (1–2 mcg/kg) and midazolam (0.07–0.08 mg/kg). Vital parameters (heart rate, respiratory rate, blood pressure), together with oxygen saturation and electrocardiographic tracing, were continuously monitored during the procedure.
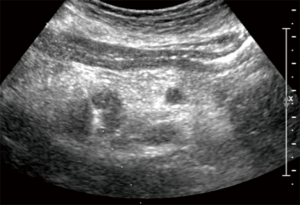
The antenna was positioned under US-guidance [Arietta V70 (Hitachi Aloka Medical)] (Figure 1). The ablation system consists of a microwave generator, capable of producing a power of 100 W at 2,450 MHz, which is connected to a 13.5-gauge straight microwave antenna with a 2.8-cm radiating section by coaxial cable. Continuous perfusion with saline solution at 60 mL/min and at room temperature is provided by the system along the proximal part of the antenna to avoid any thermal damage. The ablative procedure itself consisted, after the US-guided placement of the antenna into the target tumor, in the delivery of thermal energy to the tissue, produced by maintenance of a power of 100 W for a total time between 2.30 and 3 minutes, as specified by the manufacturer, obtaining the desired necrosis volume.
Outcomes and methods
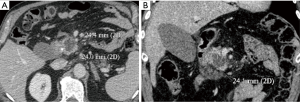
Technical success consisted in a correct position of the antenna into the target lesion, as planned before treatment. Time of ablation and overall procedure time were registered in all patients. Complications were classified according to Common Terminology Criteria for Adverse Events (17). Safety was evaluated on the basis of the complications recorded immediately after the procedure and during the follow-up. A complication was defined as “immediate” when it occurred up to 24 hours following the procedure, as “periprocedural” if it occurred within 30 days, and as “delayed” if occurred more than 30 days after the procedure (18). Major complications were defined as events that, if untreated, could leave to life threatening, or events that led to substantial morbidity and disability, or could be cause of hospital readmission or substantially lengthened the patient’s hospital stay (6,19). Minor complications included typical postablation syndrome symptoms (fever, pain, nausea and vomiting) if lasting more than 4 days after the ablation procedure. The follow up protocol included a CT abdominal examination at 1, 3, 6 and 12 months after treatment, together with the assessment of complete blood count and metabolic function tests. All the follow up CT images were evaluated by a specialized radiologist, blinded from the ones that had performed the procedures. The images obtained were classified according to the Response Evaluation Criteria in Solid Tumors (RECIST; version 1.1) (20), although this type of analysis is not the primary purpose of the present study. For a better evaluation of the ablation zone, all the portal venous phase images of the 1-month follow-up CT scans were transferred to a workstation, where manual segmentation of the MW ablation volume was performed on each axial image. Multiplanar reformatting (MPR) was performed to study the ablation volume in the three dimensions (Figure 2A,B). The longitudinal diameter was measured along the electrode axis on the plane in which the electrode shaft was located, and two transverse diameters were measured on the planes perpendicular to the first plane. Transverse diameter A was defined as the longest transverse diameter, while the other transverse diameter was defined as transverse diameter B. To evaluate the shape of the ablation zone, roundness index A was defined as transverse diameter A divided by longitudinal diameter, roundness index B was defined as transverse diameter B divided by longitudinal diameter, and roundness index transverse was defined as transverse diameter B divided by transverse diameter A. Therefore, a value near 1 represents a more spherical ablation zone shape, and a value distant from 1 implies an oval configuration (14,15). All patients underwent a clinical evaluation, which included a QoL questionnaire, and they were asked about post-ablation syndrome, which consists of transient flulike symptoms (fever, malaise, pain, myalgia, nausea and vomiting) usually resolving within 4 days post-procedure (21). A single trained interviewer registered prospectively the data about the QoL before treatment, during the first postoperative outpatient visit, and at 6-week, 3- and 6-month follow-up visits. The European Organisation for Research and Treatment of Cancer QoL Questionnaire Core 30 (22), a cancer-specific metric questionnaire, was used. The following parameters were assessed at every visit: fatigue, pain and loss of appetite.
Results
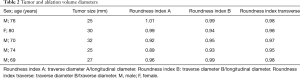
The mean follow-up period was 9.2 months (range, 6–12 months). The technical success rate was 100%, as the antennae were correctly positioned into the tumor in all cases. Histologic reports demonstrated pancreatic ductal adenocarcinoma (PDAC) in all cases (23). The mean diameter of adenocarcinomas was 27.8 mm (range, 25–32 mm). Mean ablation time was of 2 minutes and 48 seconds (range, 2 minutes and 30 seconds–3 minutes). Mean overall procedure time was 28 min (range, 25–30 min) (Table 1).

Full table
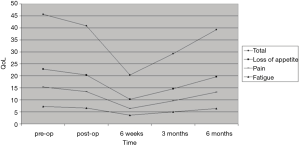
During follow-up, contrast-enhanced CT results according to RECIST were as follows: 5 partial response (PR), 0 stable disease (SDs), and 0 progressive disease (PD) at 1 month; 0 PR, 3 SDs, and 2 PD at 4 months; 0 PR, 2 SDs, and 1 PDs at 9 months; 0 PR, 1 SD, and 1 PDs at 12 months. No cases of CR were observed, and 3 patients died during follow up, respectively 2 at 6 months and 1 at 10 months, as a result of their primary cancer. Table 2 reports the Roundness indexes of the ablation zones. The mean value of the Roundness index transverse resulted 0.97, indicating a spherical shape of the ablation mean volume (Table 2). No major complications were registered. Only one minor complication, consisting of a mild peripancreatic fluid collection diagnosed at the post-procedural imaging, was registered; no associated increase of pancreatic enzymes or abdominal pain was recorded, and the collection resolved without any specific medic or surgical intervention before discharge. Pain post-procedure was not clinically relevant for any patient and in particular, any medication other than the ones used during the procedure was required. Postablation syndrome was not considered to be relevant in any patient and resolved within the expected time in all cases. QoL significantly improved in all patients after 6 weeks and tended to return to pretreatment levels in the following months (Figure 3). QoL was not influenced by minor complications. None of the patients required further surgery, and all complications were solved over the course of the hospital stay. The hospital stays after the procedure had an average duration of 4 days (range, 3–5 days). With respect to tumor response, none of the patients were eligible for a second treatment. Three days after the procedure, the internal/external biliary drain previously placed in one of the patients treated, was removed and replaced with a permanent metallic stent.
Full table
Discussion
In the last years numerous studies have described the use of local ablative techniques for the treatment of unresectable tumors in various organs, including pancreas (24-26). In particular, growing interest has been shown for microwave technology, which has the same benefits but also important advantages when compared to the more commonly known radiofrequency ablation (RFA). In fact, microwaves radiate through all biological tissues, including charred, desiccated and high electrical impedance tissues, like the ones produced by ablation itself. These technical features permit to reduce procedure times, to achieve high temperatures in the target tumor obtaining larger volumes of cellular necrosis, and to be more efficient on lesions with cystic components and/or located closer to vascular structures, with a reduction in the heat-sink effect, and less intra-procedural pain (25,27,28). However, also some disadvantages of MW have been described, one of the most limiting being the not totally predictable size and shape of the ablation field. This is crucial when considering pancreatic tumors, as the pancreas is surrounded by numerous important and delicate anatomic vascular and non-vascular structures (common bile duct, duodenum, transverse colon, portal vein, mesenteric artery and vein, celiac trunk) at real risk of thermal injury (10,25). Moreover, the nonmaleficence universal medical principle needs to be here strictly observed since, like RF ablation, MW ablation of pancreatic tumors is restricted to locally advanced, nonresectable, and nonmetastatic neoplasms, with the primary purpose being not the cure but the palliation of the disease (29). It’s important to note that also other ablative techniques have been recently described for pancreatic cancer, like irreversible electroporation (IRE), that doesn’t involve the use of thermal energy thereby reducing the risk of causing injuries to viable structures; few data were published even if IRE seems to be a very attractive option in patients with local advanced pancreatic cancer on the basis of its absence of thermal effect on the vessels. However, IRE is very expensive at the moment and not available in many centers (8).
Some immunological anti-tumor beneficial effects have been widely described for RF ablation of pancreatic and hepatic cancers, and could be translated to other heat-based ablation techniques, including MW, but this is not the aim of this study (30). To overcome the problems related to conventional microwaves, a new technology has been developed and already described. The system (EmprintTM ablation System/Covidien Ltd) includes a 2,450-MHz generator that delivers a maximum power of 100 W and includes a new technology (Thermosphere®) that allows the operator to obtain a predictable and spherical ablation volume, using only one antenna. This is obtained by three different types of control: field control, thermal control and wavelength control (14). In this study we measured the three perpendicular dimensions of the ablation volume on the MPR reconstruction images, and we performed a quantitative analysis of the ablation volume shape through the evaluation of the roundness index transverse. This method has been already described in literature (15). In this preliminary small series, the mean roundness index transverse was 0.97, demonstrating the possibility of achieving a spherical ablation volume in pancreatic tumors. This feature, combined with an accurate pre-procedure patient selection and evaluation of the predicted ablation area on the basis of the position of the antenna made by an expert interventional radiologist, sensibly increases the safety of the MWA procedure when compared to the old system. Our technical success rate was 100%, with the antenna always correctly positioned. We have registered no major complication and one minor complication (peripancreatic fluid in one patient without any increase of pancreatic enzymes or abdominal pain). The entire procedure was relatively short, having a mean time of 28 minutes, thus reducing sedation time and patients’ discomfort, and well tolerated, as no post-procedure pain drugs were needed by any patient. Few data are available in literature regarding pain relief and QoL of patients undergoing ablation techniques for unresectable pancreatic cancer (9,10,31). In our series the Treatment of Cancer QoL Questionnaire Core 30 was used (22). We registered an improvement in the QoL of all the patients at the 6 weeks and 3 months follow-up visits, with a new decrease at the 6 months control, even though not reaching pre-treatments gravity. This result is significant considering that these patients with advanced stage disease are not eligible for surgical treatment. Notably QoL wasn’t modified by minor complications. The small number of patients in this study and the paucity of literature data about the efficacy of ablation on palliation pancreatic tumors doesn’t allow significative comparisons, but our purpose is to make this comparison possible with further studies. Regarding the classification of the disease based on application of the RECIST 1.1 criteria to the contrast-enhanced CT images during follow up, it’s important to notice that MW ablation aims to reduce the tumor’s volume, thus reducing pain, in safe conditions, and it’s not intended as a curative procedure. At the same time MWA doesn’t preclude other types of treatments, being a possible bridge to chemo or radiation therapy. It doesn’t preclude surgery either, even if given the disease stage, this option is unlikely. In the current series no patient was eligible for a second treatment. In addition to the technical feasibility, safety and palliative efficacy of the procedure, the relatively short hospital stay of our patients (average 4 days) justifies the growing interest in MWA. In our opinion, the latter should be considered an advantage in order to palliate.
The limitations of the present study include its single-center, retrospective design, the small number of patients, the relatively short time of follow up and the lack of randomization for comparisons with other types of palliation.
Conclusions
The preliminary results reported in this study demonstrated feasibility, safety and effectiveness of the high-powered MWA as palliative treatment for patients with unresectable pancreatic tumors. This technique has some important advantages over other types of palliation, and has now become sensibly safer with the use of the new available technologies. Further studies are necessary to confirm effectiveness of MWA, to compare it with other palliative treatments, and to individuate the exact role it could play in the treatment of pancreatic cancer.
Acknowledgements
None.
Footnote
Conflicts of Interest: The authors have no conflicts of interest to declare.
Ethical Statement: The study was approved by our Internal Review Board (07_01_2016_M7.1). The study was conducted in accordance with the Helsinki Declaration of 1964 (revised 2008).
References
- Siegel RL, Miller KD, Jemal A. Cancer statistics, 2016. CA Cancer J Clin 2016;66:7-30. [Crossref] [PubMed]
- Kircher SM, Krantz SB, Nimeiri HS, et al. Therapy of locally advanced pancreatic adenocarcinoma: unresectable and borderline patients. Expert Rev Anticancer Ther 2011;11:1555-65. [Crossref] [PubMed]
- Hussain D, Khan MR, Azami R. Surgical palliation for unresectable pancreatic carcinoma. J Pak Med Assoc 2004;54:601-4. [PubMed]
- Callery MP, Chang KJ, Fishman EK, et al. Pretreatment assessment of resectable and borderline resectable pancreatic cancer: expert consensus statement. Ann Surg Oncol 2009;16:1727-33. [Crossref] [PubMed]
- Ierardi AM, Lucchina N, Petrillo M, et al. Systematic review of minimally invasive ablation treatment for locally advanced pancreatic cancer. Radiol Med 2014.483-98. [Crossref] [PubMed]
- D’Onofrio M, Barbi E, Girelli R, et al. Radiofrequency ablation of locally advanced pancreatic adenocarcinoma: An overview. World J Gastroenterol 2010;16:3478-83. [Crossref] [PubMed]
- Narayanan G, Hosein PJ, Arora G, et al. Percutaneous irreversible electroporation for downstaging and control of unresectable pancreatic adenocarcinoma. J Vasc Interv Radiol 2012;23:1613-21. [Crossref] [PubMed]
- Ierardi AM, Lucchina N, Bacuzzi A, et al. Percutaneous ablation therapies of inoperable pancreatic cancer: a systematic review. Ann Gastroenterol 2015;28:431-9. [PubMed]
- Carrafiello G, Ierardi AM, Fontana F, et al. Microwave ablation of pancreatic head cancer: Safety and efficacy. J Vasc Interv Radiol 2013;24:1513-20. [Crossref] [PubMed]
- Girelli R, Frigerio I, Salvia R, et al. Feasibility and safety of radiofrequency ablation for locally advanced pancreatic cancer. Br J Surg 2010;97:220-5. [Crossref] [PubMed]
- Ahmed M, Brace CL, Lee FT Jr, et al. Principles of and advances in percutaneous ablation. Radiology 2011;258:351-69. [Crossref] [PubMed]
- Rossi M, Orgera G, Hatzidakis A, et al. Minimally invasive ablation treatment for locally advanced pancreatic adenocarcinoma. Cardiovasc Intervent Radiol 2014;37:586-91. [Crossref] [PubMed]
- Brace CL. Microwave tissue ablation: biophysics, technology, and applications. Crit Rev Biomed Eng 2010;38:65-78. [Crossref] [PubMed]
- Ierardi AM, Mangano A, Floridi C, et al. A new system of microwave ablation at 2450 MHz: preliminary experience. Updates Surg 2015;67:39-45. [Crossref] [PubMed]
- Park MJ, Kim YS, Rhim H, et al. A comparison of US-guided percutaneous radiofrequency ablation of medium-sized hepatocellular carcinoma with a cluster electrode or a single electrode with a multiple overlapping ablation technique. J Vasc Interv Radiol 2011;22:771-9. [Crossref] [PubMed]
- Splatt AM, Steinke K. Major complications of high-energy microwave ablation for percutaneous CT-guided treatment of lung malignancies: Single-centre experience after 4 years. J Med Imaging Radiat Oncol 2015;59:609-16. [Crossref] [PubMed]
- National Institute of Cancer. Common terminology criteria for adverse events (CTCAE). NIH Publication 2010;2009:0-71.
- Rhim H, Dodd GD 3rd, Chintapalli KN, et al. Radiofrequency thermal ablation of abdominal tumors: lessons learned from complications. Radiographics 2004;24:41-52. [Crossref] [PubMed]
- Goldberg SN, Grassi CJ, Cardella JF, et al. Image-guided tumor ablation: standardization of terminology and reporting criteria. J Vasc Interv Radiol 2009;20:S377-90. [Crossref] [PubMed]
- Eisenhauer EA, Therasse P, Bogaerts J, et al. New response evaluation criteria in solid tumours: revised RECIST guideline (version 1.1). Eur J Cancer 2009;45:228-47. [Crossref] [PubMed]
- Carrafiello G, Laganà D, Ianniello A, et al. Post-radiofrequency ablation syndrome after percutaneous radiofrequency of abdominal tumours: One centre experience and review of published works. Australas Radiol 2007;51:550-4. [Crossref] [PubMed]
- Aaronson NK, Ahmedzai S, Bergman B, et al. The European organisation for research and treatment of cancer QLQ-C30: a quality-of-life instrument for use in international clinical trials in oncology. J Natl Cancer Inst 1993;85:365-76. [Crossref] [PubMed]
- Janky R, Binda MM, Allemeersch J, et al. Prognostic relevance of molecular subtypes and master regulators in pancreatic ductal adenocarcinoma. BMC Cancer 2016;16:632. [Crossref] [PubMed]
- Ierardi AM, Coppola A, Lucchina N, et al. Treatment of lung tumours with high-energy microwave ablation: a single-centre experience. Med Oncol 2017;34:5. [Crossref] [PubMed]
- Carrafiello G, Laganà D, Mangini M, et al. Microwave tumors ablation: Principles, clinical applications and review of preliminary experiences. Int J Surg 2008;6:S65-9. [Crossref] [PubMed]
- Zhang NN, Lu W, Cheng XJ, et al. High-powered microwave ablation of larger hepatocellular carcinoma: Evaluation of recurrence rate and factors related to recurrence. Clin Radiol 2015;70:1237-43. [Crossref] [PubMed]
- Simon CJ, Dupuy DE, Mayo-Smith WW. Microwave ablation: principles and applications. Radiographics 2005;25:S69-83. [Crossref] [PubMed]
- Brace CL, Hinshaw JL, Laeseke PF, et al. Pulmonary thermal ablation: comparison of radiofrequency and microwave devices by using gross pathologic and CT findings in a swine model. Radiology 2009;251:705-11. [Crossref] [PubMed]
- Hadjicostas P, Malakounides N, Varianos C, et al. Radiofrequency ablation in pancreatic cancer. HPB (Oxford) 2006;8:61-4. [Crossref] [PubMed]
- Teng LS, Jin KT, Han N, et al. Radiofrequency ablation, heat shock protein 70 and potential anti-tumor immunity in hepatic and pancreatic cancers: A minireview. Hepatobiliary Pancreat Dis Int 2010;9:361-5. [PubMed]
- Wu Y, Tang Z, Fang H, et al. High operative risk of cool-tip radiofrequency ablation for unresectable pancreatic head cancer. J Surg Oncol 2006;94:392-5. [Crossref] [PubMed]


