A nomogram to predict the likelihood of permanent hypoparathyroidism after total thyroidectomy based on delayed serum calcium and iPTH measurements
Introduction
Total thyroidectomy (TT) has become the operation of choice for most patients with thyroid cancer or bilateral benign disease. The increasing use of TT, however, has been associated with more patients developing and being treated for postoperative hypocalcemia, which is currently its most common adverse effect (1-5). Medical treatment of postoperative hypocalcemia consists in the administration of calcium salts and active vitamin D metabolites according to three different strategies: routine treatment, reactive treatment (only for patients developing symptoms) or preventive supplementation for patients with low PTH and/or serum calcium 24 h after surgery. We adhere to the latter (6,7) to avoid overtreatment of those patients with a normal postoperative parathyroid function and to prevent symptomatic hypocalcemia, often requiring hospital readmission.
There are no reliable immediate postoperative biochemical parameters that allow the identification of those patients with hypocalcemia that will develop protracted or permanent hypoparathyroidism; consequently acute postoperative parathyroid failure is medically treated until parathyroid function returns to normal and patients can be weaned off replacement therapy. Despite the lack of good evidence studies, a common medical strategy during this initial period of parathyroid insufficiency consists of keeping the serum calcium in the low-normal range to stimulate the parathyroid glands and to avoid hypercalcaemia (8). Previous retrospective studies, however, have shown that normal-high serum calcium levels and detectable iPTH well after TT are associated with an increased likelihood of recovery from protracted hypoparathyroidism (9,10). Further analysis revealed that the number of parathyroid glands remaining in situ (PGRIS) after TT had a synergistic effect with serum calcium levels in improving the likelihood of parathyroid function recovery in cases where replacement therapy had to be continued for more than 1 month after TT (11).
The cellular biology events leading either to the recovery of the functionality of the parathyroid glands or to permanent hypoparathyroidism are unknown. Typically, the parathyroid glands stop secreting PTH for a more or less prolonged period of time and then, most often, PTH serum concentration returns to normal. Although in most instances hypoparathyroidism resolves within 6 months, time to recovery may exceed 1 year (12). We have hypothesized (10) that resting the failing parathyroid glands keeping the serum calcium in the high-normal range during the initial postoperative period may help to restore the parathyroid function (parathyroid splinting). To further investigate this hypothesis, the present study aimed at assessing prospectively the influence of serum calcium concentrations under replacement therapy on the recovery of parathyroid function, and, more specifically, the ability of delayed serum calcium and iPTH concentrations to predict permanent hypoparathyroidism after the first postoperative month.
Methods
A prospective longitudinal observational cohort multicenter study was performed during the years 2013–2014 on consecutive adult patients undergoing first-time TT (either for benign or malignant conditions) followed by parathyroid failure defined as a serum calcium <8 mg/dL at 24 h and PTH <15 pg/mL at 4 h after surgery. Participant centers were Fundación Jiménez Díaz (Madrid), University of Padova, Jagiellonian University Medical College (Krakow) and Hospital del Mar (Barcelona). Patients were started on replacement treatment after parathyroid insufficiency was diagnosed and then discharged from hospital and followed for at least 1 year. Exclusion criteria were reoperations, completion thyroidectomies and associated parathyroidectomy for primary or secondary hyperparathyroidism.
The study was approved by the Institutional Review Board at each participating center. Signed consent was obtained from recruited patients at the time postoperative hypocalcemia was diagnosed.
Definitions
Protracted hypoparathyroidism was diagnosed if iPTH concentration was low (<15 pg/mL) or undetectable and medical treatment was still necessary 1 month after TT (6). Factors influencing prolonged parathyroid failure were investigated comparing patients who recovered the parathyroid function within 1 month with those needing more prolonged therapy. Permanent hypoparathyroidism was diagnosed in patients with subnormal iPTH serum levels (<15 pg/mL) and requiring medical treatment after at least 1-year follow-up. Complete recovery of the parathyroid function was diagnosed when patients could keep normal serum calcium levels without supplementation and iPTH concentration were normalized.
Patients’ management
Patients were discharged on the first or second postoperative day under replacement treatment with calcium carbonate (2–4 g/day) and calcitriol (0.5–1.5 mcg/day) according to the current protocols at the participating units. Serum calcium, vitamin D and iPTH concentrations were determined preoperatively, 24 h after TT, at 1 week and 1 month, and then every 3 months until recovery or up to 1 year. Once the pathology report was available, the number of PGRIS was calculated using the formula: 4 − (parathyroid glands autografted + parathyroid glands found in the specimen). Patients were then classified as PGRIS 1, 2, 3 or 4 (11).
Statistical analysis
An anonymized database was created and exported to the SPSS statistics software version 22.0 (IBM, Armonk, NY, USA). The main analysis was focused on the influence of serum calcium concentrations at 1 postoperative month on the proportion of patients with protracted hypoparathyroidism developing permanent parathyroid failure. Rates of recovery/permanent failure were compared at different serum calcium concentration ranges using the exact Fisher’s test. For quantitative variables, the unpaired Student’s t-test was employed. Binomial logistic regression analysis with predictors selected by a forward stepwise procedure was used to assess the risk factors for permanent hypoparathyroidism. Data are presented as mean (SD). Statistical significance was set at P<0.05.
Results
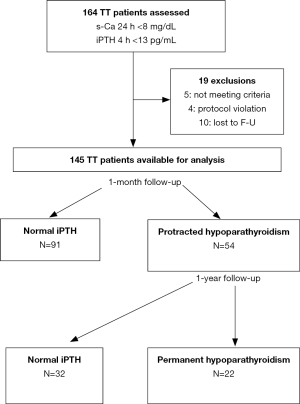
Some 164 patients were initially recruited but 19 were not included in the final analysis (Figure 1). Thus, 145 patients with postoperative hypocalcemia were investigated. Demographic and clinical characteristics of the cohort are shown in Table 1. Hypocalcemia recovered within 1 month in 91 patients (63%) and 54 patients (37%) developed protracted hypoparathyroidism of whom 32 recovered the parathyroid function and 22 were diagnosed of permanent hypoparathyroidism after at least 1-year follow-up. Factors associated with failure to recover the parathyroid function within 1 month are shown in Table 2, the most significant one being the number of PGRIS.
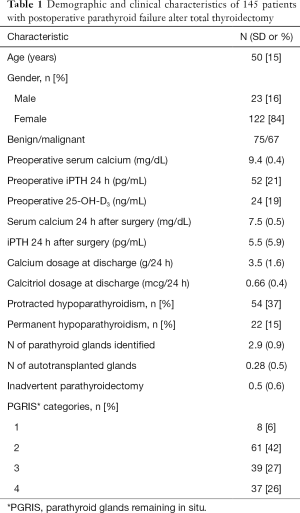
Full table
Full table
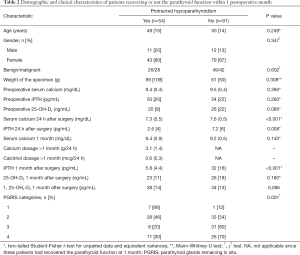
The clinical and metabolic profiles of patients with protracted hypoparathyroidism who did or did not recover the parathyroid function within 1 year are shown in Table 3. Those with a serum calcium concentration above 9 mg/dL at 1 postoperative month had a 77% iPTH recovery rate whereas lower concentrations were associated with only a 26% chance of parathyroid function restortation (P<0.001). No association was found between preoperative vitamin D levels and the development of protracted or permanent hypoparathyroidism.
Full table
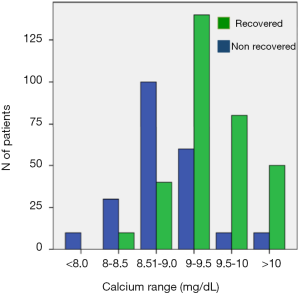
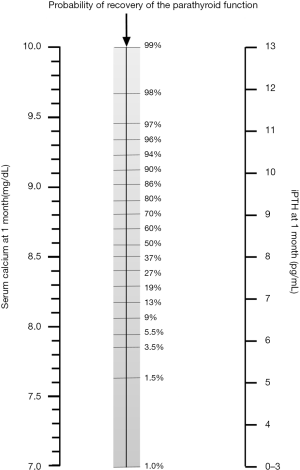
The number of patients with protracted hypoparathyroidism developing permanent hypoparathyroidism was calculated for each of five serum calcium concentration ranges 1 month after surgery (Figure 2). There was a parallel increase in the serum calcium concentration and the rate of parathyroid function recovery. No correlation was found between serum calcium and iPTH in patients with detectable PTH at 1 month (r=−0.119) suggesting that calcium concentrations, at this time point, play no role in determining iPTH concentrations. In addition, there were no differences in the serum calcium concentration in patients with detectable or undetectable iPTH at 1 month [9.2 (0.6) vs. 9.6 (1.1) mg/dL; P=0.105]. A multivariate analysis was carried out to predict the final parathyroid status in patients with protracted hypoparathyroidism and two independent variables were identified: the iPTH and serum calcium concentrations determined at 1 postoperative month; these classified correctly the final parathyroid function of 94% of the patients. An orientative nomogram to calculate the likelihood of recovery of the parathyroid function based on the multivariate analysis is shown in Figure 3 where the probability of permanent hypoparathyroidism is displayed as the intersection value when a straight line is drawn between calcium and iPTH levels.
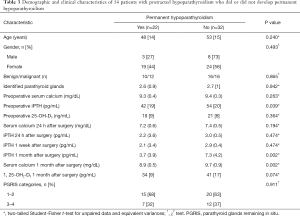
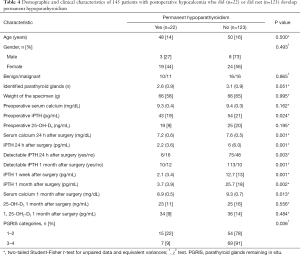
To clarify the risk factors for permanent parathyroid failure in the whole cohort, those patients developing permanent hypoparathyroidism were compared with those with a normal parathyroid function at the end of the study (Table 4). Lower values for postoperative biochemical parameters and fewer PGRIS in patients who ultimately developed permanent hypoparathyroidism, indicate a more severe intraoperative injury to the parathyroid glands in these cases. Pre and postoperative vitamin D serum levels were similar in both groups. Remarkably, iPTH concentrations at 1 month were below preoperative values {32 [16] vs. 53 [21] pg/mL; P<0.001} in patients recovering early from hypocalcemia, suggesting that despite the parathyroid function was restored they had lost a significant iPTH reserve.
Full table
Discussion
Parathyroid failure after TT is a dynamic syndrome expanding from transient postoperative hypocalcemia that recovers within a month to permanent hypoparathyroidism requiring life-long medical replacement therapy. The main causes leading to acute postoperative fall of iPTH have been progressively elucidated, the most relevant being PGRIS resulting from both inadvertent parathyroidectomy and parathyroid autotransplantation (9-12,14-16). Other, probably less important or common variables are weight of the specimen, reoperation for bleeding, thyroidectomy for Graves’s disease and female sex (5).
Two thirds of postoperative hypocalcemias resolve within the first weeks after thyroidectomy but the rest persist after 1 month and require prolonged medical treatment. At this moment in time, the baseline clinical variables lose significance and few PGRIS appears to be the main factor leading to protracted hypoparathyroidism (11,16). In other words, disease-related and pathology variables appear to lose relevance as parathyroid failure moves away from the immediate postoperative period.
Late (>1 month) recovery of the parathyroid function is a poorly understood phenomenon. Over 75–80% of patients with protracted hypoparathyroidism will eventually recover, but time to parathyroid function return is highly variable and controversial. The current clinical guidelines state that the diagnosis of permanent hypoparathyroidism should be made if replacement therapy is still needed 6 months (European) (17) or even 1 year (USA) (18) after surgery, and some anecdotal reports have been published of resolution of “permanent” hypoparathyroidism well beyond this time period (12). This implies that in some patients, viable parathyroid tissue remains in the neck for months but is not secreting PTH for unknown reasons.
In the absence of a better hypothesis, it seems logical to attribute this long-term inactivity of the parathyroid glands to an ischemic injury at the time of surgery, probably resulting in partial necrosis of the glandular parenchyma. In previous retrospective reports, we found that a normal-high serum calcium concentration was an independent good predictor of recovery of the parathyroid function (9,10). This has been confirmed in the present prospective study where patients with protracted hypoparathyroidism and a 1-month serum calcium concentration in the high-normal range showed a threefold recovery rate compared to those with levels below 9 mg/dL. Since all these patients were on replacement therapy it may be that this beneficial effect may result from the absence of metabolic stress on the failing parathyroid glands. To overcome the criticism that protracted hypoparathyroidism may be induced spuriously by iPTH-suppressive doses of calcium and calcitriol, we investigated a possible correlation between iPTH and serum calcium at 1 month and found none. This, however, cannot be completely ruled out for the occasional patient with serum calcium above 10 mg/dL.
We can only speculate on why serum calcium concentrations at 1 month are significantly different between patients with protracted hypoparathyroidism who do or do not recover the parathyroid function. The serum calcitriol serum concentrations were slightly higher in patients who recovered suggesting some parathyroid splinting function of the replacement therapy in this group. A main limitation of the present study, however, is that calcium and calcitriol dosages were left at the discretion of the participating units and that in some patients’ calcitriol was administered in a single daily dose while others received it twice daily. This limits the interpretation of the serum calcitriol concentrations. Nevertheless, this is the first study reporting calcitriol serum concentrations in the management of postoperative hypocalcemia and the data suggest that this drug is helpful in supporting the failing parathyroid and that it should be routinely added to calcium salts when treating acute postoperative parathyroid failure.
In patients recovering from hypocalcemia, the final iPTH concentrations were significantly lower than the preoperative baseline values. A significant drop from baseline iPTH concentrations was observed even in patients recovering from hypocalcemia within the first postoperative week. Thus, even though most patients recover from parathyroid failure after TT, the secretory capacity of the parathyroid glands is left impaired. This is in agreement with the seminal 1965 contribution of Wade et al. (19) and other recent observations (16,20). This relationship between intraoperative surgical injury of the parathyroid glands and reduced iPTH reserve has been further confirmed in the study of Lang et al. (21) who found that after TT for benign disease, PGRIS was the major determinant of protracted hypoparathyroidism and that aggressive parathyroid identification resulted in more patients developing postoperative hypocalcemia and protracted hypoparathyroidism. Put together, these studies suggest that, in the absence of renal impairment or medication interfering with the parathyroid function, iPTH serum concentrations long after TT correlate with the amount of functioning (surviving) parathyroid parenchyma.
In summary, the present prospective study emphasizes the relevance of serum calcium concentration 1 month after TT as prognostic factor for the recovery of the parathyroid function in patients with protracted hypoparathyroidism. A sensible interpretation of this finding is that, by providing a more aggressive medical replacement therapy (parathyroid splinting), the ischemic non-functional parathyroid glands have a better chance to regain their secretory capacity as they rest after surgery in an environment of normal-high serum calcium concentration.
Acknowledgements
We thank Marta Gimeno-López, our clinical research assistant, for her careful database and web application management.
Footnote
Conflicts of Interest: The authors have no conflicts of interest to declare.
Ethical Statement: The study protocol was approved by the Hospital del Mar Ethics Committee (Ref. 2011/4362/l) and was conducted in accordance with the Helsinki Declaration of 1964 (revised 2008).
References
- British Association of Endocrine and Thyroid Surgeons (BAETS) Audit 2012. Available online: www.baets.org.uk
- Bergenfelz A, Jansson S, Kristoffersson A, et al. Complications to thyroid surgery: results as reported in a database from a multicenter audit comprising 3,660 patients. Langenbecks Arch Surg 2008;393:667-73. [Crossref] [PubMed]
- Hundahl SA, Cady B, Cunningham MP, et al. Initial results from a prospective cohort study of 5583 cases of thyroid carcinoma treated in the United States during 1996. U.S. and German Thyroid Cancer Study Group. An American College of Surgeons Commission on Cancer Patient Care Evaluation study. Cancer 2000;89:202-17. [Crossref] [PubMed]
- De Pasquale L, Sartori PV, Vicentini L, et al. Necessity of therapy for post-thyroidectomy hypocalcemia: a multi-centre experience. Langenbecks Arch Surg 2015;400:319-24. [Crossref] [PubMed]
- Edafe O, Antakia R, Laskar N, et al. Systematic review and meta-analysis of predictors of post-thyroidectomy hypocalcemia. Br J Surg 2014;101:307-20. [Crossref] [PubMed]
- Lorente-Poch L, Sancho JJ, Muñoz-Nova JL, et al. Defining the syndromes of parathyroid failure after total thyroidectomy. Gland Surg 2015;4:82-90. [PubMed]
- Landry CS, Grubbs EG, Hernandez M, et al. Predictable criteria for selective, rather than routine, calcium supplementation following thyroidectomy. Arch Surg 2012;147:338-44. [Crossref] [PubMed]
- Walker Harris V, Jan De Beur S. Postoperative hypoparathyroidism: medical and surgical therapeutic options. Thyroid 2009;19:967-73. [Crossref] [PubMed]
- Pattou F, Combemale F, Fabre S, et al. Hypocalcemia following thyroid surgery: incidence and prediction of outcome. World J Surg 1998;22:718-24. [Crossref] [PubMed]
- Sitges-Serra A, Ruiz S, Girvent M, et al. Outcome of protracted hypoparathyroidism after total thyroidectomy. Br J Surg 2010;97:1687-95. [Crossref] [PubMed]
- Lorente-Poch L, Sancho JJ, Ruiz S, et al. Importance of in situ preservation of parathyroid glands during total thyroidectomy. Br J Surg 2015;102:359-67. [Crossref] [PubMed]
- Kim SM, Kim HK, Kim KJ, et al. Recovery from permanent hypoparathyroidism after total thyroidectomy. Thyroid 2015;25:830-3. [Crossref] [PubMed]
- Dreiseitl S, Harbauer A, Binder M, et al. Nomographic representation of logistic regression models: a case study using patient self-assessment data. J Biomed Inform 2005;38:389-94. [Crossref] [PubMed]
- Applewhite MK, White MG, Xiong M, et al. Incidence, risk factors, and clinical outcomes of incidental parathyroidectomy during thyroid surgery. Ann Surg Oncol 2016;23:4310-5. [Crossref] [PubMed]
- Sitges-Serra A, Gallego-Otaegui L, Suárez S, et al. Inadvertent parathyroidectomy during total thyroidectomy and central neck dissection for papillary thyroid carcinoma. Surgery 2017;161:712-9. [Crossref] [PubMed]
- Kihara M, Yokomise H, Miyauchi A, et al. Recovery of parathyroid function after total thyroidectomy. Surg Today 2000;30:333-8. [Crossref] [PubMed]
- Bollerslev J, Rejnmark L, Marcocci C, et al. European Society of Endocrinology Clinical Guideline: Treatment of chronic hypoparathyroidism in adults. Eur J Endocrinol 2015;173:G1-20. [Crossref] [PubMed]
- Stack BC Jr, Bimston DN, Bodenner DL, et al. American Association of Clinical Endocrinologists and American College of Endocrinology disease state clinical review: postoperative hypoparathyroidism - Definitions and management. Endocr Pract 2015;21:674-85. [Crossref] [PubMed]
- Wade JS, Fourman P, Deane L. Recovery of parathyroid function in patients with "transient" hypoparathyroidism after thyroidectomy. Br J Surg 1965;52:493-6. [Crossref] [PubMed]
- Anastasiou OE, Yavropoulou MP, Papavramidis TS, et al. Secretory capacity of the parathyroid glands after total thyroidectomy in normocalcemic subjects. J Clin Endocrinol Metab 2012;97:2341-6. [Crossref] [PubMed]
- Lang BH, Chan DT, Chow FC. Visualizing fewer parathyroid glands may be associated with lower hypoparathyroidism following total thyroidectomy. Langenbecks Arch Surg 2016;401:231-8. [Crossref] [PubMed]


