
To each his own: a personalized vaccine for metastatic melanoma
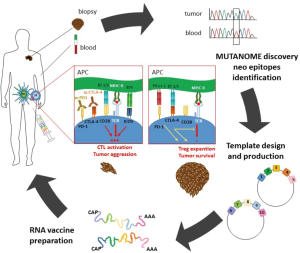
By combining bioinformatics and sequencing analysis, it is now possible to identify the private tumor neoantigens derived from an individual patient’s tumor, and use these antigens to manufacture personalized vaccines. A sophisticated example of this novel immunotherapeutic approach has been described by Sahin et al. (1), who reported the first in-human clinical trial using personalized RNA-based vaccines generated on the basis of the “mutanome” (i.e., the whole pattern of mutations inside the tumor mass) displayed by each individual patient with malignant melanoma. Results showed that these vaccines can induce a wide anti-tumor antigen-specific T cell response and positive clinical outcomes in terms of cumulative rate of metastatic events and progression-free survival (Figure 1).
The main goal of personalized oncology, also known as precision cancer medicine, is to tailor the most effective treatment to each individual patient based on both its genetic profile and that of its cancer cells. Tumors, even those belonging to the same type, have long been known to exhibit distinct and highly variable genetic profiles, which inevitably vary during cancer progression. This, in turn, leads to reduced anti-tumor therapeutic efficacy and increased risk of toxicity due to dose adjustment. Thus, in this context, genome-guided therapy has not only increased treatment success rate but has also contributed significantly towards reducing potential adverse drug effects. Moreover, this approach has become increasingly relevant for cancer immunotherapy due to the high variability in antigenicity and immunogenicity of tumor cells as well as the heterogeneous interindividual immune response. First and foremost, precision cancer medicine holds promise for highly mutated and immunogenic cancers such as melanomas, which, for the most part, are refractory to treatment.
According to the American Cancer Society (ACS), 87,000 new melanoma cases will be diagnosed in 2017 (2), with a similar trend expected in Europe (3), indicating that the incidence of this cancer is rapidly increasing. Standard treatment options for melanoma include surgery, radiotherapy, and chemotherapy, albeit their efficacy is quite poor and rarely associated with a complete and long-lasting response. Recently, promising results have been obtained with novel immunotherapy approaches based on the stimulation of the endogenous antitumor immune response of the host, which then leads to tumor reduction and improved clinical outcome (4).
The immunosurveillance theory formulated in the late 1950s holds that the immune response routinely keeps in check tumor development. Despite a large body of in vivo evidence showing that the immune system can be shaped to generate a response against tumors, there has been an ongoing debate as to what the true role of immunosurveillance might be. In this regard, the observation that tumor cells can activate multiple mechanisms of immune escape through a process known as immunoediting strongly favors a model whereby the immune system plays a central role in the anti-tumoral response (5). Furthermore, emerging evidence strongly indicates that tumors can activate key immune checkpoints, which prevent autoimmunity and regulate immune homeostasis, in order to evade immunosurveillance and progress unchecked. Thus, blockade of immune checkpoints represents one of the major goals of anti-tumor therapy.
The various immunotherapeutic approaches utilized to elicit an effective anti-tumor immune response can be broadly categorized into two main groups. The first group comprises non antigen-specific immunostimulators such as cytokines, ligands of costimulatory molecules, and checkpoint inhibitors. The second group comprises anti-tumor vaccines, which stimulate the immune response against selected tumor antigens.
Among the drugs belonging to the first group, besides a few cytokines used successfully to treat specific tumors (e.g., IL-2 for metastatic melanoma and renal cell carcinoma, and type-I IFNs for some hematological malignancies and recurrent melanoma), checkpoint inhibitors represent the most promising class of therapeutics, initially developed as anti-CTLA-4 agents, and then as anti-PD-1 agents (6), along with several others currently on clinical trial. CTLA-4 is a T cells surface receptor expressed by activated T cells and regulatory T (Treg) cells involved in shutting down activated effector T cells and activating Treg cells (7). Likewise, PD-1 is expressed on activated T cells and delivers a co-inhibitory signal that limits the T cell response (8). Therefore, it is not surprising that monoclonal antibodies (mAb) blocking either CTLA-4 or PD-1 potentiate the immune responses by inhibiting these suppressor mechanisms (Figure 1). CTLA-4 and PD-1 belong to the CD28 family of immune receptors, which also includes key positive costimulatory molecules such as CD28 and ICOS, which may also be targeted in several types of immune diseases including tumors (9-11). However, a major limitation for the use of checkpoint inhibitors is that they act unspecifically and may affect the immune response of the patient against self antigens, thereby causing autoimmune diseases.
Anti-tumor vaccines instead target a plethora of tumor-specific antigens which can arise from oncoviruses, hyperexpression of normal antigens, re-expression of embryonic or fetal molecules (i.e., oncofetal antigens), or mutated proteins involved in neoplastic transformation. Through tumor-specific antigen recognition, these vaccines boost the patient’s immune system to induce a pro-inflammatory adaptive immune response targeting selected cancer cells expressing such antigens. One of the major advantages of anti-tumor vaccines is that they potentially represent specific, safe, and well-tolerated therapeutic options capable of circumventing drug resistance while obtaining a durable response due to immunological memory.
Anti-tumor vaccines can be grouped in four main categories: (I) peptide vaccines; (II) cellular vaccines, including tumor cell and immune cell vaccines; (III) viral vector vaccines; and (IV) nucleic acid vaccines, including DNA and RNA vaccines. The tumor antigens targeted by these vaccines are generally represented by common tumor antigens frequently expressed by a given type of tumor, or by neoantigens, which are “private” antigens arising from rare mutations characterizing each individual tumor. The latter are often highly immunogenic (12) since they have not been browsed by central tolerance.
The general trend is to use these vaccines as an adjuvant therapy after surgery, radiotherapy and chemotherapy since their efficacy is expected to be maximal against minimal residual disease. Theoretically, vaccines targeting common tumor antigens would be preferable since they could be used to immunize the general patient population. These vaccines may also include a mixture of common antigens to increase the vaccine efficacy in both the individual patient and the general patient population. They may be traditional protein/peptide vaccines or innovative vaccines such as those obtained using recombinant infectious vectors or naked DNA/RNA. However, an unresolved issue about these vaccines is their apparent inability to overcome the antigenic heterogeneity of tumor cells not only among different individuals but also within a given tumor mass due to immunoediting (5). This intra and inter-tumor heterogeneity accounts for the limited efficacy of these vaccines in the general patient population.
An alternative approach is represented by the preparation of personalized vaccines based on the antigen profile of the tumor cells of each individual patient, a strategy that adheres to the principles of precision medicine. For instance, positive results have been obtained by cloning the specific Ig gene expressed by a B cell neoplastic cell clone and using it as a “private” tumor antigen for either protein or DNA vaccine preparation (13). Another useful strategy is to use irradiated tumor cells (14), total tumor lysates plus adjuvants, or autologous dendritic cells, which may be modified in several ways to increase their immunogenicity (15). Nonetheless, a major limitation for the use of these vaccines is represented by their relatively weak immunogenicity and, thus, low efficacy. Moreover, two key aspects of personalized vaccines should also be taken into account: time and cost-effectiveness. While the time necessary to produce an anti-tumor vaccine must be compatible with disease evolution, the overall vaccine cost-effectiveness must be consistent with resource allocation policies for the general patient population.
RNA vaccines seem to be ideal to face these problems since they would be safe and immunologically effective, with a convenient time and cost-effectiveness. RNA vaccines are second generation vaccines consisting of protein-encoding nucleic acid sequences (16). RNA transcripts, once internalized by the host cell, are translated into antigens which then elicit the host immune response. These vaccines were developed shortly after the introduction of first generation DNA vaccines, which proved to be effective in animal models. However, the validity of DNA vaccination in humans still remains highly debated due to practical difficulties in controlling the expression levels and duration of the encoded antigens, and the possibility that random integration of the vaccine DNA into the host genome may dysregulate the expression of endogenous genes and cause diseases (17).
In this scenario, malignant melanoma constitutes the ideal model to test novel immunotherapeutic approaches due to its high immunogenicity, metastatic potential and poor prognosis. However, cancer vaccine immunotherapy for melanoma still faces a number of challenges due to the heterogeneity and high mutation rate of this tumor (18). In this regard, checkpoint antagonists were initially developed and validated in melanoma, and later on then their use was extended to other tumors refractory to conventional therapies.
In a paper featured in the July 13th issue of Nature, Sahin and co-workers have successfully generated personalized RNA vaccines against melanoma (1). Thanks to a multi-omic approach combining exome, RNA sequencing, and algorithm analyses, the authors first identified the pattern of non-synonymous mutations of the tumors from 13 patients with advanced malignant melanoma. Next, they selected about 10 neoantigens for each patient based on a predicted high-affinity binding to the endogenous MHC class II and class-I molecules. Thanks to this approach, they were able to obtain a mix of antigens that could activate both T helper and cytotoxic T cells, which are restricted to MHC class-II and class-I, respectively. The nucleic acid sequences were then engineered to produce RNAs encoding peptides corresponding to the selected neoantigens. The whole vaccine development process lasted anywhere from 89 to 160 days. The personalized RNA vaccines were then injected percutaneously into the inguinal lymph nodes of each patient who received 8–20 doses. Remarkably, each vaccine elicited a sustained response of both CD4+ and CD8+ T cells, displaying effective anti-tumor activities. The authors recorded immunological responses against 60% of the predicted neo-epitopes, and each individual responded to at least three mutations. Clinically, the vaccines were safe and without side effects during the follow-up (>23 months). Moreover, 8 out of 13 patients who were tumor-free at the time of vaccination remained tumor-free for the following 23 months. Given that more than 50% of melanoma patients are expected to experience relapse (19), the results reported by Sahin and co-workers appear to be very encouraging. Only 5 out of the 13 patients experienced melanoma relapses, with one of them displaying a complete response to a second line anti PD-1 therapy and showing persistence of vaccine-induced T cells for up to 9 months after the end of vaccination (1).
The findings by Sahin are in good agreement with a study published in the same issue of Nature by Ott et al., where the authors succeeded in selecting as many as 20 neoantigens for each patient using a similar multi-omic approach (20). The clinical-grade long peptides corresponding to the neoantigens predicted to bind the patient’s MHC were then synthesized and used to immunize 6 melanoma patients (20). In good agreement with Sahin et al. (1), patient vaccination induced a polyfunctional and durable CD4+ and CD8+ T cell response against the neoantigens. Moreover, 4 patients displayed no recurrence at 25 months after recurrence, and the two patients who relapsed displayed a full response to subsequent anti-PD-1 treatment with expansion of neoantigen-specific T cells (20).
In conclusion, both studies show the safety and feasibility of personalized vaccination in human melanoma and pave the way for the use of this approach for the treatment of other tumors displaying high rates of mutations and high metastatic potential. Intriguingly, this vaccination approach may work in synergy with checkpoint inhibitor-based immunotherapies, which may cooperate in unbreaking the response to vaccine neoantigens.
Acknowledgments
None.
Footnote
Conflicts of Interest: The authors have no conflicts of interest to declare.
References
- Sahin U, Derhovanessian E, Miller M, et al. Personalized RNA mutanome vaccines mobilize poly-specific therapeutic immunity against cancer. Nature 2017;547:222-6. [Crossref] [PubMed]
- Siegel RL, Miller KD, Jemal A. Cancer Statistics, 2017. Available online: http://onlinelibrary.wiley.com/doi/10.3322/caac.21387/pdf
- Ferlay J, Soerjomataram I, Ervik M, et al. GLOBOCAN 2012 v1.0, Cancer Incidence and Mortality Worldwide: IARC CancerBase No. 11. Lyon, France: International Agency for Research on Cancer, 2013. Available online: http://globocan.iarc.fr
- Achkar T, Tarhini AA. The use of immunotherapy in the treatment of melanoma. J Hematol Oncol 2017;10:88. [Crossref] [PubMed]
- Dunn GP, Bruce AT, Ikeda H, et al. Cancer immunoediting: from immunosurveillance to tumor escape. Nat Immunol 2002;3:991-8. [Crossref] [PubMed]
- Callahan MK, Postow MA, Wolchok JD. CTLA-4 and PD-1 Pathway Blockade: Combinations in the Clinic. Front Oncol 2015;4:385. [Crossref] [PubMed]
- Krummel MF, Allison JP. CD28 and CTLA-4 have opposing effects on the response of T cells to stimulation. J Exp Med 1995;182:459-65. [Crossref] [PubMed]
- Keir ME, Butte MJ, Freeman GJ, et al. PD-1 and its ligands in tolerance and immunity. Annu Rev Immunol 2008;26:677-704. [Crossref] [PubMed]
- Redoglia V, Dianzani U, Rojo JM, et al. Characterization of H4: a mouse T lymphocyte activation molecule functionally associated with the CD3/T cell receptor. Eur J Immunol 1996;26:2781-9. [Crossref] [PubMed]
- Dianzani C, Minelli R, Gigliotti CL, et al. B7h triggering inhibits the migration of tumor cell lines. J Immunol 2014;192:4921-31. [Crossref] [PubMed]
- Dianzani C, Minelli R, Mesturini R, et al. B7h triggering inhibits umbilical vascular endothelial cell adhesiveness to tumor cell lines and polymorphonuclear cells. J Immunol 2010;185:3970-9. [Crossref] [PubMed]
- Chen DS, Mellman I. Elements of cancer immunity and the cancer-immune set point. Nature 2017;541:321-30. [Crossref] [PubMed]
- Hawkins RE, Zhu D, Ovecka M, et al. Idiotypic vaccination against human B-cell lymphoma. Rescue of variable region gene sequences from biopsy material for assembly as single-chain Fv personal vaccines. Blood 1994;83:3279-88. [PubMed]
- Lipson EJ, Sharfman WH, Chen S, et al. Safety and immunologic correlates of Melanoma GVAX, a GM-CSF secreting allogeneic melanoma cell vaccine administered in the adjuvant setting. J Transl Med 2015;13:214. [Crossref] [PubMed]
- González FE, Gleisner A, Falcón-Beas F, et al. Tumor cell lysates as immunogenic sources for cancer vaccine design. Hum Vaccin Immunother 2014;10:3261-9. [Crossref] [PubMed]
- Schlake T, Thess A, Fotin-Mleczek M, et al. Developing mRNA-vaccine technologies. RNA Biol 2012;9:1319-30. [Crossref] [PubMed]
- Nichols WW, Ledwith BJ, Manam SV, et al. Potential DNA vaccine integration into host cell genome. Ann N Y Acad Sci 1995;772:30-9. [Crossref] [PubMed]
- Hodis E, Watson IR, Kryukov GV, et al. A landscape of driver mutations in melanoma. Cell 2012;150:251-63. [Crossref] [PubMed]
- Leiter U, Meier F, Schittek B, et al. The natural course of cutaneous melanoma. J Surg Oncol 2004;86:172-8. [Crossref] [PubMed]
- Ott PA, Hu Z, Keskin DB, et al. An immunogenic personal neoantigen vaccine for patients with melanoma. Nature 2017;547:217-21. [Crossref] [PubMed]


