The role of interventional radiology in the treatment of epiphora
Introduction
The lacrimal drainage system (LDS) serves as a conduit for tear flow from the external eye to the nasal cavity. It consists of superior and inferior punctum and canaliculi, common canaliculus, lacrimal sac and nasolacrimal duct (1). The nasolacrimal duct extends from the inferior portion of the lacrimal sac through the bony lacrimal canal and opens into the inferior meatus of the nasal cavity (1,2).
Nasolacrimal duct obstruction (NLDO) is the complete or partial obstruction of nasolacrimal duct that leads to insufficient drainage of tears, called epiphora (3,4). Excessive tearing is the most common symptom of patients with NLDO, followed by acute or chronic infection (3-5).
NLDO could be either congenital or acquired (related to trauma, inflammatory disease, dacryoliths, neoplasm) (3,4,6). However, the most common cause of NLDO in adults is idiopathic inflammatory obstruction of the nasolacrimal duct, that according to clinic-pathologic studies maybe induced by the compression of the lumen by inflammatory infiltrates and edema, as the possible result of an autoimmune disease or an unidentified infection (7).
This condition affects women twice as frequently as men and peaks in the elderly age (3,4,6). It is a relatively common ophthalmologic affection representing less than 5% of clinical consultations in ophthalmology (3,6).
Surgery constitutes the usual treatment of LDS obstructions below the common canaliculus, namely, external dacryocystorhinostomy (DCR) (6). This is an invasive surgical procedure requiring skin incision and osteotomy to create an anastomosis between the sac and the nasal mucosa to bypass the obstruction. Despite the high success rate (79–99%), DCR has many disadvantages and limitations (8,9). It requires general anesthesia and it may arise in a permanent facial scar. In addition, the reobstruction of the anastomotic tract by fibrotic scars and osteogenic activity, is the major long-term drawback (6,9).
Described for the first time in 1978 (10), fluoroscopically guided interventional procedures are a therapeutic alternative to surgery for LDS obstructions. They are generally performed by the interventional radiologist and include two different techniques: the balloon dacryocystoplasty or the nasolacrimal stent placement (6).
In both cases, a pre-operative characterization of the occlusion is needed for a correct treatment planning (11). Widely employed in clinical practice, computed tomography dacryocystography (CTD) represents one the most useful radiological option for the depiction of NLDO, enabling the assessment of the site and the severity of the stenosis (11).
In this paper, we aim to report the contribution either of diagnostic and interventional radiological approaches in non-surgical therapy of epiphora.
Methods
Using the terms “epiphora” or “nasolacrimal duct obstructions” and “interventional radiology” or “fluoroscopy” and “dacryocystoplasty” and “nasolacrimal stent placement” and “computed tomography dacryocystography” contained in title and/or abstract and/or keywords, a comprehensive literature review was conducted through Medline, Scopus and Google Scholar (01/01/1990–01/07/2017) databases. Additional from authors’ bibliographies were hand searched.
CTD
Imaging of the LSD is required for the preoperative assessment of epiphora.
Various imaging modalities, including conventional fluoroscopic dacryocystography, nuclear scintigraphy, computed tomography and magnetic resonance imaging (MRI) are available (12), but CTD is certainly one of the most utilized (11,13-16).
Combining CT with dacryocystography, CTD is able to enhance the relationship between the nasolacrimal drainage system and the surrounding soft tissue and bony structures, defining the lacrimal system anatomy, assessing the level and the severity of the stenosis and facilitating preoperative planning (11,15,17).
CTD technique consists of LDS opacification by administration of an iodinate contrast medium by cannulation or direct instillation in the conjunctival cul de sac (18). Cannulation of the lacrimal canaliculi (typically the inferior) should be preceded by irrigation and compression of the lacrimal sac to flush out retained secretions within the LDS, which may lead to interpretive difficulties and misdiagnosis (16,18); then the injection of 0.5–1 mL of contrast medium can be performed through a lacrimal cannula, instilling one or two drops per minute, per eye, for 5 minutes (18).
Images are acquired immediately after contrast administration; patient is scanned in a supine position for axial imaging and then the acquired data are reformatted into 3D and 2D coronal and parasagittal planes along the major axis of the lacrimal drainage apparatus (18).
In a patent system, the contrast medium is supposed to immediately drain from the lacrimal sac into the nose. Thus, a delayed appearance of contrast medium in the nasal cavities can be interpreted as a partial obstruction; while its complete absence associated to its retention in the lacrimal sac should be referred to a complete occlusion of the nasolacrimal duct (11-16,18) (Figures 1,2).
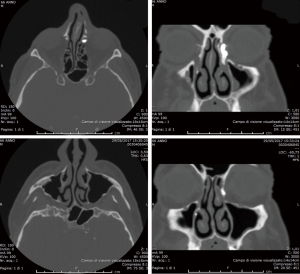
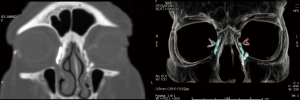
Interestingly, it should be noticed that there is not a strict correlation between the radiological and the clinical findings. Indeed, the lacrimal production decreases with age, so that an occlusion of the LDS can be even depicted in completely asymptomatic patients (19).
The main advantages of CTD over the other modalities, especially over MRI dacryocystography, are wide availability, lower costs, short scanning times, high reproducibility and most of all its ability to provide a precise definition of bone morphology, even showing smaller drainage structures (12). However, since any radiation dose cannot be considered completely safe, other techniques that do not involve administration of ionizing radiation (i.e., MRI dacryocystography) could be advisable in younger patients (12).
Furthermore, CTD should be avoided in patients with allergic diathesis to iodinate contrast media (12,16).
Techniques
Balloon dacryocystoplasty
Introduced by Becker and Berry in 1989 (20), the balloon dacryocystoplasty is an interventional radiological procedure consisting in the dilatation of a NLDO with the employ of a dedicated balloon catheter, able to apply a radial force on the thickened duct wall (21-23).
After a preliminary digital subtraction dacryocystography (DSD) to confirm the CTD reports, a 20 gauge soft plastic arterial sheath is introduced over a flexible tip metallic guidewire through the superior canaliculus into the LDS and then advanced to the level of the stenosis, where the probe is gently manipulated to cross the obstruction (22). Some authors suggest the use of the plastic sheath for the negotiation of the stenosis in order to avoid potential damages of the lacrimal duct walls, induced by the relatively stiff part of the guidewire (24). Once the obstruction is crossed, a 0.016 in steerable guidewire is advanced through the sheath into the inferior nasal meatus till the nare (22,24). This part of the intervention can be challenging and may require the employ of specially designed hooks or hemostat tweezers to catch the guidewire. However, the use of these instruments is controversial, since it increases the risk of parietal damages (6).
Thus, under fluoroscopic monitoring in lateral projection, an angioplasty balloon catheter with a 3-French shaft, 2–4 mm in diameter and 2 cm in length, is passed over the guidewire to the site of the stenosis (6,24). The introduction of the balloon can be performed either through an antegrade (from the canaliculus) or a retrograde (from the nasal cavities) approach (25,26).
When the balloon is right positioned, it can be inflated at 5 atm pressure with a water-iodinate contrast medium solution. Effective dilatations are supposed to last for 30 s–5 min, according to the severity of the obstruction (6). The results of the procedure must be checked with a final DSD to verify the patency of the treated duct lumen (22).
The choice of balloon dacryocystoplasty for treatment of epiphora has some major advantages over surgery. Indeed, the procedure does not require hospitalization as it can be performed under topical anesthesia and most of the side effects can be prevented by the prophylactic administration of oral or topical antibiotics and steroid eye drops for 1 week (6). Complications are generally negligible such as temporary mild pain during the intervention or circumscribed bleeding from the nasal cavity or the lacrimal punctum (6).
Another interesting aspect of balloon dacryocystoplasty is that it does not preclude a further surgical intervention (6).
However, the effectiveness of balloon dacryocystoplasty seems to be strictly related to patient selection. Absolute contraindications include LDS neoplasias, acute infective dacryocystitis risking to involve the surrounding tissues and structural deformations of the bony lacrimal canal (20,21,27). Furthermore, balloon dacryocystoplasty success rate seems to be influenced by the extension and the severity of the NLDO (6).
Best outcomes are reported for some favorable conditions such as acquired focal, incomplete obstructions and short distal nasolacrimal duct occlusions (5,19,24,28,29-37) (Table 1).
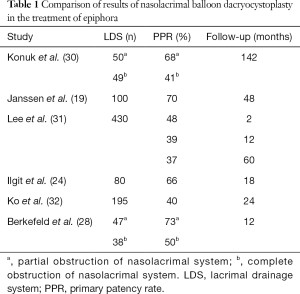
Full table
Indeed, in one on the largest series (31), 350 patients with idiopathic acquired epiphora were enrolled to evaluate the initial and long-term results of balloon dacryocystoplasty. The lesions were classified according to cause, severity, site of the obstruction, the diameter and inflation time of the balloon. The investigators achieved an overall technical success rate of 95.3% and an overall initial success rate was 57.4%. They also stated that the initial success depended by the severity, the site of the stenosis and the diameter of the balloon, while the long-term patency was influenced by the site of the obstruction and the balloon dilatation time.
Similarly, Konuk et al. (30) employed for 99 cases of idiopathic-acquired LDS obstruction. The mean follow-up period ranged 36–142 months. The investigators experienced a higher long-term overall success rate for partial and distal obstruction (73.3%). They concluded that the long-term success rate of balloon dacryocystoplasty for the treatment of epiphora is disappointing compared to surgery, but positive outcomes can be gained in carefully selected patients with partial obstruction of the distal nasolacrimal duct.
Altogether, these results suggest that balloon dacryocystoplasty cannot ensure the same effectiveness of traditional surgical treatment, but can still be helpful as first line therapy in some selected cases.
Nasolacrimal stent placement
Proposed for the first time by Song et al. in 1994 (38), the positioning of nasolacrimal plastic stent is a minimally invasive radiological procedure, developed with the aim of overcoming the failures of dacryocystoplasty in treatment of epiphora.
The device introduced by Song is a 6-French polyurethane nasolacrimal stent, equipped with a mushroom proximal tip (5 mm in diameter and length), which can be compressed during the delivery phase and expanded during the deployment. The stent is also 35 mm long, with an outer diameter of 2 mm and luminal diameter of 1.5 mm.
Compared with the expandable metallic stents previously tested by the same Song with deceiving results (39), the polyurethane device revealed higher elasticity and less foreign body inflammatory reaction (38,40).
The stent placement is performed under fluoroscopic guidance in lateral projection and can be preceded by a former dacryocystoplasty (6).
As well as in balloon dacryocystoplasty, an initial DSD is needed to plan the intervention. Then, after crossing the stenosis with a metallic probe, a 6-French sheath with a dilator is advanced retrograde over the guidewire until its tip achieves the lacrimal sac (6,41). This is a crucial moment and the position of the tip must be controlled with injection of contrast medium through the sheath, removing the dilator (41). After this check, the stent can be threaded in the sheath and when its proximal tip gains the lacrimal sac, the sheath can be withdrawn, while the stent pusher is held. The result is the stent deployment and the expansion of its mushroom tip in the lacrimal sac (6,41). Ciampi et al. in 2011 proposed a little variant of this technique, suggesting the delivery of the stent advancing the device over the wire inside a specially designed tapered dacryocystography catheter (42). However, in all cases the procedure must be concluded with a final DSD to verify the position and the patency of the stent (6,41). Furthermore, irrigation of the LSD is required the day after the intervention in order to wash the stent and assure its patency (6).
Like dacryocystoplasty, stent placement is a procedure that can be performed simply under local anesthesia during the day hospital recovery, involving a prophylactic one week therapy with topical corticosteroids and antibiotics (43). However, in this case the risk of complications is mildly increased compared to balloon dilatation, as possible side effects include also foreign body sensation, headache, stent mal-positioning or migration and acute dacryocystitis (40,43).
Interestingly, the only contraindication for nasolacrimal stent placement is acute dacryocystitis, while canalicular obstruction, LDS structural malformations, dacryolithiasis, sac neoplasm or previous sinus surgery, which generally preclude balloon dacryocystoplasty, can be considered as relative exclusion criteria (40).
Table 2 reports several experiences of nasolacrimal stenting (17,40-42,44-49).
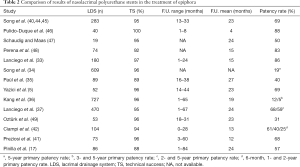
Full table
The most important limit of this procedure is the occlusion of the stent within one year from the intervention, induced by a chronic low-grade inflammation leading to the growth of granulation tissue inside the mesh of the stent (49). In 2003, 10 years after the invention of nasolacrimal stenting technique, Song published a study (50) aimed to compare the clinical effectiveness of a covered nitinol stent with that of a polyurethane stent for treatment of epiphora in 68 patients. The results were deceiving: even if technical success was achieved in more than 94% of patients, with the immediate resolution of epiphora in all cases, during the mean follow-up period of 40 months, the recurrence of the NLDO was assessed in 30 of 31 patients treated with nitinol stent and 26 of 35 patients who received the plastic one. The authors concluded that despite the polyurethane stent used for treatment seemed to be more effective than the nitinol stent, in both cases the long-term patency rates were not encouraging (50).
Different strategies were suggested in order to overcome this limit.
Lee et al. (51) achieved the highest long-term patency rate in the literature (93.2%) after a mean follow-up of 22 months at 1, 3, and 6 months, performing a periodical irrigation of the LDS through the canaliculi after stent placement with a saline solution containing antibiotics and mucolytics to avoid the inflammatory occlusion of the stent (51).
Moreover, Ciampi et al. in 2011 (42) described an experience, employing in 115 cases of epiphora a new polyurethane stent featured by a special S-shaped configuration to assist placement. The median duration of primary patency was 11 months, while the percentage of patency at 6 months was 60.8%, at 1 year was 39.6%, and at 2 years was 25%. The authors considered these results as a demonstration of the benefit of the stent positioning for NLDO (42).
Eventually in 2017 some investigators (52), proposed in a preclinical study on rabbits the utilization of biodegradable stents containing poly-L-lactic acid-polycaprolactone-polyethylene glycol (PLLA-PCL-PEG) complexes for therapeutic applications in epiphora. The histopathological testing indicated that the selected stent was biodegradable and caused minimal stimulation and earlier tissue restoration in the lacrimal epithelium, so that the authors concluded that it could represent an interesting alternative choice in treatment of NLDO (52).
However, it should be considered that even when the stent occlusion occurs, this affects, but does not preclude a further surgical intervention (6). Furthermore, stent removal is a well-tolerable procedure, that can be provided either with fluoroscopic, endoscopic or surgical approach (43,53).
Conclusions
Interventional radiological procedures represent a minimal invasive option for treatment of epiphora. Despite the long-term success rate is certainly lower than the surgical therapy, these procedures can still constitute a useful treatment option in NLDO, considering their low morbidity rate, their repeatability and the possibility of a further surgical approach in case of failure.
Acknowledgements
None.
Footnote
Conflicts of Interest: The authors have no conflicts of interest to declare.
References
- Kominami R, Yasutaka S, Taniguchi Y, et al. Anatomy and histology of the lacrimal fluid drainage system. Okajimas Folia Anat Jpn 2000;77:155-60. [Crossref] [PubMed]
- Paulsen F, Thale A, Kohla G, et al. Functional anatomy of human lacrimal duct epithelium. Anat Embryol (Berl) 1998;198:1-12. [Crossref] [PubMed]
- Mandeville JT, Woog JJ. Obstruction of the lacrimal drainage system. Curr Opin Ophthalmol 2002;13:303-9. [Crossref] [PubMed]
- Dantas RR. Lacrimal drainage system obstruction. Semin Ophthalmol 2010;25:98-103. [Crossref] [PubMed]
- Yazici Z, Yazici B, Parlak M, et al. Treatment of nasolacrimal duct obstruction with polyurethane stent placement: long-term results. AJR Am J Roentgenol 2002;179:491-4. [Crossref] [PubMed]
- Ilgit ET, Onal B, Coskun B. Interventional radiology in the lacrimal drainage system. Eur J Radiol 2005;55:331-9. [Crossref] [PubMed]
- Linberg JV, McCormick SA. Primary acquired nasolacrimal duct obstruction. A clinicopathologic report and biopsy technique. Ophthalmology 1986;93:1055-63. [Crossref] [PubMed]
- Allen K, Berlin AJ. Dacryocystorhinostomy failure: association with nasolacrimal silicone intubation. Ophthalmic Surg 1989;20:486-9. [PubMed]
- McMurray CJ, McNab AA, Selva D. Late failure of dacryocystorhinostomy. Ophthal Plast Reconstr Surg 2011;27:99-101. [Crossref] [PubMed]
- Hanafee WN, Dayton GO Jr. Dilatation of the nasolacrimal duct under radiographic control. Radiology 1978;127:813-5. [Crossref] [PubMed]
- Nykamp SG, Scrivani PV, Pease AP. Computed tomography dacryocystography evaluation of the nasolacrimal apparatus. Vet Radiol Ultrasound 2004;45:23-8. [Crossref] [PubMed]
- Caldemeyer KS, Stockberger SM Jr, Broderick LS. Topical contrast-enhanced CT and MR dacryocystography: imaging the lacrimal drainage apparatus of healthy volunteers. AJR Am J Roentgenol 1998;171:1501-4. [Crossref] [PubMed]
- Weber AL, Rodriguez-DeVelasquez A, Lucarelli MJ, et al. Normal anatomy and lesions of the lacrimal sac and duct: evaluated by dacryocystography, computed tomography, and MR imaging. Neuroimaging Clin N Am 1996;6:199-217. [PubMed]
- Saraç K, Hepsen IF, Bayramlar H, et al. Computed tomography dacryocystography. Eur J Radiol 1995;19:128-31. [Crossref] [PubMed]
- Freitag SK, Woog JJ, Kousoubris PD, et al. Helical computed tomographic dacryocystography with three-dimensional reconstruction: a new view of the lacrimal drainage system. Ophthal Plast Reconstr Surg 2002;18:121-32. [Crossref] [PubMed]
- Shweel M, Elshafei A, AbdelRahman RM, et al. Evaluation of lacrimal drainage system obstruction using combined multidetector CT and instillation dacryocystography. Egypt J Radiol Nucl Med 2012;43:413-20. [Crossref]
- Pinilla I, Fernández-Prieto AF, Asencio M, et al. Nasolacrimal stents for the treatment of epiphora: Technical problems and long-term results. Orbit 2006;25:75-81. [Crossref] [PubMed]
- Udhay P, Noronha OV, Mohan RE. Helical computed tomographic dacryocystography and its role in the diagnosis and management of lacrimal drainage system blocks and medial canthal masses. Indian J Ophthalmol 2008;56:31-7. [Crossref] [PubMed]
- Janssen AG, Mansour K, Bos JJ. Obstructed nasolacrimal duct system in epiphora: long-term results of dacryocystoplasty by means of balloon dilation. Radiology 1997;205:791-6. [Crossref] [PubMed]
- Becker BB, Berry FD. Balloon catheter dilatation in lacrimal surgery. Ophthalmic Surg 1989;20:193-8. [PubMed]
- Song HY, Ahn HS, Park CK, et al. Complete obstruction of the nasolacrimal system. Part I. Treatment with balloon dilation. Radiology 1993;186:367-71. [Crossref] [PubMed]
- Tien DR, Young D. Balloon dilation of the nasolacrimal duct. J AAPOS 2005;9:465-7. [Crossref] [PubMed]
- Perry JD, Maus M, Nowinski TS, et al. Balloon catheter dilation for treatment of adults with partial nasolacrimal duct obstruction: A preliminary report. Am J Ophthalmol 1998;126:811-6. [Crossref] [PubMed]
- Ilgit ET, Yüksel D, Unal M, et al. Transluminal balloon dilatation of the lacrimal drainage system for the treatment of epiphora. AJR Am J Roentgenol 1995;165:1517-24. [Crossref] [PubMed]
- Kuchar A, Steinkogler FJ. Antegrade balloon dilatation of nasolacrimal duct obstruction in adults. Br J Ophthalmol 2001;85:200-4. [Crossref] [PubMed]
- Steinkogler FJ, Huber E, Kuchar A, et al. Retrograde dilation of postsaccal lacrimal stenosis. Ann Otol Rhinol Laryngol 1994;103:110-4. [Crossref] [PubMed]
- Janssen AG, Mansour K, Krabbe GJ, et al. Dacryocystoplasty: treatment of epiphora by means of balloon dilation of the obstructed nasolacrimal duct system. Radiology 1994;193:453-6. [Crossref] [PubMed]
- Berkefeld J, Kirchner J, Müller HM, et al. Balloon dacryocystoplasty: indications and contraindications. Radiology 1997;205:785-90. [Crossref] [PubMed]
- Müller HM, Fries U, Berkefeld J, et al. Indications and contraindications of lacrimal duct balloon dilatation. Ophthalmologe 1999;96:97-101. [Crossref] [PubMed]
- Konuk O, Ilgit E, Erdinc A, et al. Long-term results of balloon dacryocystoplasty: success rates according to the site and severity of the obstruction. Eye (Lond) 2008;22:1483-7. [Crossref] [PubMed]
- Lee DH, Song HY, Ahn H, et al. Balloon dacryocystoplasty: Results and factors influencing outcome in 350 patients. J Vasc Interv Radiol 2001;12:500-6. [Crossref] [PubMed]
- Ko GY, Lee DH, Ahn HS, et al. Balloon catheter dilation in common canalicular obstruction of the lacrimal system: safety and long-term effectiveness. Radiology 2000;214:781-6. [Crossref] [PubMed]
- Lanciego C, De Miguel S, Perea M, et al. Nasolacrimal stents in the management of epiphora: medium-term results of a multicenter prospective study. J Vasc Interv Radiol 2001;12:701-10. [Crossref] [PubMed]
- Song HY, Lee DH, Ahn H, et al. Intervention in the lacrimal drainage system. Cardiovasc Intervent Radiol 2002;25:165-70. [Crossref] [PubMed]
- Paúl L, Pinto I, Vicente JM, et al. Nasolacrimal stents in the treatment of epiphora: long-term results. J Vasc Interv Radiol 2002;13:83-8. [Crossref] [PubMed]
- Kang SG, Song HY, Lee DH, et al. Nonsurgically placed nasolacrimal stents for epiphora: Long-term results and factors favoring stent patency. J Vasc Interv Radiol 2002;13:293-300. [Crossref] [PubMed]
- Lanciego C, Toledano N, De Miguel S, et al. Resolution of epiphora with nasolacrimal stents: results of long-term follow-up in a multicenter prospective study. J Vasc Interv Radiol 2003;14:1417-25. [Crossref] [PubMed]
- Song HY, Jin YH, Kim JH, et al. Nasolacrimal duct obstruction treated nonsurgically with use of plastic stents. Radiology 1994;190:535-9. [Crossref] [PubMed]
- Song HY, Ahn HS, Park CK, et al. Complete obstruction of the nasolacrimal system. Part II. Treatment with expandable metallic stents. Radiology 1993;186:372-6. [Crossref] [PubMed]
- Song HY, Lee CO, Park S, et al. Lacrimal canaliculus obstruction: nonsurgical treatment with a newly designed polyurethane stent. Radiology 1996;199:280-2. [Crossref] [PubMed]
- Preziosi P, Di Primio M, Erdembileg T, et al. Treatment of lacrimal stenoses obstructions with interventional radiology: immediate and 5-year follow-up results. Radiol Med 2008;113:1211-8. [Crossref] [PubMed]
- Ciampi JJ, Lanciego C, Navarro S, et al. Treating epiphora in adults with the wilhelm plastic nasolacrimal stent: Mid-term results of a prospective study. Cardiovasc Intervent Radiol 2011;34:124-31. [Crossref] [PubMed]
- Pinto IT, Paul L, Grande C. Nasolacrimal polyurethane stent: Complications with CT correlation. Cardiovasc Intervent Radiol 1998;21:450-3. [Crossref] [PubMed]
- Song HY, Lee CO, Park S, et al. Lacrimal Canalicular Obstructions: Safety and Effectiveness of Balloon Dilation. J Vasc Interv Radiol 1996;7:929-34. [Crossref] [PubMed]
- Song HY, Jin YH, Kim JH, et al. Nonsurgical placement of a nasolacrimal polyurethane stent: long-term effectiveness. Radiology 1996;200:759-63. [Crossref] [PubMed]
- Pulido-Duque JM, Reyes R, Carreira JM, et al. Treatment of complete and partial obstruction of the nasolacrimal system with polyurethane stents: Initial experience. Cardiovasc Intervent Radiol 1998;21:41-4. [Crossref] [PubMed]
- Schaudig U, Maas R. The polyurethane nasolacrimal duct stent for lower tear duct obstruction: long-term success rate and complications. Graefes Arch Clin Exp Ophthalmol 2000;238:733-7. [Crossref] [PubMed]
- Perena MF, Castillo J, Medrano J, et al. Nasolacrimal polyurethane stent placement: preliminary results. Eur J Ophthalmol 2001;11:25-30. [Crossref] [PubMed]
- Oztürk S, Konuk O, Ilgit ET, et al. Outcome of patients with nasolacrimal polyurethane stent implantation: do they keep tearing? Ophthal Plast Reconstr Surg 2004;20:130-5. [Crossref] [PubMed]
- Ko GY, Song HY, Seo TS, et al. Obstruction of the lacrimal system: treatment with a covered, retrievable, expandable nitinol stent versus a lacrimal polyurethane stent. Radiology 2003;227:270-6. [Crossref] [PubMed]
- Lee JS, Jung G, Oum BS, et al. Clinical efficacy of the polyurethane stent without fluoroscopic guidance in the treatment of nasolacrimal duct obstruction. Ophthalmology 2000;107:1666-70. [Crossref] [PubMed]
- Zhan X, Guo X, Liu R, et al. Intervention using a novel biodegradable hollow stent containing polylactic acidpolyprolactone-polyethylene glycol complexes against lacrimal duct obstruction disease. PLoS One 2017;12:e0178679. [Crossref] [PubMed]
- Attas-Fox L, Codère F. Nonsurgical retrieval of retained lacrimal stenting material. Ophthal Plast Reconstr Surg 2012;28:303-4. [Crossref] [PubMed]


