Concordance between preoperative computed tomography angiographic mapping and intraoperative perforator selection for deep inferior epigastric artery perforator flap breast reconstructions
Introduction
Breast reconstructions have become an integral part of breast cancer treatment. The preferred method of autologous breast reconstruction in our teaching hospital (Gelre Hospital, Apeldoorn, the Netherlands) has been the utilization of a lower abdominal free flap with a pedicle based on the deep inferior epigastric artery perforator (DIEP). This is due to a highly reliable outcome with the loss of one flap out of 49 (success rate of 98.0%) in the last four years. However, 16.7% of our cases (8 out of 48) did require an early revision. The indications were, for example, a hematoma, a second look at anastomoses because of venous congestion, fat necrosis or partial flap loss. Some of these revisions may be related to our technique; however, some could be related to the selection of the perforator.
Preoperative imaging has repeatedly been shown to be of value as it provides the surgeon with detailed information about the location of specific perforators. It significantly reduces flap harvest time (1-4), total operative time (1,5-12), flap complication rates (1,10,12), and donor site morbidity (6,12,13).
To date, multiple-detector row computed tomography (CT) angiography, which has the advantages of widespread availability and high spatial resolution, has become the gold standard for mapping the perforators preoperatively. Protocols and interpretation may, however, vary. In our hospital we are fortunate to have an excellent working relationship between the Departments of Plastic Surgery and Radiology. We have adopted a well-established Belgian CT angiography protocol for mapping perforators preoperatively to our DIEP flap surgeries.
The primary objective of this study is to assess the concordance between the perforator(s) advised by the radiologist based on the CT angiography findings and the quality of this perforator during the operation. In other words, does the surgeon use the same perforator as was selected by the radiologist? Secondly, we wanted to compare our results with those of other, similar studies. The third objective was to identify those factors which could possibly assist in reaching a higher concordance. To the best of our knowledge, this has not been done before.
Methods
The Institutional Review Board (IRB) of Gelre Hospital, Apeldoorn, the Netherlands, approved the study (RF 17.60). The study followed the tenets of the Declaration of Helsinki (DoH). A waiver of informed consent was granted as the study was a retrospective chart review and not a prospective interventional study. Informed consent had been obtained from all patients for both the imaging and reconstructive surgery. The waiver was granted on the basis of the ethics guidelines and in accordance with the rules laid down in Dutch law: the Medical Research Involving Human Subjects Act (WMO).
Patient population
Data of 49 consecutive female patients who underwent a clinically indicated CT angiography and a unilateral DIEP flap breast reconstruction at our institution between January 2013 and May 2017 were included in this retrospective analysis. All operations had been undertaken by a single reconstructive surgical unit consisting of four core surgeons; either one of two of the surgeons (Carolien F. Wever, Michiel R. Beets) routinely selected the perforator(s) intraoperatively.
CT angiography
Exclusion criteria for CT angiography included an allergy to iodinated contrast media, renal impairment and claustrophobia.
All CT angiograms were acquired and reported by one designated radiologist (Joost J. Kardux), who has 15 years of experience in CT angiography. The radiologist would identify one or more potentially suitable perforators and was not limited in the number of perforators that could be selected. The surgeon responsible for the actual microvascular anastomosis studied the CT angiogram separately. In only a few cases the CT angiogram was read and interpreted by both, together.
Scanning protocol
A dedicated lower abdominal wall CT angiographic acquisition and contrast medium protocol was established for the Siemens Somatom® Definition Flash CT scanner (Siemens Healthcare Nederland B.V.) located in the Gelre Hospital in Apeldoorn, the Netherlands. Patients were scanned in a supine position, with their arms raised. Clothing and other straps around the abdomen were removed to prevent changes of the abdominal contours. The scanning range was from the pubic symphysis to 10 cm above the umbilicus. Table 1 provides an overview of our CT angiography acquisition parameters. As contrast medium, 100 mL of intravenous iomeprol 400 (Iomeron®, Bracco UK Ltd.) (contains 81.65% w/v of iomeprol equivalent to 40% iodine or 400 mg iodine/mL) or iomeprol 350 was injected into an antecubital vein with a flow of 5 mL/s. No oral contrast medium was used. As the time interval between the beginning of the intravenous contrast material injection and the arrival of the bolus in the aorta may vary between patients, bolus tracking was used to ascertain the scanning delay, which was then set to start at 7 seconds after reaching 100 Hounsfield units (HU) at the region of interest, namely the infrarenal aorta (contrast medium transit time +7 seconds).

Full table
Axial 4/3 mm images were reviewed to exclude other pathologic processes as previous research has shown that the percentage of DIEP patients with a radiographically suspicious incidentaloma or unexpected finding on CT angiography, unrelated to their breast cancer, can be high (14,15) and, in some cases, may significantly alter the surgical management plan (15).
The protocol for postprocessing of the CT angiography consisted of axial and coronal 16/10 mm maximum-intensity projections (MIP) as this allowed the arterial perforator tree to be rapidly reconstructed without the need for a complicated modification of multiple parameters. Thus the voxels of highest attenuation within a volume in the direction of view were displayed to provide an angiographic-like image (16).
Multiplanar reformations (MPR) give the radiologist the opportunity to display the data in a different plane from that in which the scan was originally taken. Sagittal 4/3 mm MPR were used to display the intramuscular course in one image.
An axial 1.5/1 mm view was used to look at the smaller vessels and finer details. In addition, an axial 0.75/0.4 mm view was analyzed consensually on a dedicated workstation [Vitrea® 6.7.2 (024.1), Vital Images, Minnetonka, USA] used for further analyzing the perforating branches. Images of the abdominal wall were reconstructed to mark the perforators.
Interpretation of CT angiogram
The radiologist reported several items following the steps explained below.
Step 1
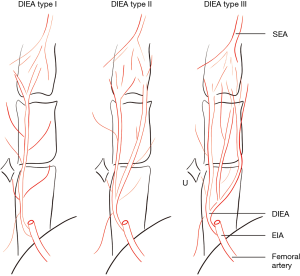
The caliber and branching type of the deep inferior epigastric artery (DIEA) was described for the left and right hemi-abdomen. The branching pattern of the DIEA was specified according to the classification of Moon and Taylor (17), which is summarized thus (see Figure 1)—type I: a single DIEA; type II: a DIEA that bifurcates below the level of the umbilicus; type III: a DIEA that splits into three or more branches below the level of the umbilicus.
Step 2
One or more suitable perforators per hemi-abdomen were selected. The point location of where each perforator penetrates the anterior fascia of the rectus muscle was marked with X- and Y-coordinates, using a grid with the umbilicus set as 0-point.
By selecting perforators the following factors were taken into consideration: the caliber of the DIEA, the caliber and location of the perforator, the length of the intramuscular course of the perforator, and the form of this course (see Table 2).

Full table
Preference was given to perforators that (I) split from a large DIEA; (II) had a larger caliber; (III) were not too close to (3 cm), and were inferior to, the umbilicus or central in the flap; (IV) had a short intramuscular course; (V) had a straight, rather than a tortuous course.
Scars were described in the reports, but were not taken into account during the radiologist’s process of selecting the most suitable perforator(s). The radiologist was aware of a predilection on the part of the surgeons to harvesting a flap from the contralateral hemi-abdomen.
Perioperative perforator selection
Immediately prior to surgery, the lead surgeon would mark the perforators on the abdominal skin with the aid of a handheld Doppler (Dopplex® SD2, 8 MHz VP8HS, Cardiff, UK). Intraoperatively, the preferred perforator was selected by visual perception and palpation during flap elevation.
Data collection and analysis
Data were collected from patient files, along with CT angiogram reports and operation reports.
To determine whether allowance should be made for a possible change in application or interpretation of the protocol over a period of time, the cohort was divided into two groups. Group I consisted of all the DIEP surgeries done in 2013 and 2014. Group II included those who had had the same procedure done in 2015 and 2016. We compared the distribution within the two groups with a Pearson chi square test. Statistical analyses were made using SPSS 22 (SPSS, Chicago, IL, USA) with a P value <0.5 considered significant.
Results
The study included 49 women with a mean age of 51 (range, 32–67) years and a mean body mass index of 26.4 (range, 19.4–32.5) kg/m2. All patients had a unilateral breast reconstruction by means of a DIEP flap. In all, but two patients (4.1%), the reconstruction was done as a delayed procedure, following their mastectomy. In two cases the surgery was preoperatively converted into a muscle-sparing transverse rectus abdominis myocutaneous flap. A single perforator was found to be insufficient and no second perforator was located vertically in line. Therefore, a small part of the rectus muscle was harvested within the flap.
In 33 of the 49 (67.3%) reconstructions, the perforator chosen by the surgeon intraoperatively agreed with the perforator advised by the radiologist. In the other 16 cases, the surgeon chose a perforator that was not described in the radiologist’s report. In 2 cases no perforators had been selected by the radiologist.
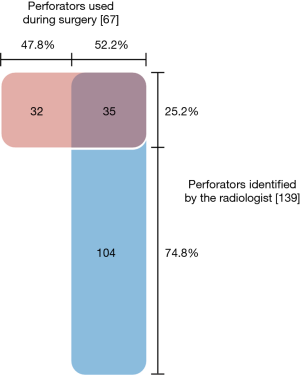
In total, 139 potentially suitable perforators were identified by the radiologist, ranging from 0 to 8 perforators (median 3) per case. Altogether 67 perforators were used in 49 reconstructions: 1 to 3 perforators per flap (median 1). Thirty-five of the perforators used were among those recommended by the radiologist (see Figure 2). Thirty-two of the perforators used had not been advised by the radiologist. Only 25.2% (35 out of 139) of the suggested perforators were ultimately used. In all, 52.2% of the perforators used during surgery were marked preoperatively by the radiologist.
In 2013 and 2014, in 14 cases out of 21 (66.7%) the surgeon used a perforator advised by the radiologist. In 2015 and 2016, the surgeon chose one of the advised vessels 15 times out of 23 (65.2%). There was no significant difference between these two periods, Χ2 (1, N=44) =0.01, P=0.92.
Discussion
Since its introduction by Alonso-Burgos et al. (18) and Masia et al. (19) in 2006, CT angiography has become widely accepted for preoperative perforator mapping for DIEP flap planning. A systematic review of 678 separate articles and a meta-analysis of 6 studies reaffirmed the significant clinical benefit of preoperative CT angiography prior to DIEP flap breast reconstruction (20).
Intraoperatively, the surgeons in our department had an aim similar to that of the radiologist, which was to select the most reliable perforator. Their, and the final, decision on the best perforator was based on visual perception and palpations intraoperatively.
The median of the perforators selected by the radiologist was 3 per case. Of the 139 suitable perforators selected by the radiologist, only 35 perforators suggested were ultimately used for the DIEP flaps. This low percentage of 25.2% can, in part, be explained by the fact that in most cases the surgeon used only one perforator, whereas the radiologist was not limited in the number of perforators to be selected. In addition, it may well have been possible that one perforator was given different coordinates by the surgeon and the radiologist, and was then recorded as two different perforators.
Furthermore, almost half of the perforators selected during surgery had not been marked as potentially suitable perforators preoperatively. In these cases, insufficient information was obtained from the CT angiography to provide the surgeon with a preoperative plan of certitude. This figure is somewhat higher than that found by Keys et al. (21) who intraoperatively added 27 perforators out of a total of the 100 perforators used in 52 flaps that had not been previously marked using CT angiography.
Our study revealed a concordance of 67.3% between one or more perforators advised preoperatively by the radiologist and those chosen intraoperatively by the surgeon. In 2 cases none of the perforators was thought to be suitable by the radiologist and therefore none was suggested.
Nowadays, it is widely known that preoperative imaging may result in better outcomes for perforator flaps. Yet most published articles focus on the sensitivity and specificity of imaging techniques. There are only a few other reports where the translation of the accuracy of the CT angiography into actual clinical utility has been studied.
Keys et al. (21) retrospectively researched a group of 37 women receiving 52 free flap breast reconstructions (30 bilateral and 22 unilateral). The preoperative imaging modality was CT angiography. The three largest perforators were reported by the radiologist. Thereafter the surgeon reviewed the scans and chose the perforators that he was willing to use. The researchers found that 62 of the 76 (82%) perforators planned by the surgeon (1–3 per flap) were ultimately used intraoperatively. Most perforators that were not used (71%) were rejected due to insufficient information from the CT angiogram: the vessels appeared to be insufficient or the intramuscular course was found to be too long. The radiologist reported 132 perforators in total, of which 73 were used by the surgeon (55.3%). The surgeons used 27 extra perforators not marked preoperatively by the radiologist. Overall, in 44% of the cases the surgeon used different or extra perforators that were not part of the initial preoperative plan.
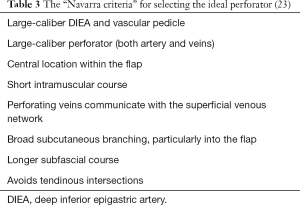
In 2014, Casares Santiago et al. (22) reported a concordance of 95.2% (100 out of 105 cases) between the perforator chosen by the radiologist based on CT angiography and the perforator chosen by the surgeon during surgery. For their purpose, Casares Santiago et al. adopted and adapted the criteria established during an international meeting held in Navarra, a province of northern Spain, in 2008 (Table 3) (23). The goal of the “Navarra meeting” was to develop a consensus amongst surgeons about the most important factors in selecting the “right” perforator for the planning and during the execution of these reconstructions. The aim of this framework was to arrive at improved flap outcomes and less dissection through the rectus muscle, with fewer donor-site complications such as abdominal bulges.
As far as we could ascertain, the report by Casares Santiago et al. (22) is still the only publication taking all factors proposed during the Navarra consensus meeting into account. While most centers of excellence have reported on the reliability of CT angiography for the purpose of preoperative perforator identification, most of these reports were based on one or a combination of factors and many were published before the actual meeting.
There are a number of differences between our study and others.
First, ours is the only study where the radiologist was not limited in the number of perforators that could be selected. In the earlier study by Keys et al. (21), the radiologist was limited to three perforators. In the adaptation of the ‘Navarra criteria’ presented by Casares Santiago et al. (22), the radiologists were limited to two perforators per patient: one “main perforator” and one “rescue perforator”. Only the “main perforator” was used to calculate the concordance.
Secondly, our scanning range was wider: up to 10 cm above the umbilicus. Keys et al. (21) and Casares Santiago et al. (22) maintained a scanning range of respectively 4 and 5 cm above the umbilicus.
In their retrospective study of preoperative CT angiograms of the abdominal wall, Saad et al. (24) found that 83% of all perforators of choice were found within 3 cm of the umbilicus on the preoperative CT angiograms. They were confident enough in their ability to identify more cranially located dominant perforators on preoperative CT angiograms to modify their flap design in order to avoid the perforator being on the edge of the superior aspect of a normally designed flap. However, only 9% of the dominant perforators were found more than 2 cm above the level of the umbilicus.
These two factors, namely the number of perforators and the scanning range, combined may explain the more liberal selection by our radiologist as it is not unusual to find perforators of a good caliber beyond 3 cm from the umbilicus. Casares Santiago et al. (22) scanned up to 5 cm above the umbilicus, yet suggested replacing the original Navarra criterion of a “centrally located perforator within the flap” (Table 3) with “a cranial limitation of 3 cm above the umbilicus”. Limiting either the number, the upper range of scanning, or both, may thus make sense, provided allowance is made for a certain percentage falling outside this range.
Thirdly, in their selection and recommendation of perforators, the radiologists of the Casares Santiago group (22), like our group, took more factors than one into consideration. Their group adapted, but largely adhered to, the “Navarra criteria” (23). In the case of Keys et al. (21), we learn that their radiologist selected the perforators based on size. Selecting perforators solely on size can be deceptive and may explain their intraoperative changes in some cases. A study published by Cina et al. (25) reported that CT angiography overestimates perforator size by up to 1.18±0.35 mm.
Where a choice has to be made between two analogous perforators, it makes sense to give preference to the one with a broader branching pattern subcutaneously. However, a subcutaneous branching pattern was not visible in all of our angiograms. It was most definitely taken into account for selecting suitable perforators. If no network, and more particularly no “main trunk” of the perforator was identifiable subcutaneously, the perforator was considered to be insufficient, since the vessels were thought to be too small to supply the flap.
Although a longer subfascial segment of the perforator is preferable and was duly reported whenever present, it was seldom a decisive factor in the radiologist’s choice of the ideal perforator. More weight was given to the length of the intramuscular course. In this regard it may be worth mentioning the importance of taking cognizance of the branching pattern of the DIEA on the CT angiogram. Previous studies have demonstrated that the bifurcating DIEA pattern (Moon & Taylor type II) has the shortest intramuscular course (26,27).
We, contrary to the recommendation of the Navarra group (23) and its adherents (22), prefer to use a perforator that passes through a tendinous intersection. In this we are not alone as their presence, which may range from 5% (28) to 12.4% (29), and their preferential use has been well described elsewhere (28,29). For some of these perforators, the course is completely retromuscular instead of intramuscular (9). Although the initial dissection may prove to be more challenging (22,23), it does not necessarily lead to an increase in perforator dissection time (29).
Fourthly, venous enhancement was not part of our protocol. Based upon previous research (30) which suggested that less postoperative venous congestion can be expected wherever communication between the deep and superficial venous systems was evident on angiography, the Navarra group suggested that this communication between the deep and superficial inferior epigastric veins is clearly demonstrated preoperatively. However, Rozen and Ashton (31) reported that the demonstration of such communication is notoriously difficult as it is often too small to detect with CT angiography. This can be partly explained by the challenge posed by determining the optimal image-acquisition timing. Exceeding the time limit for optimal image acquisition can result in arteries and veins showing similar opacification (also described as venous contamination) (32). Venous contamination may be a reason why many other centers have focused on obtaining only the best possible arterial perforator images.
If we were to include more information regarding the venous outflow and, more particularly, the communication between the deep and superficial inferior epigastric veins, we would have to revise our scanning protocol.
We conduct an “arterial-phase” scan with a 7-second delay. This could be one of the reasons why it is difficult for us to evaluate the venous network and subcutaneous branching pattern of the perforators. Keys et al. (21) and Casares Santiago et al. (22) use scanning delays of 10 and 15–20 seconds respectively. Whereas Keys et al. (21) did not describe the region of interest for the bolus tracking, Casares Santiago et al. (22) set the region of interest on the abdominal aorta 2 cm above bifurcation, and used a higher bolus peak of 180 HU (vs. 100 HU in our study). Image quality is directly related to HU-density in the femoral artery. A previous study suggested a significant correlation regarding the image quality score at postprocessing between volume rendering, especially MIP, and the HU value, and thus less interobserver variability (33).
Changing our CT angiography protocol, in other words seeking an alternative solution, may be attractive but implementation could be more time consuming. A disadvantage of CT angiography is the lack of information regarding flow direction, which would make it possible to distinguish clearly between the arterial and venous systems. Gravvanis et al. (34) have offered a welcome alternative that is both cost-effective and does not increase radiation exposure. Apart from using CT angiography to study the arterial conduit, they used color Doppler ultrasonography (Duplex) to evaluate the accompanying venous tributaries within the “perforator complex” [the triad of an arterial perforator, venous perforator(s) and nerve in any combination]. They concluded that fewer perfusion problems intraoperatively and less fat necrosis postoperatively could be expected if venous dominance, in terms of size and peak flow velocity, is correctly identified by CT angiography-guided color Doppler ultrasonography. They warned, as have some others (35), that the arterial perforator with the largest size is not always accompanied by the largest venous conduit, and vice versa.
Finally, whether one or more radiologists should be involved in reporting the CT angiogram for preoperative perforator screening has, to the best of our knowledge, not been studied. Previous studies have shown that interobserver variability can be statistically significant, regardless of excellent intraobserver agreement where only one, very specific CT arteriographic interpretation is required (36).
We therefore believe, until proven otherwise, that one dedicated and experienced radiologist may suffice, because whatever is lost in depth of knowledge when two or more are involved, is gained in restriction of argument.
Conclusions
Our study was intended as a methodology developmental study. It is limited in its population size. Nevertheless, we are confident in recommending a change in our scanning protocol, whereby scanning range and selection of the preoperative number of perforators are limited and image acquisition timing is optimized. One, or a combination of these factors, may be required for future prospective clinical trials based on this study and will facilitate the translation of CT angiography accuracy into actual clinical utility.
Finally, we would still encourage closer communication between the radiologist and the senior surgeon for studying the vasculature of the lower abdomen of each case. Because, to dissect without proper imaging is to sail an uncharted sea, whilst studying the images without actual vascular dissection is not to set sail at all.
Acknowledgements
None.
Footnote
Conflicts of Interest: The authors have no conflicts of interest to declare.
Ethical Statement: The Institutional Review Board (IRB) of Gelre Hospital, Apeldoorn, the Netherlands, approved the study (RF 17.60). Informed consent had been obtained from all patients for both the imaging and reconstructive surgery.
References
- Masia J, Kosutic D, Clavero JA, et al. Preoperative computed tomographic angiogram for deep inferior epigastric artery perforator flap breast reconstruction. J Reconstr Microsurg 2010;26:21-8. [Crossref] [PubMed]
- Fitzgerald O’Connor E, Rozen WM, Chowdhry M, et al. Preoperative computed tomography angiography for planning DIEP flap breast reconstruction reduces operative time and overall complications. Gland Surg 2016;5:93-8. [PubMed]
- Minqiang X, Lanhua M, Jie L, et al. The value of multidetector-row CT angiography for pre-operative planning of breast reconstruction with deep inferior epigastric arterial perforator flaps. Br J Radiol 2010;83:40-3. [Crossref] [PubMed]
- Fansa H, Schirmer S, Frerichs O, et al. Significance of abdominal wall CT-angiography in planning DIEA perforator flaps, TRAM flaps and SIEA flaps. Handchir Mikrochir Plast Chir 2011;43:81-7. [Crossref] [PubMed]
- Clavero JA, Masia J, Larrañaga J, et al. MDCT in the preoperative planning of abdominal perforator surgery for postmastectomy breast reconstruction. AJR Am J Roentgenol 2008;191:670-6. [Crossref] [PubMed]
- Casey WJ, Chew RT, Rebecca AM, et al. Advantages of preoperative computed tomography in deep inferior epigastric artery perforator flap breast reconstruction. Plast Reconstr Surg 2009;123:1148-55. [Crossref] [PubMed]
- Smit JM, Dimopoulou A, Liss AG, et al. Preoperative CT angiography reduces surgery time in perforator flap reconstruction. J Plast Reconstr Aesthet Surg 2009;62:1112-7. [Crossref] [PubMed]
- Tong WM, Dixon R, Ekis H, et al. The Impact of preoperative CT angiography on breast reconstruction with abdominal perforator flaps. Ann Plast Surg 2012;68:525-30. [Crossref] [PubMed]
- Masia J, Larrañaga J, Clavero JA, et al. The value of the multidetector row computed tomography for the preoperative planning of deep inferior epigastric artery perforator flap: our experience in 162 cases. Ann Plast Surg 2008;60:29-36. [Crossref] [PubMed]
- Malhotra A, Chhaya N, Nsiah-Sarbeng P, et al. CT-guided deep inferior epigastric perforator (DIEP) flap localization - Better for the patient, the surgeon, and the hospital. Clin Radiol 2013;68:131-8. [Crossref] [PubMed]
- Uppal RS, Casaer B, Van Landuyt K, et al. The efficacy of preoperative mapping of perforators in reducing operative times and complications in perforator flap breast reconstruction. J Plast Reconstr Aesthet Surg 2009;62:859-64. [Crossref] [PubMed]
- Rozen WM, Anavekar NS, Ashton MW, et al. Does the preoperative imaging of perforators with CT angiography improve operative outcomes in breast reconstruction? Microsurgery 2008;28:516-23. [Crossref] [PubMed]
- Ghattaura A, Henton J, Jallali N, et al. One hundred cases of abdominal-based free flaps in breast reconstruction. The impact of preoperative computed tomographic angiography. J Plast Reconstr Aesthet Surg 2010;63:1597-601. [Crossref] [PubMed]
- Ho OA, Bagher S, Jaskolka J, et al. Incidentalomas associated with abdominal and pelvic CT angiograms for abdominal-based breast free flap reconstruction. J Plast Reconstr Aesthet Surg 2016;69:e97-102. [Crossref] [PubMed]
- Hughes JM, Smith JR, Jones L, et al. Incidental findings in CT angiograms for free DIEP flap breast reconstruction - Do they change our management? Eur J Surg Oncol 2016;42:59-63. [Crossref] [PubMed]
- Karlo CA, Leschka S, Stolzmann P, et al. A systematic approach for analysis, interpretation, and reporting of coronary CTA studies. Insights Imaging 2012;3:215-28. [Crossref] [PubMed]
- Moon HK, Taylor GI. The vascular anatomy of rectus abdominis musculocutaneous flaps based on the deep superior epigastric system. Plast Reconstr Surg 1988;82:815-32. [Crossref] [PubMed]
- Alonso-Burgos A, García-Tutor E, Bastarrika G, et al. Preoperative planning of deep inferior epigastric artery perforator flap reconstruction with multislice-CT angiography: imaging findings and initial experience. J Plast Reconstr Aesthet Surg 2006;59:585-93. [Crossref] [PubMed]
- Masia J, Clavero JA, Larrañaga JR, et al. Multidetector-row computed tomography in the planning of abdominal perforator flaps. J Plast Reconstr Aesthet Surg 2006;59:594-9. [Crossref] [PubMed]
- Teunis T, Heerma van Voss MR, Kon M, et al. CT-angiography prior to DIEP flap breast reconstruction: a systematic review and meta-analysis. Microsurgery 2013;33:496-502. [Crossref] [PubMed]
- Keys KA, Louie O, Said HK, et al. Clinical utility of CT angiography in DIEP breast reconstruction. J Plast Reconstr Aesthet Surg 2013;66:e61-5. [Crossref] [PubMed]
- Casares Santiago M, García-Tutor E, Rodríguez Caravaca G, et al. Optimising the preoperative planning of deep inferior epigastric perforator flaps for breast reconstruction. Eur Radiol 2014;24:2097-108. [Crossref] [PubMed]
- Rozen WM, Garcia-Tutor E, Alonso-Burgos A, et al. Planning and optimising DIEP flaps with virtual surgery: the Navarra experience. J Plast Reconstr Aesthet Surg 2010;63:289-97. [Crossref] [PubMed]
- Saad A, Rebowe RE, Hogan ME, et al. Localization of the dominant deep inferior epigastric artery perforator by computed tomography angiogram. Ann Plast Surg 2014;72:670-3. [Crossref] [PubMed]
- Cina A, Barone-Adesi L, Rinaldi P, et al. Planning deep inferior epigastric perforator flaps for breast reconstruction: a comparison between multidetector computed tomography and magnetic resonance angiography. Eur Radiol 2013;23:2333-43. [Crossref] [PubMed]
- Rozen WM, Palmer KP, Suami H, et al. The DIEA branching pattern and its relationship to perforators: the importance of preoperative computed tomographic angiography for DIEA perforator flaps. Plast Reconstr Surg 2008;121:367-73. [Crossref] [PubMed]
- Molina AR, Jones ME, Hazari A, et al. Correlating the deep inferior epigastric artery branching pattern with type of abdominal free flap performed in a series of 145 breast reconstruction patients. Ann R Coll Surg Engl 2012;94:493-5. [Crossref] [PubMed]
- Vandevoort M, Vranckx JJ, Fabre G. Perforator topography of the deep inferior epigastric perforator flap in 100 cases of breast reconstruction. Plast Reconstr Surg 2002;109:1912-8. [Crossref] [PubMed]
- Pons G, Masia J, Sanchez-Porro L, et al. Paramuscular perforators in DIEAP flap for breast reconstruction. Ann Plast Surg 2014;73:659-61. [Crossref] [PubMed]
- Blondeel PN, Arnstein M, Verstraete K, et al. Venous congestion and blood flow in free transverse rectus abdominis myocutaneous and deep inferior epigastric perforator flaps. Plast Reconstr Surg 2000;106:1295-9. [Crossref] [PubMed]
- Rozen WM, Ashton MW. The venous anatomy of the abdominal wall for deep inferior epigastric artery (DIEP) flaps in breast reconstruction. Gland Surg 2012;1:92-110. [PubMed]
- Pellegrin A, Stocca T, Belgrano M, et al. Preoperative vascular mapping with multislice CT of deep inferiorepigastric artery perforators in planning breast reconstruction after mastectomy. Radiol Med 2013;118:732-43. [Crossref] [PubMed]
- Saba L, Atzeni M, Ribuffo D, et al. Analysis of deep inferior epigastric perforator (DIEP) arteries by using MDCTA: comparison between 2 post-processing techniques. Eur J Radiol 2012;81:1828-33. [Crossref] [PubMed]
- Gravvanis A, Tsoutsos D, Papanikolaou G, et al. Refining perforator selection for deep inferior epigastric perforator flap: The impact of the dominant venous perforator. Microsurgery 2014;34:169-76. [Crossref] [PubMed]
- Figus A, Wade RG, Gorton L, et al. Venous perforators in DIEAP flaps: An observational anatomical study using duplex ultrasonography. J Plast Reconstr Aesthet Surg 2012;65:1051-9. [Crossref] [PubMed]
- Øvrehus KA, Munkholm H, Bøttcher M, et al. Coronary computed tomographic angiography in patients suspected of coronary artery disease: impact of observer experience on diagnostic performance and interobserver reproducibility. J Cardiovasc Comput Tomogr 2010;4:186-94. [Crossref] [PubMed]