Comparison of indocyanine green fluorescence and parathyroid autofluorescence imaging in the identification of parathyroid glands during thyroidectomy
Introduction
Intraoperative identification and preservation of parathyroid glands (PG) are critical for preventing postoperative hypocalcemia following thyroidectomy. Traditionally, surgeons have relied on visual cues for this purpose. Despite the advances in surgical technique, postoperative hypocalcemia remains to be one of the well-recognized complications of thyroidectomy. In today’s practice, transient postoperative hypocalcemia rates are around 15–30% and permanent postoperative hypocalcemia rates are around 1–3% (1). Indocyanine green (ICG) fluorescence and parathyroid autofluorescence (AF) are two recent techniques that aim to help with the intraoperative identification of PGs during thyroidectomy.
ICG is an amphiphilic tricarbocyanine dye with near-infrared fluorescent properties at around 820 nm wavelength. It is administered intravenously during thyroidectomy, and the resulting fluorescence can be captured using commercially available near-infrared cameras (2). ICG use in thyroidectomy has recently become a new topic in endocrine surgery with several groups reporting their experiences. It allows intraoperative detection as well as perfusion assessment of PGs (3,4). This technique looks promising given the high detection rate of PGs (5) and fewer incidental parathyroidectomies (6) with ICG fluorescence (ICGF) imaging compared to traditional detection methods. On the other hand, its limitations include interference from background thyroid fluorescence hindering PG detection, false negative results where a visibly viable PG would not retain the dye, and the lack of knowledge on direct correlation between intraoperative ICGF characteristics and postoperative hypocalcemia (7).
Parathyroid AF is a more recently defined property which can be captured using a spectrometer (8) or a modified near-infrared imaging camera (9). It has been reported to consistently identify PGs across various disease states, and unlike ICGF, does not require administration of a fluorescent dye (10). Of note, parathyroid AF persists regardless of gland viability and can be detected even after surgical resection of the gland (11).
Despite the growing popularity of these techniques, to our knowledge, there is no study comparing ICGF and parathyroid AF. With this study, we aim to compare the utility of these techniques in the intraoperative detection of PGs during thyroidectomy.
Methods
This is an IRB-approved study conducted between June 2015 and December 2016. Written informed consent was obtained from all patients prior to participation. Patients undergoing total thyroidectomy or thyroid lobectomy for benign and malignant thyroid diseases were eligible for enrollment. Between June 2015 and December 2015, ICG was used for fluorescence imaging. Parathyroid AF imaging was used between June 2016 and December 2016. Two separate prospective databases for ICGF and AF imaging were utilized. For each modality, 22 patients with similar clinical and demographic parameters were selected for comparison.
Both techniques were performed after the exposure of the central neck, but before any further dissection. The ability of each technique to identify PGs in comparison with the naked eye (NE) was recorded in the operating room. Recorded variables included number and location of PGs visualized with NE, with ICGF/AF, and if any, with ICGF/AF before NE. For ICGF imaging, 5 mg ICG was given intravenously after central neck exposure and the Pinpoint system (Novadaq, Toronto, Canada) was used for fluorescence imaging. For AF imaging, Fluobeam imaging system (Fluoptics, Grenoble, France) was used without contrast administration. Operating room lights were turned off for AF measurements.
In addition to ICGF/AF imaging characteristics, other study variables included age, sex, body mass index, pathology, type of surgery, and postoperative day 1 parathyroid hormone and calcium levels. Postoperative hypocalcemia was defined as calcium level of less than 8 mg/dL. Data were analyzed using student t-test, Wilcoxon rank sum test, Chi-square/Fisher test as appropriate, and regression analysis.
Results
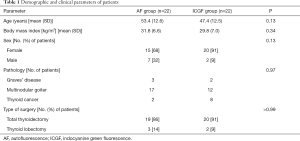
A total of 44 patients [35 of 44 (80%) female] underwent total thyroidectomy or thyroid lobectomy with either ICGF (n=22) or AF (n=22) imaging years. In the whole series, the mean age was 50.4 (SD, 12.9) years and body mass index 30.8 kg/m2 (SD, 6.9). Thirty-nine (89%) patients underwent total thyroidectomy, while the remaining 5 (11%) underwent thyroid lobectomy. Final pathology included Graves’ disease (n=5), multinodular goiter (n=29), and thyroid cancer (n=10). The groups were comparable in terms of demographics, type of surgery, and pathology (Table 1).
Full table
Both techniques had similar ability to detect PGs. Overall, ICGF imaging detected 95% (60 of 63) of PGs identified by NE, while parathyroid AF imaging detected 98% (61 of 62) (P=0.31) (Table 2). The two techniques mainly differed in the timing of identification. The location of a PG before detection by NE was more frequently suggested by AF than ICGF. Overall, AF detected 52% (32 of 62) of PGs before NE, while ICGF did 6% (4 of 63) (P<0.001). In per patient analysis, at least one PG was detected before NE with AF in 82% (18 of 22) of patients, as opposed to 14% (3 of 22) with ICGF (P<0.001). In line with these findings, the median (range) number of PGs detected before NE per patient was greater with AF than ICGF [2 (range, 0–3) vs. 0 (range, 0–2); P<0.001].

Full table
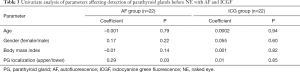
On regression analysis, no parameter was found to increase the likelihood of detection of PGs by ICGF before recognition with NE (Table 3). For AF, the only predictive factor was the location of the PGs. The upper glands were more likely to be detected before NE with AF than the lower ones (P=0.03). Transient postoperative hypocalcemia rates were similar in both groups [2 of 22 (9%) patients in AF vs. 1 of 22 (5%) in ICGF; P>0.99]. There was no permanent hypocalcemia in either group.
Full table
Discussion
To our knowledge, this is the first study that compares the utility of ICGF and AF imaging in PG identification during thyroid surgery. Our results indicate that both techniques enable high PG detection rates and low rates of postoperative hypocalcemia. The limitation of this study is the small sample size.
In previous studies, PG detection rates have been reported to range between 84–100% with ICGF (2-6) and between 77–100% with AF (9-12) imaging. Detection rates of 98% and 95% in our experience with AF and ICGF imaging, respectively, compared favorably with the literature. Also, the transient postoperative hypocalcemia rates of 9% with AF and 5% with ICGF imaging were in line with these previous reports.
In the current study, both AF and ICGF were associated with high detection rates for PGs (98% and 95%). The main difference between the modalities was related to the timing of detection. Due to less interference from background thyroid, AF enabled the detection of PGs before NE more often (52% of all PGs) compared to ICGF (6%). In the latter modality, the ICG uptake in the thyroid gland significantly limits the ability to distinguish PGs. In addition, unlike AF, ICGF requires intravenous contrast administration after central neck exposure. Injection of ICG can cause allergic urticarial reactions and rarely anaphylaxis. Furthermore, since the ICG solution contains sodium iodine for solubility, its use may be unsafe in patients with iodine allergy and renal insufficiency (13).
Despite its certain inferiorities to AF imaging, ICGF imaging has a potential role in the assessment of parathyroid perfusion. Other groups have reported utility in preventing postoperative hypocalcemia with the administration of ICG after completion of thyroidectomy (4).
Cost is always a barrier to incorporation of new technologies to conventional surgery, and has not been assessed in the current study.
Conclusions
In conclusion, this study reports the utility of AF and ICGF in the identification of PGs during thyroidectomy procedures in a comparative fashion. Due to different physical properties, each modality is associated with different pros and cons. As these modalities are gaining more popularity, we hope that this manuscript serves as an objective comparison.
Acknowledgements
None.
Footnote
Conflicts of Interest: The authors have no conflicts of interest to declare.
Ethical Statement: The study was approved by the Institutional Review Board (No. 14-1503) and written informed consent was obtained from all patients.
References
- Edafe O, Antakia R, Laskar N, et al. Systematic review and meta-analysis of predictors of post-thyroidectomy hypocalcaemia. Br J Surg 2014;101:307-20. [Crossref] [PubMed]
- Lavazza M, Liu X, Wu C, et al. Indocyanine green-enhanced fluorescence for assessing parathyroid perfusion during thyroidectomy. Gland Surg 2016;5:512-21. [Crossref] [PubMed]
- Vidal Fortuny J, Belfontali V, Sadowski SM, et al. Parathyroid gland angiography with indocyanine green fluorescence to predict parathyroid function after thyroid surgery. Br J Surg 2016;103:537-43. [Crossref] [PubMed]
- Lang BH, Wong CK, Hung HT, et al. Indocyanine green fluorescence angiography for quantitative evaluation of in situ parathyroid gland perfusion and function after total thyroidectomy. Surgery 2017;161:87-95. [Crossref] [PubMed]
- Zaidi N, Bucak E, Yazici P, et al. The feasibility of indocyanine green fluorescence imaging for identifying and assessing the perfusion of parathyroid glands during total thyroidectomy. J Surg Oncol 2016;113:775-8. [Crossref] [PubMed]
- Yu HW, Chung JW, Yi JW, et al. Intraoperative localization of the parathyroid glands with indocyanine green and Firefly(R) technology during BABA robotic thyroidectomy. Surg Endosc 2017;31:3020-7. [Crossref] [PubMed]
- Kahramangil B, Berber E. The use of near-infrared fluorescence imaging in endocrine surgical procedures. J Surg Oncol 2017;115:848-55. [Crossref] [PubMed]
- Paras C, Keller M, White L, et al. Near-infrared autofluorescence for the detection of parathyroid glands. J Biomed Opt 2011;16:067012. [Crossref] [PubMed]
- Ladurner R, Sommerey S, Arabi NA, et al. Intraoperative near-infrared autofluorescence imaging of parathyroid glands. Surg Endosc 2017;31:3140-5. [Crossref] [PubMed]
- McWade MA, Sanders ME, Broome JT, et al. Establishing the clinical utility of autofluorescence spectroscopy for parathyroid detection. Surgery 2016;159:193-202. [Crossref] [PubMed]
- De Leeuw F, Breuskin I, Abbaci M, et al. Intraoperative Near-infrared Imaging for Parathyroid Gland Identification by Auto-fluorescence: A Feasibility Study. World J Surg 2016;40:2131-8. [Crossref] [PubMed]
- Falco J, Dip F, Quadri P, et al. Cutting Edge in Thyroid Surgery: Autofluorescence of Parathyroid Glands. J Am Coll Surg 2016;223:374-80. [Crossref] [PubMed]
- Sound S, Okoh A, Yigitbas H, et al. Utility of Indocyanine Green Fluorescence Imaging for Intraoperative Localization in Reoperative Parathyroid Surgery. Surg Innov 2015. [Epub ahead of print].


