Near total parathyroidectomy for the treatment of renal hyperparathyroidism
Introduction
Secondary hyperparathyroidism (SHPT) represents an evolution of chronic kidney disease (CKD) that emerges progressively and proportionally with the duration and the severity of the underlying condition. SHPT in advanced stage CKD causes the gradual onset of debilitating symptoms and is associated to higher mortality. Several medical strategies have been developed to prevent or to control renal hyperparathyroidism, and new drugs are steadily introduced, but as SHPT become significantly symptomatic or refractory to medical treatment, the only effective therapeutic alternative remains the surgical ablation of the parathyroids.
Since the beginning of surgery of renal hyperparathyroidism, a diatribe has emerged and never resolved between those who propose the removal of all parathyroid glands [total parathyroidectomy (tPTX)], those who prefer to leave a parathyroid remnant in situ [subtotal parathyroidectomy (sPTX)] and those who graft an amount of parathyroid tissue in a heterotopic site [tPTX with autotransplantation (tPTX + AT)], each method having a number of technical variants (1). Many studies have been published comparing the techniques, but none has demonstrated its superiority. Lacking this evidence, current view is more and more oriented to leave the choice of the technique to the surgeon, according to his personal experience and judgment.
tPTX is associated to a lower recurrence rate but carries on a higher incidence of chronic hypocalcemia and some authors fears the consequences of an adynamic bone disease; the techniques with tissue preservation (sPTX and tPTX + AT) are associated to a higher incidence of relapsing hyperparathyroidism (proportional to time from parathyroidectomy) but a lower rate of chronic hypoparathyroidism. In advanced stage CKD the difference in results depend, at least theoretically, on the amount of parathyroid tissue left, which can vary from none to more than one gland (in case of missing or supernumerary glands). However, the differences between the techniques are more marked in the short term, but then vanish on the long run.
We prefer to adopt different policies in different kind of patients (individualized surgery): we perform tPTX in patients with end stage CKD who have poor chance to undergo renal transplantation (RTx) and who have severe symptoms. In patients with a realistic chance to receive a functioning kidney and in those with low compliance with medical therapy we have developed a technique that we define “near total parathyroidectomy” (ntPTX), in which a very small (3 mm × 3 mm × 3 mm) parathyroid remnant is left in situ. The aim of this strategy is to provide a long-lasting cure of hyperparathyroidism (minimal relapse rate) though leaving a residual parathyroid hormone (PTH) production promptly available after surgery, useful in case of a successful RTx shortly after parathyroidectomy.
We analyze and report here our results with ntPTX for renal hyperparathyroidism.
Methods
We reviewed all SHPT patients submitted to ntPTX in a tertiary referral center during the period 2001–2015 (retrospective, single arm study). Surgical technique (ntPTX): the goal is the identification of at least 4 parathyroid glands; the most suitable gland is chosen (according to its grade of hypertrophy, consistency and nodularity) and a small remnant visually established as a cuboid of about 3 mm × 3 mm × 3 mm is left at the vascular pole of the gland in situ (Figure 1). The thymus is incised and palpated bilaterally but not removed unless there is an abnormal finding. If less than 4 glands are found or the palpation of the thymus reveals a nodule, we perform a trans-cervical thymectomy and eventually a thyroid lobectomy on the side of the missing gland; if we are not able to identify at least 4 glands in the specimens, then we don’t leave the small parathyroid remnant in the neck. Feedback by intraoperative quick measurement of PTH has not been obtained in this study (unavailability).
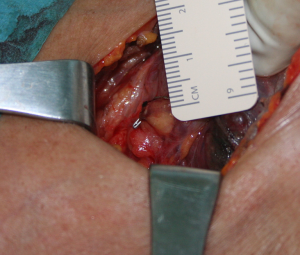
We extracted from a prospectively compiled data base the demographic data, biochemical markers of CKD-MBD before and after ntPTX, the time on dialysis before surgery, the presence of clinically prominent symptoms, the number of glands removed, the removal of thymus/thyroid, surgical complications, symptomatic hypocalcemia, the 30-day mortality, the eventual RTx, the time of follow-up, the function of renal graft at follow-up, survival rate, relapse rate, and any medical treatment aiming at modify circulating calcium or PTH at last follow-up.
Surveillance consisted in out-patient clinic visits, request of information to referring nephrologist and personal telephone interview. Variations of clinical pictures were harvested by personal interview of patients (no questionnaires were used, apart a visual analogic scale system for symptoms at operation). The primary outcome was the cure rate after ntPTX; we furthermore were interested to evaluate the effects of this technique on calcium homeostasis in those patients receiving a renal transplant, as compared to those remaining on dialysis.
We defined persistence the presence of PTH values >350 pg/mL less than 6 months after ntPTX, and recurrence PTH >350 thereafter. This value has been conventionally established for this study, and is grossly representative of values considered in more recent guide-lines.
Statistical analysis
The Shapiro-Wilk test was carried out to verify normality of the distributions. ANOVA test and Bonferroni’s test for multiple comparisons were performed to study quantitative variables. Chi-squared test with Yate’s correction for continuity was used to analyze categorical variables. Post-hoc power tests were conducted to estimate the sample sizes of the groups, the 1-β values of the significant variables were >0.8, assuring appropriate sample sizes. The statistical analysis was carried out using the IBM SPSS software package, version 17.0.1.
Results
Forty-seven patients with a mean age of 47 years (range 23–77 years) were submitted to ntPTX during the observation period. Thirty-two were males. Seventeen patients (36%) were frankly symptomatic, and 18 (38%) were referred for parathyroidectomy to have access to a transplantation waiting list. The mean time on dialysis before the operation was 6 years.
At histology 4 glands were documented in 40 patients, 3 glands in 5 patients, only 2 glands in 1, and 5 glands in another. The thymus was resected in 26 patients, the thyroid in 9. The operation was uneventful in all but two subjects: a young woman submitted to video-assisted ntPTX and total thyroidectomy for papillary carcinoma had a permanent unilateral vocal cord paralysis; another patient had a postoperative cervical hematoma requiring surgical evacuation. Nine patients (19%) developed a postoperative symptomatic hypocalcemia requiring higher doses of calcium and vitamin D replacement and a longer in-hospital monitoring. The 30-day perioperative mortality was null. No patient experienced persistent disease. Perioperatively, mean ± sd PTH fell from 1,603.5±1,740.3 to 37.3±53.3 pg/mL (P<0.0001), calcium from 9.9±1.2 to 8.1±1.2 mg/dL (P<0.0001), alkaline phosphatase (ALP) raised from 715.1±932.4 to 847.9±1,096.6 U/L (P=0.023) (Table 1).
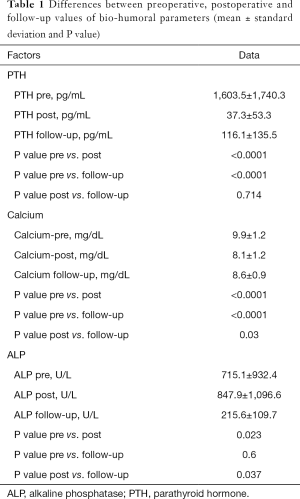
Full table
Follow-up time is 8.5±4.1 years (mean ± sd). Thirty-five patients (74%) are alive and 12 are dead (mostly due to cardio-vascular complications). Among patients (n=17) with prominent preoperative symptoms—weakness, bone pain, pruritus—they were still dramatically reduced in 82% at follow-up. PTH at follow-up was 116.1±135.5 pg/mL, and calcium 8.6±0.9 mg/dL (Table 1). During the observation period we registered 4 (8.5%) recurrences of SHPT: one patient developed an hyperplasia of the remnant, and was cured by its removal; a second patient had an elevation of PTH after RTx, also probably caused by remnant hyperplasia (according to sestamibi scintigraphy images), and it has been effectively controlled by cinacalcet 30 mg/day (current PTH 122 pg/mL); the third patient has a PTH of 429 pg/mL under cinacalcet, and the fourth patient has a PTH of 712 pg/mL and is refusing both medical therapy and surgical re-exploration. To summarize, three of them are currently under hemodialysis and one has a functioning graft. So, at last follow-up 3 patients (6.4%) have a PTH ≥350 pg/mL; Paradoxically, none of the five patients with less than 4 glands removed at operation showed persistence or recurrence of disease.
One patient had a functioning renal transplant at time of ntPTX (tertiary hyperparathyroidism), and other 27 subsequently underwent RTx thereafter. The mean time to RTx was 2.8 years. The renal graft was still functioning (at last follow-up or at death) in 20 (71% of transplanted, 43% of the whole study population). The PTH levels in subjects who never received a transplantation, in those submitted to Rtx and in those who had a functioning transplant at last follow-up were 107, 110 and 83 pg/mL respectively (Table 2). Statistical analysis did not show a correlation between the renal functional status and the relapse of hyperparathyroidism. As far as pharmacological treatment regards, 32 subjects are assuming calcium and/or vitamin D (in the form of cholecalciferol and calcitriol) and 4 cinacalcet or vitamin D analogues. Hypocalcemia (<8 mg/dL) at follow-up was observed in 7 patients (2 transplanted, 5 on hemodialysis), all under substitutive treatment and asymptomatic. Subjects with a functioning transplant are less linked to vitamin D/calcium supplementation (P=0.015, Chi-squared test with Yates’ correction), while there was not a significant association of the functional status of the kidney at last follow-up and the cure or the relapse of hyperparathyroidism (Table 3).

Full table
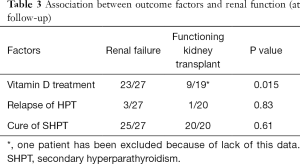
Full table
Discussion
parathyroidectomy for renal hyperparathyroidism should ideally lower PTH levels back in the “normal” range (150–300 pg/mL according to KDOQI guidelines, or a wider range according to the KDIGO recommendations) (2) and prevent the relapse of hyperparathyroidism with time. None of the surgical procedures developed to treat this condition has served perfectly this purpose: sPTX and tPTX + AT are associated with a rate of recurrent SHPT (5–20%) that grows with time from PTX (3-7); tPTX is associated to lower relapse rates (0-12%), but a higher risk of hypoparathyroidism (8-12). In the past decades, several studies compared the various surgical techniques. All these studies were hampered by a less than optimal methodology and several weak points, and they were not able to show an evidence of the superiority of one technique over the others, including a recent meta-analysis on 1,589 patents (13). The impression is that there is not a single, universally valid approach, but rather that any different technique is better applied to different subgroups of patients (individual therapy) (1). So, remnant preserving techniques seems more suitable for patients with realistic odds to recover a normal renal function (following RTx), while total PTX could be a better choice for patients with debilitating symptoms and a poor chance of receiving a RTx. A recently published multicenter non-confirmatory randomized trial from Germany comparing tPTX + AT and thymectomy versus tPTX alone reported a recurrence rate of 8.3% and 0% respectively, concluding that both strategies seem to be safe and effective for the treatment of otherwise uncontrollable SHPT and that tPTX alone is a feasible alternative therapeutic option. The patients that subsequently received a RTx did not show any difference regarding the kind of parathyroidectomy received (14).
tPTX, sPTX and tPTX + AT have all been in our surgical armamentarium, with satisfying results. Since the end of ‘90s nephrologist started to refer us patients with relatively asymptomatic renal hyperparathyroidism precluded to RTx list because of high PTH levels. Consequently, we decided to test the effect of leaving a very small amount of parathyroid tissue in the neck (a cuboid of about 3 mm × 3 mm × 3 mm at the hilum of the macroscopically better appearing parathyroid gland) with the purpose to cure the hyperparathyroidism while leaving a readily functioning cluster of parathyroid cells, useful in case of RTx being performed shortly thereafter (that is to maintain normal calcium levels with little/no oral supplementation).
In such case, we thought ntPTX could perform better than: (I) tPTX, which needs years to eventually recover some parathyroid production; (II) tPTX + AT, the viability of implanted tissue being less predictable, and to avoid infiltration of surrounding soft tissues sometimes associated with ectopic implants; (III) the “classic” 7/8 sPTX, which fares worse in terms of recurrence. In a word, we glimpsed ntPTX as a bridge solution between tPTX and the other practiced form of parathyroidectomy with residual tissue.
Recurrence rates vary widely depending on the definition of recurrence and on the length of follow-up. Regarding to this, the results we present here are satisfying, with very low rates at a mean follow-up of 8.5 years: only 1 patient requiring a reoperation, 4 subjects controlled by cinacalcet, 3 patients with a PTH >350 pg/mL (or 4 with a PTH >300 pg/mL). Furthermore, the hypoparathyroidism rate (23%) is acceptable since it was easily managed and well tolerated. The effect on circulating PTH levels was satisfactory both in patients who received RTx (mean PTH at follow-up 110 pg/mL), representing more than half of the sample, and in those who remained on hemodialysis (mean PTH at follow-up 107 pg/mL) (Table 2). The dramatic drop in PTH levels observed immediately after surgery is maintained also on the long-term (Table 1). If we consider the length of follow-up of the study, these results could be substantially considered as definitive.
Other authors have explored techniques implementing a very small remnant of parathyroid tissue in surgery for SHPT. Milas proposed a “ntPTX” for patients with secondary (n=93) and tertiary (n=49) hyperparathyroidism (15). The technique she described consisted in leaving a vascularized parathyroid remnant is left in situ which approximates the size of 2 normal parathyroid glands (80–100 mg), associated to intraoperative PTH monitoring and cryopreservation. Their results were excellent: after a 23-month follow-up mean PTH was 101 pg/mL. Sharma later published the results of this technique on a sample of 150 patients on hemodialysis with a mean follow-up of 42 months, reporting a PTH of 301±285.7 pg/mL (16). According to the description, their remnant seems bigger (5 mm × 5 mm × 5 mm) than the one we leave (3 mm × 3 mm × 3 mm).
Dr. He and his group from Jinan (China) developed a tPTX with trace amounts of parathyroid tissue autotransplantation (about 30 mg, cutted in pieces of 1 mm3, implanted into the sternocleidomastoid muscle) and compared it to tPTX, in a non-randomized fashion. At 6 months, the biochemical profile between the two groups didn’t show any statistical difference (PTH 13.7 and 8.9 ng/L respectively). At follow-up (mean 42 months) no graft-dependent recurrence was observed, but no biochemical data were reported (17).
Our study has some methodological limitations: it is retrospective, the amount of preserved tissue is evaluated in a grossly macroscopic way, the harvest of data after discharge has not been scheduled systematically so it can be sometimes heterogeneous, the compliance in collaborating hemodialysis services and nephrology units varied considerably. This is probably the reason why multicenter randomized studies have been rarely produced till now. Nevertheless, we think the information extracted from this analysis is an interesting contribution to the open debate on the surgical treatment of SHPT. Although the choice of the surgical technique is generally left to the single surgeon, the overall trend seems to aim toward a progressive reduction of the residual parathyroid tissue (or its total ablation) in patients with renal hyperparathyroidism refractory to medical treatment.
In conclusion, our data confirm that ntPTX is another effective operation to treat renal hyperparathyroidism, especially in a long-term perspective. We think the target patients for this technique are the subset of those waiting for the kidney transplantation; nonetheless it seems to serve well also those who continue to live with an insufficient renal function.
Acknowledgements
None.
Footnote
Conflicts of Interest: The authors have no conflicts of interest to declare.
Ethical Statement: This is a retrospective study. All patients were treated in a National Reference Hospital according to international standard rules, including privacy respect. No data included in this study allow the identification of participating subjects. All patients signed an informed consent prior to the operation and consented to be included in the study.
References
- Lorenz K, Bartsch DK, Sancho JJ, et al. Surgical management of secondary hyperparathyroidism in chronic kidney disease--a consensus report of the European Society of Endocrine Surgeons. Langenbecks Arch Surg 2015;400:907-27. [Crossref] [PubMed]
- Uhlig K, Berns JS, Kestenbaum B, et al. KDOQI US commentary on the 2009 KDIGO Clinical Practice Guideline for the Diagnosis, Evaluation, and Treatment of CKD-Mineral and Bone Disorder (CKD-MBD). Am J Kidney Dis 2010;55:773-99. [Crossref] [PubMed]
- Konturek A, Barczyński M, Stopa M, et al. Subtotal parathyroidectomy for secondary renal hyperparathyroidism: a 20-year surgical outcome study. Langenbecks Arch Surg 2016;401:965-74. [Crossref] [PubMed]
- Tominaga Y, Matsuoka S, Uno N, et al. Removal of autografted parathyroid tissue for recurrent renal hyperparathyroidism in hemodialysis patients. World J Surg 2010;34:1312-7. [Crossref] [PubMed]
- Oltmann SC, Madkhali TM, Sippel RS, et al. Kidney Disease Improving Global Outcomes guidelines and parathyroidectomy for renal hyperparathyroidism. J Surg Res 2015;199:115-20. [Crossref] [PubMed]
- Agha A, Loss M, Schlitt HJ, et al. Recurrence of secondary hyperparathyroidism in patients after total parathyroidectomy with autotransplantation: technical and therapeutic aspects. Eur Arch Otorhinolaryngol 2012;269:1519-25. [Crossref] [PubMed]
- Low TH, Clark J, Gao K, et al. Outcome of parathyroidectomy for patients with renal disease and hyperparathyroidism: predictors for recurrent hyperparathyroidism. ANZ J Surg 2009;79:378-82. [Crossref] [PubMed]
- Puccini M, Carpi A, Cupisti A, et al. Total parathyroidectomy without autotransplantation for the treatment of secondary hyperparathyroidism associated with chronic kidney disease: clinical and laboratory long-term follow-up. Biomed Pharmacother 2010;64:359-62. [Crossref] [PubMed]
- Schneider R, Slater EP, Karakas E, et al. Initial parathyroid surgery in 606 patients with renal hyperparathyroidism. World J Surg 2012;36:318-26. [Crossref] [PubMed]
- Conzo G, Perna AF, Sinisi AA, et al. Total parathyroidectomy without autotransplantation in the surgical treatment of secondary hyperparathyroidism of chronic kidney disease. J Endocrinol Invest 2012;35:8-13. [PubMed]
- Shih ML, Duh QY, Hsieh CB, et al. Total parathyroidectomy without autotransplantation for secondary hyperparathyroidism. World J Surg 2009;33:248-54. [Crossref] [PubMed]
- Coulston JE, Egan R, Willis E, et al. Total parathyroidectomy without autotransplantation for renal hyperparathyroidism. Br J Surg 2010;97:1674-9. [Crossref] [PubMed]
- Chen J, Jia X, Kong X, et al. Total parathyroidectomy with autotransplantation versus subtotal parathyroidectomy for renal hyperparathyroidism: A systematic review and meta-analysis. Nephrology (Carlton) 2017;22:388-96. [Crossref] [PubMed]
- Schlosser K, Bartsch DK, Diener MK, et al. Total Parathyroidectomy With Routine Thymectomy and Autotransplantation Versus Total Parathyroidectomy Alone for Secondary Hyperparathyroidism: Results of a Nonconfirmatory Multicenter Prospective Randomized Controlled Pilot Trial. Ann Surg 2016;264:745-53. [Crossref] [PubMed]
- Milas M, Weber CJ. Near-total parathyroidectomy is beneficial for patients with secondary and tertiary hyperparathyroidism. Surgery 2004;136:1252-60. [Crossref] [PubMed]
- Sharma J, Raggi P, Kutner N, et al. Improved long-term survival of dialysis patients after near-total parathyroidectomy. J Am Coll Surg 2012;214:400-7; discussion 407-8. [Crossref] [PubMed]
- He Q, Zhuang D, Zheng L, et al. Total parathyroidectomy with trace amounts of parathyroid tissue autotransplantation as the treatment of choice for secondary hyperparathyroidism: a single-center experience. BMC Surg 2014;14:26. [Crossref] [PubMed]


