A critical look at the effect of hyperbaric oxygen on the ischemic nipple following nipple sparing mastectomy and implant based reconstruction: a case series
Introduction
The advent of nipple preservation for oncologic and prophylactic mastectomy has enhanced aesthetic outcomes in breast reconstruction. Nipple sparing mastectomy (NSM) has been demonstrated to increase patient satisfaction and result in superior cosmetic outcomes compared to skin sparing mastectomy with nipple reconstruction (1,2). Over the past ten years, numerous studies have demonstrated NSM to be oncologically safe in appropriately selected patients and a viable alternative to skin-sparing mastectomy (3-6). Based on the safety and efficacy of NSM and the possibility of aesthetic superiority, nipple preservation has become the preferred approach in the highest volume breast centers in the United States.
Although NSM is preferred in many situations, it is not without risk based on the perfusion requirements of the additional skin and nipple areolar complex (NAC). Mastectomy skin flap ischemia/necrosis is a well-known complication following mastectomy with prevalence in the literature ranging from 2–30% (7-10). In the setting of NSM and direct to implant or autologous reconstruction, the risks of nipple ischemia may be compounded because of the added internal volume of the device or flap that may further compromise the perfusion of the tissues following NSM. Mastectomy skin flap necrosis as well as NAC necrosis may be increased in this setting (11-13).
As oncologic surgeons become increasingly comfortable with nipple-sparing mastectomy, their indications have expanded to larger and more ptotic breasts and in some patients, even previously radiated breasts (14,15). Skin flap necrosis following skin sparing mastectomy is usually managed with immediate excision and closure; however, ischemia of the NAC following NSM presents a unique challenge in that excision of the all or a portion of the areola usually results in a deforming asymmetry (10). NAC ischemia is often evident within 24–48 hours following NSM, however the degree of non-viable tissue (i.e., depth of impending tissue loss), as well as the timeline for progression or healing may be difficult to predict. It is during this time period that adjunct methods aimed at improving tissue perfusion are often attempted. Such therapies have traditionally included the application of topical vasodilators, local wound care with hydrating gels and/or antibacterial compounds; however, newer strategies such as supplemental oxygen administration and hyperbaric oxygen are now being considered more often. Unfortunately, there are few studies evaluating the role of hyperbaric oxygen for nipple ischemia following nipple-sparing mastectomy and reconstruction and of the few studies in the literature, most are isolated case reports that demonstrate eventual healing and device salvage (16-19).
Hyperbaric oxygen therapy comes at a significant cost to both the patient and the health care system as a whole. Although dive regimens vary, a standard course consists of 1–2 dives per day. Dive times range from 90 minutes to 4 hours and may continue for up to 30 days. Depending on the setting, the cost of each individual dive is approximately $400 but may vary from $100–1,000 (20,21). Recently, Dent et al. demonstrated high rates of healing in the setting of both partial and full thickness ischemia in patients treated with conservative management alone (22). Given the paucity of high quality studies and the need for additional inquiry, the purpose of this study is to critically examine the effects of hyperbaric oxygen therapy on the survival of the NAC following NSM and compare that to conservative treatment alone.
Methods
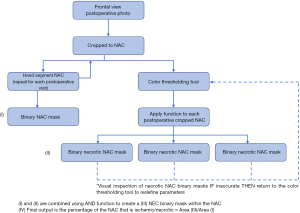
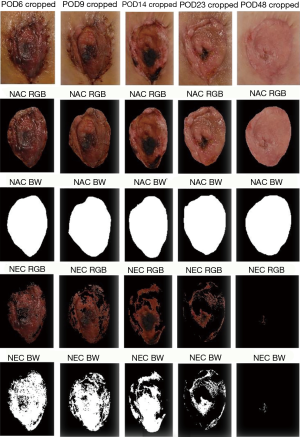
An IRB approved retrospective review was conducted to identify patients who experienced NAC ischemia following implant based breast reconstruction at MedStar Georgetown University Hospital from 2009 to 2013. Patients who were treated with or without hyperbaric oxygen therapy were included. Chart review was then performed to include weekly post-operative photographs, patient demographics, the use of adjuvant radiation therapy, smoking history, prior breast surgery, volume of breast tissue removed, initial expander fill volume, and type of mastectomy (therapeutic vs. prophylactic). Only patients with weekly post-operative photographs documenting the progression of NAC ischemia were included. Patients treated with HBOT received two 4-hour dives per day. Patients who received conservative management were treated with local wound care including petroleum gauze and/or topical antibiotic ointment. Post-operative photographs were then analyzed using a MATLAB (MathWorks, Natick, MA, USA) data processing pathway (Figure 1). Each NAC was hand defined using the image Segmenter tool producing a binary mask for each NAC at each post-operative visit (Figure 2, rows 2 and 3). A region of interest was then defined by visually selecting pixels in the ischemic portion of the NAC. A script based on the hue, saturation, and value of visually identified ischemic pixels was then applied to each post-operative visit cropped photograph (Figure 2, rows 4 and 5). Finally, by computing the number of pixels in the ischemic region of interest and dividing them by the number of pixels in the entire NAC, a percentage of ischemia relative to the whole NAC was generated. These values were then plotted to assess time to healing in each group. Treatment was discontinued once complete healing or progression to full thickness necrosis was documented.
Results
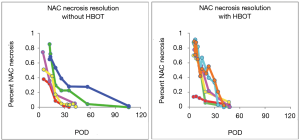
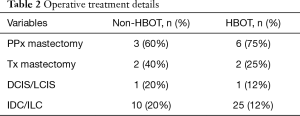
A total of 13 patients with adequate post-operative photographs for accurate documentation were included in the study. Of these, 8 patients were treated with HBOT while 5 were treated with conservative management. There were no significant differences in the prevalence of risk factors for NAC ischemia between groups (Table 1). Differences between prophylactic and therapeutic NSM or invasive versus in situ disease between groups is shown in Table 2. A single patient in the HBOT cohort had a free nipple graft at the time of NSM. There was no significant difference between degree of initial ischemia defined as the average delta ischemia (64.9% in the HBOT groups vs. 61.7% in the non-HBOT group, P=0.85) All patients in both groups, demonstrated good healing of the NAC. The average time (days) to complete healing was 35.9 days in the HBOT group and 65.4 days in the non-HBOT group. This difference was not found to be statistically significant (Figure 3).

Full table
Full table
Discussion
One of the most devastating complications following nipple-sparing mastectomy is partial or total loss of the NAC. The psychological advantages of nipple preservation have been demonstrated in numerous studies based on body image, self-esteem, and retention of an important aesthetic structure (1,23). Given that many women are choosing NSM for prophylactic and therapeutic indications, it is prudent for surgeons to do everything possible to preserve the vascularity of the NAC. This can be accomplished intraoperatively as well as postoperatively. Intraoperative maneuvers include optimizing incision location to minimize disruption of perfusion, maintaining the normal subcutaneous layer of the breast to avoid injury to the subdermal plexus of vessels, and assessment of perfusion to the NAC following NSM technology focused on visualization of blood flow and perfusion (22,24). Studies have demonstrated that these techniques can be beneficial postoperative maneuvers to improve the circulation to the NAC include nitro paste and HBO. The effect of nitro paste is to vasodilate and to improve the circulation.
Postoperative nipple ischemia can manifest in various ways and can be secondary to arterial or venous insufficiency. Arterial insufficiency may result in a pale NAC, whereas venous insufficiency often manifests in a congested appearance. The first step in the assessment of nipple perfusion is to make sure that this is not a pressure related phenomenon such as an implant that is too large or abnormal pressure around the nipple. Operative exploration may sometimes be indicated. If reversible causes are ruled out, then secondary maneuvers such as nitro paste or HBO are considered. This study has demonstrated that although HBO therapy did not prove to be advantageous for the ischemic NAC following NSM compared to conventional management based on eventual healing, it did appear to accelerate the rate at which the ischemic tissues recovered.
Hyperbaric oxygen therapy has been studied in animal models, and several mechanisms have been proposed to support its use in wound management. First, HBOT raises dissolved plasma oxygen concentration from 0.3 to nearly 7%, thus increasing the diffusion distance of O2 in the interstitial space by 4×–5× (25,26). Zamboni et al. found using in vitro and in vivo animal studies that ischemia-reperfusion injury was minimized through a nitric oxide mediated mechanism that resulted in decreased polarization of the CD18 on neutrophils (Zamboni2). Additionally, several animal studies have shown the benefit of HBOT in neovascularization of ischemic tissue flaps (27-29).
The main limitation of the current study is the small sample size given the relatively low rate of NAC ischemia. A further limitation of this study is the variability in degree and presentation of NAC ischemia between patients. Although not standardized in this study, it is our general practice to refer patients with more severe ischemia to HBOT. Although the patients treated with HBOT on average achieved complete healing in nearly half the time, this was not statistically significant. The considerable healthcare cost and time investment with HBOT must be weighed against the potential benefit of more rapid healing, return to work, and faster initiation of adjuvant radiation therapy. To this end, further larger scale studies are warranted. This initial case series may aid surgeons in patient counseling and decision making when HBOT is available. Furthermore, this series demonstrates the clinical utility of a data processing pathway using MATLAB (MathWorks) software to assess the progression of ischemic tissue. This may prove useful in future studies pertaining to mastectomy/NAC ischemia.
Conclusions
Ischemia of the NAC following NSM and reconstruction remains a challenging problem. Hyperbaric oxygen therapy has been shown to increase tissue oxygenation, prevent ischemia-reperfusion injury, and improve neovascularization in in-vitro as well as in animal studies. In the current study, we were not able to demonstrate a statistically significant benefit in patients treated with HBOT vs. conservative treatment for NAC ischemia. Further large-scale studies are warranted to further determine clinical utility and cost-effectiveness of HBOT for nipple ischemia following NSM and implant based reconstruction.
Acknowledgements
None.
Footnote
Conflicts of Interest: The authors have no conflicts of interest to declare.
Ethical Statement: The IRB used was the Georgetown University Institutional Review Board (No. 2016-0999).
References
- Satteson ES, Brown BJ, Nahabedian MY. Nipple-areolar complex reconstruction and patient satisfaction: a systematic review and meta-analysis. Gland Surg 2017;6:4-13. [Crossref] [PubMed]
- Spear SL, Shuck J, Hannan L, et al. Evaluating long-term outcomes following nipple-sparing mastectomy and reconstruction in the irradiated breast. Plast Reconstr Surg 2014;133:605e-14e. [PubMed]
- Spear SL, Willey SC, Feldman ED, et al. Nipple-sparing mastectomy for prophylactic and therapeutic indications. Plast Reconstr Surg 2011;128:1005-14. [Crossref] [PubMed]
- Patel KM, Hill LM, Gatti ME, et al. Management of massive mastectomy skin flap necrosis following autologous breast reconstruction. Ann Plast Surg 2012;69:139-44. [Crossref] [PubMed]
- Endara M, Chen D, Verma K, et al. Breast reconstruction following nipple-sparing mastectomy: a systematic review of the literature with pooled analysis. Plast Reconstr Surg 2013;132:1043-54. [Crossref] [PubMed]
- Shimo A, Tsugawa K, Tsuchiya S, et al. Oncologic outcomes and technical considerations of nipple-sparing mastectomies in breast cancer: experience of 425 cases from a single institution. Breast Cancer 2016;23:851-60. [Crossref] [PubMed]
- Warren Peled A, Foster RD, Stover AC, et al. Outcomes after total skin-sparing mastectomy and immediate reconstruction in 657 breasts. Ann Surg Oncol 2012;19:3402-9. [Crossref] [PubMed]
- Margulies AG, Hochberg J, Kepple J, et al. Total skin-sparing mastectomy without preservation of the nipple-areola complex. Am J Surg 2005;190:907-12. [Crossref] [PubMed]
- Garwood ER, Moore D, Ewing C, et al. Total skin-sparing mastectomy: complications and local recurrence rates in 2 cohorts of patients. Ann Surg 2009;249:26-32. [Crossref] [PubMed]
- Antony AK, Mehrara BM, McCarthy CM, et al. Salvage of tissue expander in the setting of mastectomy flap necrosis: a 13-year experience using timed excision with continued expansion. Plast Reconstr Surg 2009;124:356-63. [Crossref] [PubMed]
- Lee KT, Pyon JK, Bang SI, et al. Does the reconstruction method influence development of mastectomy flap complications in nipple-sparing mastectomy? J Plast Reconstr Aesthet Surg 2013;66:1543-50. [Crossref] [PubMed]
- Khavanin N, Jordan S, Lovecchio F, et al. Synergistic interactions with a high intraoperative expander fill volume increase the risk for mastectomy flap necrosis. J Breast Cancer 2013;16:426-31. [Crossref] [PubMed]
- Wong RK, Morrison SD, Momeni A, et al. Outcomes of breast reconstruction in breast cancer patients with a history of mantle radiation for Hodgkin lymphoma. Ann Plast Surg 2014;72 Suppl 1:S46-50. [Crossref] [PubMed]
- Schneider LF, Chen CM, Stolier AJ, et al. Nipple-sparing mastectomy and immediate free-flap reconstruction in the large ptotic breast. Ann Plast Surg 2012;69:425-8. [Crossref] [PubMed]
- Alperovich M, Choi M, Frey JD, et al. Nipple-sparing mastectomy in patients with prior breast irradiation: are patients at higher risk for reconstructive complications? Plast Reconstr Surg 2014;134:202e-6e. [Crossref] [PubMed]
- Fredman R, Wise I, Friedman T, et al. Skin-sparing mastectomy flap ischemia salvage using urgent hyperbaric chamber oxygen therapy: a case report. Undersea Hyperb Med 2014;41:145-7. [PubMed]
- Mermans JF, Tuinder S, von Meyenfeldt MF, et al. Hyperbaric oxygen treatment for skin flap necrosis after a mastectomy: a case study. Undersea Hyperb Med 2012;39:719-23. [PubMed]
- Alperovich M, Harmaty M, Chiu ES. Treatment of nipple-sparing mastectomy necrosis using hyperbaric oxygen therapy. Plast Reconstr Surg 2015;135:1071e-2e. [Crossref] [PubMed]
- Copeland-Halperin LR, Bruce SB, Mesbahi AN. Hyperbaric oxygen following bilateral skin-sparing mastectomies: A Case Report. Plast Reconstr Surg Glob Open 2016;4:e680. [Crossref] [PubMed]
- Guo S, Counte MA, Gillespie KN, et al. Cost-effectiveness of adjunctive hyperbaric oxygen in the treatment of diabetic ulcers. Int J Technol Assess Health Care 2003;19:731-7. [Crossref] [PubMed]
- Chuck AW, Hailey D, Jacobs P, et al. Cost-effectiveness and budget impact of adjunctive hyperbaric oxygen therapy for diabetic foot ulcers. Int J Technol Assess Health Care 2008;24:178-83. [Crossref] [PubMed]
- Dent BL, Small K, Swistel A, et al. Nipple-areolar complex ischemia after nipple-sparing mastectomy with immediate implant-based reconstruction: risk factors and the success of conservative treatment. Aesthet Surg J 2014;34:560-70. [Crossref] [PubMed]
- Qureshi AA, Odom EB, Parikh RP, et al. Patient-Reported Outcomes of Aesthetics and Satisfaction in Immediate Breast Reconstruction After Nipple-Sparing Mastectomy With Implants and Fat Grafting. Aesthet Surg J 2017;37:999-1008. [Crossref] [PubMed]
- Coopey SB, Tang R, Lei L, et al. Increasing eligibility for nipple-sparing mastectomy. Ann Surg Oncol 2013;20:3218-22. [Crossref] [PubMed]
- Jones SR, Carpin KM, Woodward SM, et al. Hyperbaric oxygen inhibits ischemia-reperfusion-induced neutrophil CD18 polarization by a nitric oxide mechanism. Plast Reconstr Surg 2010;126:403-11. [Crossref] [PubMed]
- Eskes A, Vermeulen H, Lucas C, et al. Hyperbaric oxygen therapy for treating acute surgical and traumatic wounds. Cochrane Database Syst Rev 2013;16:CD008059. [PubMed]
- Zamboni WA, Roth AC, Russell RC, et al. The effect of hyperbaric oxygen on reperfusion of ischemic axial skin flaps: a laser Doppler analysis. Ann Plast Surg 1992;28:339-41. [Crossref] [PubMed]
- Helmers R, Milstein DM, van Hulst RA, et al. Hyperbaric oxygen therapy accelerates vascularization in keratinized oral mucosal surgical flaps. Head Neck 2014;36:1241-7. [PubMed]
- Liu X, Yang J, Li Z, et al. Hyperbaric oxygen preconditioning promotes neovascularization of transplanted skin flaps in rats. Int J Clin Exp Pathol 2014;7:4734-44. [PubMed]