Chyle fistula in advanced and metastatic thyroid cancer
Introduction
Chyle fistula (CF) is a rare complication after head and neck surgery. Not only affects the postoperative recovery prolonging the hospitalization period due to difficult management needed to avoid further medical problems such as infections, wound necrosis, sepsis, etc., but also because of the severe nutritional disarray that it may cause (1).
Even though it is more common in advanced infiltrating metastatic lymph nodes from a larynx or hypo pharyngeal squamous cell carcinoma occurring in almost 2% to 8% of all neck dissections, it does occur in thyroid related surgeries (especially malignant conditions) with a 0.5–1.4% prevalence (2,3).
The first line of treatment of such complication is preventing it as the surgeon must always be aware of the risk of injuring the thoracic duct in the neck, especially in the presence of large infiltrating lymph nodes in the left supraclavicular area (2). However, regardless of such careful management of surrounding tissues around the thoracic duct, the fistula might not be apparent during surgery, until is diagnosed in the hospitalization ward, requiring a set of measures such as surgical re-intervention, drainage, a fat-free diet, compressive bandages, etc. (1-4).
The chyle
Once a person has a meal and swallows it, the food is processed in the digestive tract (5). The fatty component of such diet is divided into two parts, one goes directly to the liver through the portal system and the rest is collected as chylomicrons in the lymphatic vessels surrounding the small intestine forming the so-called cisterna chyle to drain finally in the thoracic duct, circulating in the blood for a period, giving the liver time to process the first part of the ingested fatty component of the diet (6,7). Depending on diet habits, every day an average person produces 2 to 4 liters of this substance, which essentially is a sterile alkaline whitish fluid made basically from proteins, triglycerides, cholesterol, glucose, electrolytes and lymphocytes (4-7).
Thoracic duct
In brief, once the cisterna chyle is formed beneath the diaphragm muscle, it turns in to a duct that travels upwards through the esophageal hiatus crossing from a right position to a left one ascending para vertebral and extra pleural towards the superior mediastinum between the aortic arch and the subclavian artery to continue up to the area were the left internal jugular vein meets the subclavian vein forming the left thoracic duct draining the entire body lymph except for the area superior to the right diaphragm muscle, upper right thorax, arms and right side of the face and skull which is drained to the right side duct connecting the right subclavian vein as well (6,7).
Methods
A retrospective review of surgical data base of one surgeon (Carlos S. Duque) working at the Head and Neck Division, Hospital Pablo Tobón Uribe, and Clinical Las Americas (Medellin, Colombia) from July 1st 2005 through May 2017 was performed.
All of the thyroid related surgeries were revised including benign disease, solitary thyroid nodules, malignancy, thyroiditis or multinodular goiter, first surgery, re-do surgery, along with mediastinal dissection, neck dissection or a neck or mediastinal dissection performed to treat recurrent disease.
Even though the patient demographics were looked at, the main goal was to search for those patients on whom a diagnosis of CF was observed. The medical records of those patients were extensively reviewed collecting data such as surgery performed, problems throughout the surgery, time lapse between surgery and the moment that the fistula was seen, the treatment established to control the problem, etc.
Institutional review board was obtained, all patients consented to have their case reviewed and published.
Results
A total of 1,984 thyroid related surgeries were done during this period. Out of this number, 1,050 procedures were done due to thyroid cancer mainly papillary thyroid cancer, 14 patients had medullary thyroid cancer, 4 of them with advanced bilateral neck disease without any postoperative complications along with 2 female patients with poorly differentiated thyroid cancers. The majority of the patients were females, the age range was 11 to 85 years old, during this time, 4 patients younger than 18 years were operated, out of three female patients two of them had left metastatic neck nodes without any inconvenience in their postoperative care nor there was any problem with the other two patients who had right neck nodes requiring also a neck dissection along with their thyroidectomy.
As there were no CFs in the benign group regardless that some of the cases were patients with big multinodular goiters displacing the trachea and extending out to the supraclavicular fossae and into the mediastinum, the attention was focused mainly in the 1,050 patients operated due to a malignant condition. After reviewing this large set of procedures which included isolated thyroidectomies with or without mediastinal dissections, thyroidectomies with left, right neck dissection, bilateral neck dissection or mediastinal and neck dissections performed as a single or combined procedure due to newly diagnosed or recurrent papillary thyroid carcinoma, only three (3) patients were found with a diagnosis of a left CF. Two patients (male and female) with recurrent left neck with infiltrating diffuse lymphadenopathy and one female patient with a first-time thyroidectomy with mediastinal disease along with advanced supraclavicular neck lymphadenopathy. The three patients’ hospitalization time ranged from 5 to almost 12 days, all of them were completely recovered once the CF was controlled (Table 1).
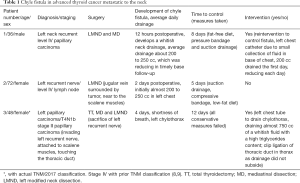
Full table
Patient cases
A 48-year-old white female underwent a total thyroidectomy, mediastinal and left modified neck dissection under intraoperative neuromonitorization NIM 3.0 (Medtronic®, Jacksonville, USA) along with the sacrifice of her left recurrent nerve involved by tumor due to a T2N1b papillary cancer (on 2017 TNM Staging Classification or stage IV for the prior classification due to her age). Even though large metastatic lymph nodes were resected, some of them almost infiltrating the scalene muscles, phrenic nerve and the area of the left thoracic duct which was handled with careful dissection and suture ligature, not revealing a CF at any moment during the procedure. She had an uneventful recovery and was discharged home without a drain as her neck drainage has less than 30 cc of normal serous fluids on postoperative day 2. Four days after she was released she seeks medical attention due to shortness of breath, as a pulmonary embolus should be ruled out, she was admitted. A chest X-ray showed a left thoracic pleural effusion occupying almost half of the left thorax and a normal right lung. Her left neck was completely flat and no collection was seen. A thoracic surgery consultation was obtained and a left chest tube was placed draining almost 750 cc of a whitish milky fluid, an analysis of such sample showed a high content of triglycerides confirming the diagnosis of a chylothorax. As all of the medical measures such as a fat-free diet, elevated head position failed, embolization of the duct, was tried without being able to control the fistula as it continued to be active with a daily thorax drainage of almost 400 cc, it was decided that and endoscopic clip placement of the duct in the left side was conducted controlling the fistula, as the chest tube finally dropped the drainage of the Chyle, the patient was released from the hospital almost 12 days after she was readmitted.
Patient No. 2
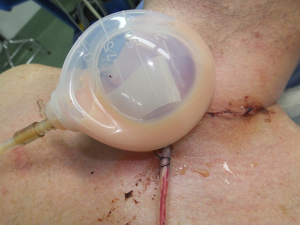
A 38-year-old male underwent a total thyroidectomy with mediastinal dissection due to a left thyroid lobe papillary carcinoma with 8 out of 9 paratracheal positive nodes with extra nodal extension, for which he was given 150 mCi of radioactive iodine. Fifteen months after, his thyroglobulin started to increase, so a neck ultrasound was obtained showing indeed suspicious left lymph nodes, from which a fine needle biopsy revealed recurrent papillary thyroid cancer. He underwent a left modified neck dissection levels II through V under intraoperative neuromonitorization NIM 3.0 (Medtronic®) resecting large lymphadenopathy measuring no more than 2.5 cc loose, not infiltrating lymph nodes, the thoracic duct was left uninjured and all tissues above it were suture ligated. Twelve hours after surgery he had a normal diet and 1 to 2 hours after he noticed a whitish colored fluid in his Blake drainage system (Ethicon®, Johnson and Johnson®, Somerville, NJ, USA) that partially filled his drainage collector. As a CF was diagnosed he was put on a free-fat diet and scheduled for revision neck surgery 26 hours after the initial procedure. On intervention, a white milky viscous fluid was seen in the neck upon de aperture of the wound. Careful exploration was done followed by meticulous ligation or suture ligation of the areas where the leak was found. A new drainage system was placed and the patient was sent to his room. A chest X-ray showed a small collection of fluid in his left basal lung for which a small drainage system was placed by interventional radiology draining about 200 cc of chyle. On postoperative day 6, the thorax drainage reduced to less than 40 cc over a 24-hour period as well as the neck drainage had less than 30 cc of a serous fluid. A normal diet was given for 24 hours without showing any increment on both drainage systems so they were removed and that patient was discharged on day 8 to continue his treatment with radioactive iodine (Figure 1).
Discussion
Even though recurrent laryngeal nerve injury and hypocalcaemia remain the most common complications after thyroidectomy, CF is a rare event but should not be underestimated. Surprisingly, as it may sound, it may occur even when a surgeon is performing a left hemi thyroidectomy with or without paratracheal dissection, a procedure considered as having a very low risk for this problem. Even though this is a quite rare scenario, it happens as aberrant lymphatic vessels might be present (4,10,11).
Most of the thyroid related CF cases reported are related to malignant disease in patients with multiple positive nodes in the left mediastinum extending to the neck, especially near the anatomical area where the thoracic duct and the lower neck lymphatic vessels join the drainage system. It is wise thinking from a surgeon resecting an enlarged left thyroid lobe along with a conglomerate of metastatic neck nodes near the lower aspects of the common carotid artery and the internal jugular vein to be in the look for those small clear lymphatic vessels forming part of the intricate lymph drainage in the neck. As careful as a surgeon might be, the oncologic outcome of the procedure overcomes the risk of developing a fistula while handling the tissues in order to resect all gross disease to control the tumor spread (4-6,10-13).
The three cases described here fit this description, even though careful dissection was done, their large and infiltrated lymph nodes were in close contact with the lymphatic draining systems, forming an intricate web of virtually undetectable tiny lymphatic vessel not showing at the moment of the surgery an evident leak. This may be due in part to the fact that the patient usually comes to surgery with a fast of minimum 6 hours or even more which have been preceded by a light meal or breakfast without the many fatty components leaving this active system at rest, thus in this regard an apparent lesion might not be noticed and secured—ligated. Once a normal diet is resumed, the chyle flows and spills through the unnoticed area with the injury, depending on how large the trauma to the duct or lymphatic is, the patients’ diet and activity, etc. The chyle might be seen in the drain or as a collection in the neck mimicking a hematoma in less than 24 hours as it happened to our male patient on whom no apparent fistula was seen on site during the procedure (10,11).
But even that a surgeon handles the tissues as careful and vigilant, this complication may be expected especially when one is confronted with large infiltrating neck nodes in the lower neck, supraclavicular area surrounding the jugular vein, scalene muscles and phrenic nerve. Though the main goal of the surgery is to remove the gross disease, a surgeon might be sure at the end of the procedure that the wound was clear of any potential untied or unsealed blood vessels or lymphatic leakage, to find later in the postoperative period that a CF has developed. Indeed, one of the main goals of a complex left lower neck dissection for gross metastatic disease is to be always on the look for the lymphatic leakage as the surgeon is resecting the affected lesions, as the principal ways to prevent it from happening. If there is doubt regarding the presence of a CF in the postoperative period, one can always order a lab analysis of such fluid in order to measure the fatty component to confirm the presence of triglycerides in the liquid (1,2,12-14).
All sorts of treatment measures have been recommended and published extensively: again the main treatment is to prevent it from happening, handle the tissues as gentle as possible, ligate the small lymphatic vessels, identify areas at risk, use or rotate local sternocleidomastoid muscle flaps to cover and seal an evident area of leakage, place a drainage, etc., but even though all of these measures are established and followed, the fistula might reveal itself later in the postoperative care. The treatment can be initially conservative starting with a non-fatty diet with a consult to the nutrition department as well as compressing neck bandages and continue suction drainage, head elevated and bed rest. Even if those measures fail, the patient might undergo total parental nutrition (TPN). At some point, octreotide or somatostatin can be used. Although this has been advocated, we certainly could not use it in our cases for various reasons, among them are price and availability. When all of these conservative measures fail and the patients continues to drain in the neck or the chylothorax does not resolve, a more active intervention can be useful. Deciding when to explore the patients depend basically on the surgeon’s recollection of the procedure and the time when the fistula was diagnosed, in our case patient number 1 was taken back to surgery shortly after the fistula became apparent. It is difficult to reassure whether this immediate intervention had an impact on the control of the problem but it needed to be done. There is no doubt that a chylothorax requires proper management by the thoracic surgery unit not only to drain the pleural effusion alleviating the patient’s respiratory symptoms but also to finally control the fistula if conservative measures or interventions like the ones described including embolization of the duct by interventional radiology fails like in our patient number 3, requiring a definitive treatment to control the problem (1-5,11-17).
In regards to a right thoracic duct fistula, we did not have any symptomatic fistulas in this side, and we reinstate “symptomatic” since it is a low activity duct draining less than 20–25% of the body’s lymph, limiting itself to the right upper chest, neck and craniofacial and right arm. We had similar scenarios in the right neck with nodes attached to the scalene muscles with difficult dissection almost as difficult to the ones experienced in the left side without any clear drainage collected in the drain on the days to follow. However, a surgeon should be aware that extensive and aggressive dissection along this area might eventually cause problems, however such complication might go unnoticed (4,6).
Acknowledgements
None.
Footnote
Conflicts of Interest: CS Duque gives neuromonitoring courses to surgeons in Latin American with the sponsorship of Medtronic®, though no part of this article was influenced nor changed from this activity. G Dionigi received honorarium from Medtronic®, Olympus®, Storz®, Inomed® for speeches about surgery and participated in advisory committee meetings sponsored by Medtronic®. The other author has no conflicts of interest to declare.
Ethical Statement: Authors obtained written authorization from the patients in order to publish their data with the compromise that their personal identification would not be released at any time, before submitting the paper to the IRB and/or Ethical Committee of both institutions (Hospital Pablo Tobon Uribe and Clinica Las Americas). Both institutions review boards allow authors to use such data on June 16 and July 10, 2017.
References
- Gregor RT. Management of chyle fistulization in association with neck dissection. Otolaryngol Head Neck Surg 2000;122:434-9. [PubMed]
- Alzaman N, Pittas AG, O'Leary M, et al. Post-thyroidectomy hypocalcemia exacerbated by chyle leak. Endocrinol Diabetes Metab Case Rep 2015;2015:140110. [PubMed]
- Merki V, Pichler J, Giger R, et al. Chylothorax in thyroid surgery: a very rare case and systematic review of the literature. J Otolaryngol Head Neck Surg 2016;45:52. [Crossref] [PubMed]
- Delaney SW, Shi H, Shokrani A, et al. Management of Chyle Leak after Head and Neck Surgery: Review of Current Treatment Strategies. Int J Otolaryngol 2017;2017:8362874. [PubMed]
- López Otero MJ, Fernández López MT, Outeiriño Blanco E, et al. Neck chylous fistula: conservative treatment. Nutr Hosp 2010;25:1041-4. [PubMed]
- Tutor JD. Chylothorax in infants and children. Pediatrics 2014;133:722-33. [Crossref] [PubMed]
- Duque CS, Londoño AF. Fístula de quilo: reporte de un caso. Acta de ORL y CCC 2009;37:235-41.
- Brierley JD, Gospodarowicz MK, Wittekind C. editors. UICC TNM classification of Malignant Tumors. 8th Edition. New Jersey, USA: Wiley Blackwell, 2017:51-4.
- Sobin LH, Gospodarowicz M, Wittekind C. editors. UICC TNM classification of Malignant Tumors. 7th Edition. New Jersey, USA: Wiley Blackwell, 2017:58-62.
- Johnson OW, Chick JF, Chauhan NR, et al. The thoracic duct: clinical importance, anatomic variation, imaging, and embolization. Eur Radiol 2016;26:2482-93. [Crossref] [PubMed]
- Smoke A, Delegge MH. Chyle leaks: consensus on management? Nutr Clin Pract 2008;23:529-32. [Crossref] [PubMed]
- Schild HH, Strassburg CP, Welz A, et al. Treatment options in patients with chylothorax. Dtsch Arztebl Int 2013;110:819-26. [PubMed]
- Meyer CD, McLeod IK, Gallagher DJ, et al. Conservative Management of an Intraoperative Chyle Leak: A Case Report and Literature Review. Mil Med 2016;181:e1180-4. [Crossref] [PubMed]
- Campisi CC, Boccardo F, Piazza C, et al. Evolution of chylous fistula management after neck dissection. Curr Opin Otolaryngol Head Neck Surg 2013;21:150-6. [Crossref] [PubMed]
- Kadota H, Kakiuchi Y, Yoshida T. Management of chylous fistula after neck dissection using negative-pressure wound therapy: A preliminary report. Laryngoscope 2012;122:997-9. [Crossref] [PubMed]
- Ahn D, Sohn JH, Jeong JY, et al. Chyle Fistula After Neck Dissection: An 8-Year, Single-Center, Prospective Study of Incidence, Clinical Features, and Treatment. Ann Surg Oncol 2015;22 Suppl 3:S1000-6. [Crossref] [PubMed]
- Jain A, Singh SN, Singhal P, et al. A prospective study on the role of octeotride in management of chyle fistula neck. Laryngoscope 2015;125:1624-7. [Crossref] [PubMed]


