Status quo and development trend of breast biopsy technology
Introduction
A definite diagnosis of breast cancer lays the foundation for appropriate subsequent treatment. Currently, the biopsy methods used for breast cancer are incisional biopsy, excisional biopsy, core needle biopsy, vacuum-assisted biopsy and bite biopsy.
With the development of modern science and technology, and the emergence of the bio-psycho-socio-medical model, there has been an increasing need to obtain the optimal therapeutic outcomes at the cost of minimal damage. The primary objectives in the treatment of breast disease are (I) to establish an accurate diagnosis; and (II) to achieve the ultimate therapeutic goals with minimized damage. Therefore, when evaluating a biopsy technique, the therapeutic efficacy and extent of injury should be taken into account in addition to the diagnostic accuracy. In view of various advantages and limitations associated of the existing biopsy methods, this review summarizes the current situation and development of breast biopsy technology to provide an insight into the latest details such as the safety and reliability as the basis for selection of the most appropriate techniques for specific settings.
Excisional breast biopsy (EBB)
EBB involves complete excision of the breast lesions, which may or may not include the margins of normal glands. It is not only the ultimate treatment of benign lesions, but the gold standard for the diagnosis of breast cancer. Although accurate diagnosis can be guaranteed with a large amount of samples collected by open surgery, this technique is applicable for palpable masses only. When the lesion is not palpable, preoperative localization will be required, and removal of more normal breast tissues may be needed, which can result in scars and other cosmetic imperfections.
Nevertheless, EBB is the preferred biopsy technique for certain breast disorders.
Phyllodes tumors of breast
Phyllodes tumors of breast can rarely be diagnosed before excisional biopsy/lumpectomy is performed. In most cases, it is not possible to distinguish adenofibroma from phyllodes tumor with core needle biopsy, while fine needle aspiration is unable to differentiate between the two, at all (1). Therefore, in the presence of a relatively large or rapidly growing fibroadenoma, EBB should be considered to pathologically exclude phyllodes tumors and provide treatment at the meantime.
Paget’s disease
A complete medical history, physical examination and diagnostic breast imaging studies are required for patients with clinical manifestations of Paget’s disease. Full-thickness skin biopsy of involved nipple areola complex (NAC) is necessary when Paget’s disease is considered for any breast lesion found by imaging studies or physical examination, which must include a part of the affected NAC. If NAC biopsy indicates Paget’s disease, breast MRI is recommended to determine the extent of the tumor, as well as other lesions, if any (2,3).
However, EBB still has certain limitations as investigators believe that biopsy by open surgical excision will restrict or affect the therapy options for primary breast cancer once confirmed because it is basically no longer possible to apply neoadjuvant treatment after the biopsy; and EBB also compromises the reliability of sentinel lymph node biopsy (SLND), because it significantly increases the false negative rate compared with patients undergoing aspiration biopsy. Krag and colleagues (4) reported compromised reliability of SLND related to the diagnostic method used in the NSABP B32 trial, where a higher false negative rate was found in 177 patients receiving open surgical biopsy compared with 589 patients undergoing aspiration biopsy (15.3% vs. 8.1%, P=0.0082). Additionally, EBB may also be an adverse factor in breast-conserving surgery by making it difficult to obtain negative margins. As reported by Liberman and colleagues (5), breast-conserving surgery was completed in 75-100% patients after aspiration biopsy without a need for secondary surgery, while only 45-64% of those receiving EBB did not require secondary surgery.
Fine-needle aspiration biopsy (FNAB)
FNAB is the first pathological method used for the diagnosis and screening of breast diseases. This rapid and cost-effective technique provides safe evaluation of breast lesions, but it is controversial whether the results can be used as the basis for the diagnosis of breast cancer. A meeting was held in Madrid in October 2007 to discuss the “current significance of fine needle aspiration cytology (FNAC) in clinical management of breast disorders”. Most experts suggested that, with evidence of malignancy based on clinical manifestations, imaging studies and fine needle aspiration cytology, FNAC can be regarded as the basis for confirming breast cancer despite the lack of histopathological findings, such as a core needle biopsy or traditional surgical biopsy (6).
Although FNAB has become increasingly less popular in the U.S., Quratulain et al. (7) still suggested the use of FNAB as a tool for rapid diagnosis and evaluation of palpable breast/chest wall masses and regional lymph nodes, of which the diagnostic accuracy could be identified by comparing the cytological diagnosis and subsequent surgical pathological specimens. From 2007 to 2011, 569 patients were enrolled in their study, including 485 patients with breast tumors, 14 patients with chest wall tumors, and 70 patients with regional lymph node metastases. Using FNAB, 285 cases of benign masses and 180 cases of malignant tumors were diagnosed. Among the benign cases diagnosed with FNAB, 85% were confirmed by clinical, radiographic, and subsequent pathological examinations. The FNAB results of the remaining 43 cases were considered inconsistent with clinical and radiologic findings. Subsequent surgical confirmed 35 of them to be benign. The other eight patients consisted of five cases of invasive ductal carcinoma, one case of invasive lobular carcinoma, and two cases of DCIS. Quratulain et al. believed that FNAB was highly accurate for diagnosis of palpable breast masses, and they found it a valuable tool for identifying the expression of HR and HER-2 receptors.
Nevertheless, FNAB have certain limitations: (I) inadequate tissue sample and false negative results are common; (II) although it is able to reveal the ER, PR and HER-2 status of cancer samples, the accuracy is suboptimal; (III) it can not distinguish between pathological carcinoma in situ and invasive carcinoma; (IV) an experienced cytopathologist is required for the collection and interpretation of specimens; and (V) there is no standard for the interpretation of FNAB results. While a similar five-category system is used in most European countries and the United States, a descriptive method is used in China without standardized interpretation criteria. Since there is no consensus over the diagnostic accuracy of FNAB either at home or abroad, confirmation of breast cancer is still based on the histopathology of the primary lesion. The value of FNAB in the diagnosis of breast masses is summarized in Table 1.
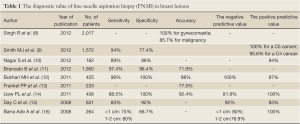
Full Table
Core needle biopsy (CNB)
A method to obtain sufficient specimens for histopathological diagnosis, CNB can not only distinguish between invasive cancer and carcinoma in situ, but also serve as an alternative to FNAC for diagnosis of a wide range of breast tumors (17). CNB is commonly used to provide a definitive diagnosis of breast cancer prior to neoadjuvant therapy. Some investigators have regarded the BARD biopsy gun as the most common CNB device, which uses a 14-gauge biopsy needle and is considered the gold standard for CNB. Gauge is a unit of measurement to describe the diameter of round in the North America. The larger the gauge, the smaller the diameter of the needle is. Diameters will increase/decrease by a factor of 2 every 6 gauges. The most common gauge, 14-gauge, represents a diameter of 1.62814 mm; and 8-gauge represents a diameter of 3.2639 mm.
In addition to similar indications as FNAB, CNB is recognized for use in: (I) patients with suspected malignant or uncertain lesions by FNAB; (II) patients negative for FNAB but with suspicious ultrasound and/or mammography findings; (III) patients for whom analysis is impossible due to inadequate samples collected by FNAB; (IV) breast lesions with microcalcifications; and (V) specimen collection for tissue banking for research purposes.
Diagnostic accuracy of CNB
A main difference between CNB and FNAC is the cross-sectional diameter of the puncture needle, which results in significantly different amounts of specimens they can collect. FNAC only collects very few cells and tissue fragments, and is unlikely to obtain a more complete tissue sample, making it a suboptimal diagnostic tool with low sensitivity and high false positive rates. CNB is able to obtain multiple tumor tissue samples, with an amount of up to 20 mg for each, which makes the diagnosis process easier based on complete tissue samples. It is reported that an accuracy rate of up to 90.1% can be achieved with the first sample collected by CNB, and this increases to 96.6% or higher with multiple sample collections, particularly in lesions less than 2 cm in diameter (18). With a limited amount of tissues and consequently limited section quality, intraoperative frozen biopsy may be prone to false positive responses, making the diagnosis difficult. In contrast, CNB results are based on paraffin sections with obviously less risk of this kind. CNB is also associated with remarkably less complications, compared with open biopsy. In clinical practice, the discomfort caused by CNB is limited to local pain and discomfort, and only a small number of patients may experience skin ecchymosis without significant hematoma or infection. Linebarger JH (19) statistically summarized the methods for diagnosis of breast cancer from 2007 to 2008, and found that, of the 360 newly diagnosed patients in the period, 350 patients (97%) were diagnosed by CNB, vacuum-assisted biopsy or other minimally invasive ways. The investigators believed that the minimally invasive techniques were efficient and accurate for diagnosing breast cancer and providing reliable guidance for the administration of treatment plans.
The accuracy of CNB is closely related to the tumor size and the amount of tissue samples collected, and it increases with the tumor diameter (20). It is recognized that at least four samples have to be collected with CNB to make a diagnosis (21). For cases with calcified lesions, some additional specimens are generally recommended, though there is no standard on the specific sample size. Hunt K et al. (22) suggested an amount of 10 specimens, all of which would be confirmed by imaging studies. For the diagnosis of ductal carcinoma in situ (DCIS), Wijeyaratne and colleagues (23) found that CNB had a detection rate for DCIS as high as that with BCS (breast conserving surgery)/mastectomy. Positive margins are present in many DCIS patients undergoing BCS. Therefore, a larger excision area is required to ensure a negative margin for patients with DCIS detected by CNB. The diagnostic sensitivity of the CNB for DCIS increases with the number of tissue samples collected.
Overall, the accuracy of CNB paraffin specimens is similar to that of pathological diagnosis based on surgically resected specimens (24,25).
CNB is also a reliable tool for revealing the ER, PR and HER-2 status of a given specimen. In regard to ER and PR diagnosis, Li S et al. (26) conducted a meta-analysis to evaluate the accuracy of CNB in identifying the expression of HR receptors in breast cancer. Twenty-one studies were included in the analysis, including 2,450 patients for detection of ER levels and 2,448 for detection of PR levels. The results showed a consistency of up to 92.8% between CNB and open surgery in ER diagnosis (K=0.78), and 85.2% in PR diagnosis (K=0.66). In the pooled data, the sensitivity and specificity of CNB were 97.3% and 82.0% for ER diagnosis, and 92.3% and 76.5% for PR diagnosis, respectively. The results translated to a positive summary likelihood ratio of 5.39% (95% CI, 2.92-9.97%) for ER and 3.93% (95% CI, 2.53-6.11%) for PR, and a negative likelihood ratio of 0.03% (95% CI, 0.02-0.05%) for ER and 0.10% (95% CI, 0.07% to PR) for PR, respectively. Li S and colleagues suggested that CNB was highly consistent with the pathological diagnosis of ER and PR based on surgical resection specimens, but a second open surgery would still be required for pathological diagnosis of cases negative for HR by CNB. However, after comparing the immunohistochemical results of ER and PR between 160 CNB and postoperative specimens, Uy et al. (27) found more negative results from the latter group, which were possibly contributed by surgical specimens that were not fixed in time. Therefore, they believed that CNB specimens were more reliable for detection of estrogen receptors. In a study of the HER-2 diagnostic accuracy, Tsuda H et al. (28) compared 100 pairs of formalin-fixed paraffin-embedded CNB specimens and surgical specimens of breast invasive cancer in terms of the diagnostic accuracy for HER-2. As a result, the concordance rates were 87% (K=0.77) and 94% (K=0.77) between CNB specimens and surgical specimens of the 3×3 and 2×2 categories in immunohistochemistry. Of the thirteen specimens with inconsistent findings, the HER-2 results were confirmed to be consistent in eleven by FISH. Therefore, the investigators believed that CNB was a reliable approach to collecting specimens for the diagnosis of HER-2.
Safety of CNB
It has been a subject of much discussion whether residual tumor cells are left along the needle tract after multiple CNB procedures, and whether they are associated with local recurrence and other safety considerations. Liebens (29) conducted a meta-analysis of 5,369 breast cancer patients undergoing CNB from 1900 to 2008, and found that although residual tumor cells were observed in 22% cases, the incidence of local recurrence was not increased consequently. The follow-up results confirmed that there was no statistical difference in the local recurrence rate between CNB patients and those receiving excision biopsy. At the same time, they also found that the incidence of residual tumor cells was independent of the size of the needle, but it was inversely proportional to the interval from CNB to surgery. However, the relationship with the number of needle biopsies and histological types was not clear. It is also a matter of concern whether CNB can give rise to distant metastasis. While investigators have found that circulating tumor cells are present in the blood and bone marrow before and during surgery, there is no evidence that they are there as a result of CNB, because surgery itself may also affect the shift and spread of tumor cells, and the presence of circulating tumor cells does not necessarily precipitate the occurrence of metastasis. Fitzal et al. (30) found that CNB did not affect the survival rate in patients receiving radiotherapy after BCS. Therefore, the current evidence suggests that CNB is not associated with increased probability of distant metastasis.
Imaging-guided CNB
Due to tissue heterogeneity in breast lesions, the reliability of needle biopsy depends on accurate collection of lesion samples, and the ability of these samples to represent the lesions are critical for the pathologist to make the correct diagnosis. The blind nature of CNB requires multiple biopsies to be performed, and is associated with a certain probability of underestimated and misdiagnosed results. Hence, sampling accuracy is essential for the success of this procedure. At present, ultrasound- or mammography-guided CNB is commonly performed for nonpalpable breast tumors or breast calcification to secure greater accuracy. In a single-center retrospective analysis of the results of ultrasound-guided 14 G hollow needle biopsy in 2,420 patients, Youk et al. (31) found 1,312 malignant cases confirmed by needle biopsy, subsequent excisional biopsy and follow-up (six were diagnosed during extended follow-up), and 1,256 malignant cases, 25 cases with high-risk lesions and 31 benign cases diagnosed by needle biopsy. According to their calculations, the false negative rate was 2.4% (31/1312), and the rates of histological underestimation were 29% (36/126) for carcinoma in situ (which was actually invasive cancer), 52% for atypical ductal hyperplasia (in fact carcinoma in situ or invasive cancer), and 17% for other high-risk lesions, respectively. As reported by Schueller et al. (32) in a single-center study of the results of ultrasound-guided 14 G hollow needle biopsy in 1,352 patients, in which 1,061 were confirmed by surgical specimens and 291 by follow-up, there were 671 cases of malignant diseases with a false negative rate of 1.6% (n=11). The rate of histological underestimation was 31.4% among the 86 high-risk lesions. Brancato et al. (11) reviewed the 5-year follow-up results of ultrasound-guided fine needle aspiration biopsy in 3,233 patients from 2003 to 2006, in which 1,950 received FNAC and 1,283 received CNB. The comparison revealed that CNB was a more reliable diagnostic approach for ultrasound-visible breast tumors, and could largely compensate for the unreliability and uncertainty of FNAC-based diagnoses.
The value of CNB in the diagnosis of breast masses is summarized in Table 2.
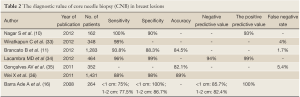
Full Table
Vacuum-assisted biopsy (VAB)
Vacuum-assisted biopsy (VAB) is a new imaging-guided biopsy approach introduced after fine needle aspiration cytology and core needle biopsy (CNB). Since it can completely resect small breast lesions and provide adequate, continuous tissue samples for pathological diagnosis via a single procedure while minimizing injury to breast tissues and maintaining the shape, it has been widely used for the early diagnosis of breast cancer and excision of benign lesions.
Common guiding technology
Selection of imaging guidance techniques is based on the visibility of lesions, difficulty of operation and cost. Commonly used approaches include stereotactic X-ray, high-frequency ultrasound and MRI.
Stereotactic X-ray
This technology is mainly used for nonpalpable lesions that are not detectable by ultrasound. Clusters of microcalcifications, structural distortion, small echogenic nodules and radial scars are the common manifestations of such lesions on a plain film. This easy-to-operate approach enables safe and precise positioning, and is particularly sensitive for microcalcifications. However, it is not applicable for small breasts (compressed thickness <2.7 cm) and lesions close to the armpit or to the chest wall. Dynamic tracking and real-time imaging is not available during the positioning process.
High frequency ultrasound
This non-radioactive, real-time guiding approach is mainly used for the diagnosis of ultrasound-visible BI-RADS 3-5 breast lesions and axillary involvement, which has certain advantages in the patient comfort, operating time, and cost. It has become the most accurate and preferred guidance modality for minimally invasive breast biopsy. Highly dependent on the patient’s cooperation, however, it is associated some difficulties in revealing microcalcifications in lesions and operating in the areola area.
Breast MRI
This is mainly used to display suspicious malignant lesions that are only visible to MRI and undetectable by X-ray, ultrasound or other testing methods. Additionally, it can be used to exclude the presence of malignancy in the case of suspected benign disease based on prior MRI examination (37). Compared with 1T and 1.5T MRI, 3T MRI has a higher sensitivity for breast cancer (38). Meeuwis and colleagues (39) indicated an underestimation rate of 6% with 3T-MRI-guided CNB histology, and no underestimated results were observed using 3T-MRI guided VAB.
Accuracy of VAB
Suitable for a wide range of applications, VAB is able to obtain sufficient and continuous histological specimens, allowing higher diagnostic accuracy and specificity, and is thus considered to be an ideal alternative to surgical biopsy. Carbognin et al. (40) performed MRI-guided VAB for 29 patients using 10G needles, resulting in a procedural success rate of 93.1%, a false negative rate of 4%, a histological underestimation rate of 4%, sensitivity and specificity of 92% and 100%, respectively, and positive and negative predictive values of 100% and 93%, respectively.
As for the detection of ER, PR and HER-2, the study showed high correlation and consistency between the parameters generated by VAB specimens and surgical biopsy specimens. Having compared the immunohistochemical results between VAB and surgical biopsy specimens, the investigators found better representation of ER and PR expression by VAB specimens than the corresponding surgical specimens (41).
Safety of VAB
Using a double-lumen catheter, VAB is completed in a way that specimens are never in contact with the biopsy channel and/or incision, which reduces the risk of tumor cell migration in channel or incision, or blood metastasis. Diaz et al. (42) observed needle track dissemination in 23% surgical specimens after VAB. It was also found that the incidence and number of cancer metastases after CNB was negatively correlated with the time from biopsy to surgical resection. The local recurrence rate did not increase with needle track dissemination after comprehensive treatment, suggesting that the tumors cells hardly survived in the needle channel. Michalopoulos et al. (43) resected 21 cases of ductal carcinoma in situ and 10 cases of invasive ductal carcinoma using VAB, and no dissemination was found in the biopsy channel. It should also be noted that an optimal surgical path designed to accommodate the maximum lesion length and properly shorten the channel length is the key to reducing the spread of tumor cells. Liberman et al. (44) compared the local recurrence rates in patients receiving core needle biopsy and fine needle aspiration for positioning and excision of breast cancer. Breast-conserving resection was performed for all patients, and the local recurrence rates were 3.70% and 3.96%, respectively, without statistically significant difference, suggesting that the local recurrence rate was not increased with needle track dissemination of tumor cells.
Limitations of VAB
Currently, VAB is mainly used for the biopsy of nonpalpable masses and calcifications, as well as excision of benign tumors (<3 cm in diameter). As with CNB, however, VAB is inevitably associated with an underestimation rate. A biopsy of microcalcifications may be very likely to be underestimated as ADH or DCIS. Zagouri et al. (45) performed VAB testing of microcalcifications, and the underestimation rates were 10.8% and 8.3% for DCIS and ADH, respectively. Varying degrees of histological underestimate are present in the diagnoses of ductal carcinoma in situ with early invasion, ductal carcinoma in situ and dysplasia via VAB, which are related with the histologic heterogeneity, size and shape of calcification, and selected lesions for the biopsy. To provide guidance for treatment decisions, Houssami and colleagues (46) established a model for DCIS patients diagnosed via stereotactic vacuum-assisted core needle biopsy (SVAB) according to the imaging scope and histological nuclear grading of their microcalcifications to evaluate the probability of a pathological diagnosis of invasive cancer based on their surgical specimens.
In the VAB procedure, lesions can not be obtained without being divided into strips. The lack of an en bloc sample makes it difficult for accurate measurement of the lesion size and scope after the procedure. In addition, VAB is associated with postoperative major complications such as pain, bleeding, skin bruising, and hematoma.
Since collection of a large amount of tissue or even complete resection of a large tumor is possible with VAB, investigators have begun to explore its clinical value for removal of small breast lesions (<0.5 cm in diameter). A recognized opinion is that complete resection shown on imaging studies does neither represent pathologically complete resection nor ensure a negative margin. Therefore, VAB is not suitable for resection of malignant tumors (47). Penco et al. (48) noted that, for any breast malignancy, VAB could serve a diagnostic purpose only rather than a therapeutic one, even if the suspicious calcifications had been completely resected. The key to successful breast-conserving surgery is a negative margin, but there is yet to be a standard definition of the distance. It is generally agreed that resection of breast tissues 1 cm from the primary tumor is required to obtain an microscopic negative margin of at least 2 mm. If this requirement is not met when using VAB, the metastasis of malignant cells is possible. In a related study, extended resection was performed for those pathologically confirmed as malignant out of 1,016 patients who had undergone VAB. Tumor cells were found in the surgical residual cavities of specimens from 882 patients (86.8%) (49). Lee et al. (50) used MRI-guided VAB to completely remove 22 cases of malignant breast lesions, and found tumor cells in the residual cavities of 14 cases. The above results suggest that complete excision does not guarantee histological negativity. However, some investigators take the opposite view. Villa and colleagues (51) performed VAB for 1,173 patients using the 11G needle. Atypical ductal hyperplasia (ADH) was confirmed in 114 patients with suspicious clustered microcalcifications, and 41 of the 49 patients with residual calcification received surgical biopsy. Eight of them were confirmed as malignant lesions, with a histological underestimate rate of 20%. Of the 65 patients without residual calcification, 26 were confirmed free of malignant lesions by surgical biopsy; only 1 of 35 patients undergoing conservative treatment was found to have malignancy through X-ray follow-up, with an underestimate rate of 1.6%. This study shows conservative treatment with X-ray follow up is feasible in patients who have no residual calcification upon VAB examination. However, it remains a pressing issue to identify a proper way to confirm complete removal of breast malignant lesions by VAB.
VAB devices
Mammotome and VaCora are the most commonly used vacuum-assisted biopsy devices in clinical settings, which are primarily based on the CNB technology.
Mammotome
The Mammotome minimally invasive biopsy system was developed by Burbank et al. (52) in 1994, and approved for clinical application by the FDA in April 1995 (53). It is a minimally invasive surgical device consisting of a rotating knife and a vacuum suction pump. Patients with solid breast masses confirmed by X-ray or ultrasound can receive monitoring-guided biopsy with Mammotome, which collects tissue samples using vacuum-assisted aspiration. This system allows repeated resection and collection of samples via a single needle procedure, while none of the specimens are in contact with the needle channel, effectively reducing the probability of tumor cell spread. Compared with CNB, the system has obvious advantages as it enables ultrasound-guided resection of tumors, particularly deep and small lesions that are not palpable by clinical assessment; precise lesion localization; adequate sample size, about 10-fold the amount with traditional CNB in a single procedure; less histological underestimation; fewer complications; and labeling of the biopsy site with a titanium clip or cyclopenthiazide gel clip for ease of follow-up studies. The diagnostic accuracy is much higher than that of core needle or open surgical biopsy. More importantly, by collecting complete and continuous tumor specimens, it yields consistent pathological and immunological results with those generated via traditional surgical specimens, providing accurate diagnostic basis for minimally invasive treatment of primary breast cancer at the early stage, thus avoiding surgery and maximizing the breast cosmetic outcome by retaining the shape and appearance more completely (54). It serves diagnostic and therapeutic purposes for benign breast lesions and severe proliferative lesions, respectively, and provides adequate specimens (8 times the amount of traditional core needle biopsy) for detection of a variety of tumor markers. The Mammotome system enables definite diagnosis of suspicious breast lesions, thereby reducing the rate of surgery for benign breast lesions. As shown in a comparison with postoperative specimens of primary breast cancer, specimens collected by this system yield an accuracy of 97.2% in pathological diagnosis and detection of ER, PR, Bcl-2 and p53. It is globally accepted that MRI is superior to other imaging studies in diagnostic accuracy of breast masses. Currently, investigators have begun to apply MRI-guided MMT biopsy in foreign countries (39,55). Luo et al. (56) performed Mammotome-based excision for 2,167 benign lesions of <10 mm, 10-19 mm, 20-29 mm and >29 mm in diameter, and found that VAB was a safe, feasible and easily accepted approach for resecting benign tumors smaller than 3 cm.
Vacora
In 2001, the Vaeora biopsy device produced by Bard has been gradually recognized by clinicians. The device was able to complete all resection procedures in a single needle setup, but the sampling chamber was unadjustable and the design was not as compact as it is today. With its two-sided-motion cutting knife, the Vacora biopsy device is now especially suitable for complete resection strong fibrotic lesions. The compact, handheld-enabled device is compatible with MRI guidance and equipped with an adjustable sample chamber. However, once the outer catheter is inserted in place, the internal core needle has to be withdrawn multiple times for resection of specimens.
INTECT
In view of the limitations of MMT, researchers have invented a new technology - INTECT® Breast Lesion Excision System (BLES). In contrast to the MMT technology, INTECT® BLES acquires the whole tumor by a single puncture, which avoids localized bleeding within the tumor and thus reduces the risk of blood dissemination of malignant cells. The sample obtained by this technology is complete with intact structure and clear boundary, facilitating a more accurate and efficient pathological assessment and comparison. In addition, it is highly correlated with mammography and can be used for pathology analysis. This technology is still under research on early-phase trials. Killebrew and Oneson from Oklahoma Breast Care Center were the first to evaluate this method in 2006 (57). As shown by the results, INTECT® BLES provides accurate pathological results of a breast biopsy with extremely high accuracy and higher diagnostic rate for intraductal carcinoma compared with core needle biopsy. The trials provided the initial basis for its application.
With the technological advances of mammography, ultrasound, MRI and other testing methods, an increasing number of nonpalpable breast lesions have become detectable via early imaging studies. Imaging-guided biopsy technology is being gradually applied in place of surgical excision biopsy as an important mean for the diagnosis of breast disease and also a treatment option of benign breast lesions.
Acknowledgements
This study was supported by National Natural Science Foundation of China (No. 81150011).
Disclosure: The authors declare no conflict of interest.
References
- Salvadori B, Cusumano F, Del Bo R, et al. Surgical treatment of phyllodes tumors of the breast. Cancer 1989;63:2532-6.
- Frei KA, Bonel HM, Pelte MF, et al. Paget disease of the breast: findings at magnetic resonance imaging and histopathologic correlation. Invest Radiol 2005;40:363-7.
- Morrogh M, Morris EA, Liberman L, et al. MRI identifies otherwise occult disease in select patients with Paget disease of the nipple. J Am Coll Surg 2008;206:316-21.
- Krag DN, Anderson SJ, Julian TB, et al. Technical outcomes of sentinel-lymph-node resection and conventional axillary-lymph-node dissection in patients with clinically node-negative breast cancer: results from the NSABP B-32 randomised phase III trial. Lancet Oncol 2007;8:881-8.
- Liberman L. Percutaneous image-guided core breast biopsy. Radiol Clin North Am 2002;40:483-500, vi.
- Kocjan G, Bourgain C, Fassina A, et al. The role of breast FNAC in diagnosis and clinical management: a survey of current practice. Cytopathology 2008;19:271-8.
- Ahmed Q, Arpin R, Pitman M, et al. Fine-Needle Aspiration Biopsy as the Initial Diagnostic Procedure in Palpable Breast Lesions. J Am Soc Cytopathol 2012;1:S5.
- Singh R, Anshu, Sharma SM, et al. Spectrum of male breast lesions diagnosed by fine needle aspiration cytology: a 5-year experience at a tertiary care rural hospital in central India. Diagn Cytopathol 2012;40:113-7.
- Smith MJ, Heffron CC, Rothwell JR, et al. Fine needle aspiration cytology in symptomatic breast lesions: still an important diagnostic modality? Breast J 2012;18:103-10.
- Nagar S, Iacco A, Riggs T, et al. An analysis of fine needle aspiration versus core needle biopsy in clinically palpable breast lesions: a report on the predictive values and a cost comparison. Am J Surg 2012;204:193-8.
- Brancato B, Crocetti E, Bianchi S, et al. Accuracy of needle biopsy of breast lesions visible on ultrasound: audit of fine needle versus core needle biopsy in 3233 consecutive samplings with ascertained outcomes. Breast 2012;21:449-54.
- Bukhari MH, Arshad M, Jamal S, et al. Use of fine-needle aspiration in the evaluation of breast lumps. Patholog Res Int 2011;2011:689521.
- Frankel PP, Esteves VF, Thuler LC, et al. Diagnostic accuracy of the fine needle aspiration cytology and core needle biopsy as a diagnostic method for breast lesions. Rev Bras Ginecol Obstet 2011;33:139-43.
- Liew PL, Liu TJ, Hsieh MC, et al. Rapid staining and immediate interpretation of fine-needle aspiration cytology for palpable breast lesions: diagnostic accuracy, mammographic, ultrasonographic and histopathologic correlations. Acta Cytol 2011;55:30-7.
- Day C, Moatamed N, Fimbres AM, et al. A retrospective study of the diagnostic accuracy of fine-needle aspiration for breast lesions and implications for future use. Diagn Cytopathol 2008;36:855-60.
- Barra Ade A, Gobbi H, de L Rezende CA, et al. A comparision of aspiration cytology and core needle biopsy according to tumor size of suspicious breast lesions. Diagn Cytopathol 2008;36:26-31.
- Goyal A. Current Trends in Breast Surgery. Indian J Surg Oncol 2012;3:287-91.
- de Lucena CE, Dos Santos Júnior JL, de Lima Resende CA, et al. Ultrasound-guided core needle biopsy of breast masses: How many cores are necessary to diagnose cancer? J Clin Ultrasound 2007;35:363-6.
- Linebarger JH, Landercasper J, Ellis RL, et al. Core needle biopsy rate for new cancer diagnosis in an interdisciplinary breast center: evaluation of quality of care 2007-2008. Ann Surg 2012;255:38-43.
- Park SM, Lee DW, Jin SY, et al. Fine-needle aspiration cytology as the first pathological diagnostic modality in breast lesions: A comparison with core needle biopsy. Basic Applied Pathology 2010;3:1-6.
- Tamaki K, Sasano H, Ishida T, et al. Comparison of core needle biopsy (CNB) and surgical specimens for accurate preoperative evaluation of ER, PgR and HER2 status of breast cancer patients. Cancer Sci 2010;101:2074-9.
- Hunt K, Robb G, Strom E, et al. eds. Breast Cancer. 2nd ed. New York: Springer Science, 2008:168-9.
- Wijeyaratne DK, Lokuhetty MD, Anthony D. Implications of diagnosing ductal carcinoma in situ in core needle biopsies of the breast. Journal of Diagnostic Pathology 2011;6:28-33.
- Mueller-Holzner E, Frede T, Daniaux M, et al. Ultrasound-guided core needle biopsy of the breast: does frozen section give an accurate diagnosis? Breast Cancer Res Treat 2007;106:399-406.
- Ljung BM, Drejet A, Chiampi N, et al. Diagnostic accuracy of fine-needle aspiration biopsy is determined by physician training in sampling technique. Cancer 2001;93:263-8.
- Li S, Yang X, Zhang Y, et al. Assessment accuracy of core needle biopsy for hormone receptors in breast cancer: a meta-analysis. Breast Cancer Res Treat 2012;135:325-34.
- Uy GB, Laudico AV, Carnate JM Jr, et al. Breast cancer hormone receptor assay results of core needle biopsy and modified radical mastectomy specimens from the same patients. Clin Breast Cancer 2010;10:154-9.
- Tsuda H, Kurosumi M, Umemura S, et al. HER2 testing on core needle biopsy specimens from primary breast cancers: interobserver reproducibility and concordance with surgically resected specimens. BMC Cancer 2010;10:534.
- Liebens F, Carly B, Cusumano P, et al. Breast cancer seeding associated with core needle biopsies: a systematic review. Maturitas 2009;62:113-23.
- Fitzal F, Sporn EP, Draxler W, et al. Preoperative core needle biopsy does not increase local recurrence rate in breast cancer patients. Breast Cancer Res Treat 2006;97:9-15.
- Youk JH, Kim EK, Kim MJ, et al. Sonographically guided 14-gauge core needle biopsy of breast masses: a review of 2,420 cases with long-term follow-up. AJR Am J Roentgenol 2008;190:202-7.
- Schueller G, Jaromi S, Ponhold L, et al. US-guided 14-gauge core-needle breast biopsy: results of a validation study in 1352 cases. Radiology 2008;248:406-13.
- Wiratkapun C, Treesit T, Wibulpolprasert B, et al. Diagnostic accuracy of ultrasonography-guided core needle biopsy for breast lesions. Singapore Med J 2012;53:40-5.
- Lacambra MD, Lam CC, Mendoza P, et al. Biopsy sampling of breast lesions: comparison of core needle- and vacuum-assisted breast biopsies. Breast Cancer Res Treat 2012;132:917-23.
- Gonçalves AV, Thuler LC, Kestelman FP, et al. Underestimation of malignancy of core needle biopsy for nonpalpable breast lesions. Rev Bras Ginecol Obstet 2011;33:123-31.
- Wei X, Li Y, Zhang S, et al. Experience in large-core needle biopsy in the diagnosis of 1431 breast lesions. Med Oncol 2011;28:429-33.
- Rauch GM, Dogan BE, Smith TB, et al. Outcome analysis of 9-gauge MRI-guided vacuum-assisted core needle breast biopsies. AJR Am J Roentgenol 2012;198:292-9.
- Meeuwis C, Veltman J, van Hall HN, et al. MR-guided breast biopsy at 3T: diagnostic yield of large core needle biopsy compared with vacuum-assisted biopsy. Eur Radiol 2012;22:341-9.
- Meeuwis C, Veltman J, van Hall HN, et al. MR-guided breast biopsy at 3T: diagnostic yield of large core needle biopsy compared with vacuum-assisted biopsy. Eur Radiol 2012;22:341-9.
- Carbognin G, Girardi V, Brandalise A, et al. MR-guided vacuum-assisted breast biopsy in the management of incidental enhancing lesions detected by breast MR imaging. Radiol Med 2011;116:876-85.
- Zagouri F, Sergentanis TN, Nonni A, et al. Comparison of molecular markers expression in vacuum-assisted biopsies and surgical specimens of human breast carcinomas. Pathol Res Pract 2010;206:30-3.
- Diaz LK, Wiley EL, Venta LA. Are malignant cells displaced by large-gauge needle core biopsy of the breast? AJR Am J Roentgenol 1999;173:1303-13.
- Michalopoulos NV, Zagouri F, Sergentanis TN, et al. Needle tract seeding after vacuum-assisted breast biopsy. Acta Radiol 2008;49:267-70.
- Liberman L, Smolkin JH, Dershaw DD, et al. Calcification retrieval at stereotactic, 11-gauge, directional, vacuum-assisted breast biopsy. Radiology 1998;208:251-60.
- Zagouri F, Sergentanis TN, Nonni A, et al. Vacuum-assisted breast biopsy: the value and limitations of cores with microcalcifications. Pathol Res Pract 2007;203:563-6.
- Houssami N, Ambrogetti D, Marinovich ML, et al. Accuracy of a preoperative model for predicting invasive breast cancer in women with ductal carcinoma-in-situ on vacuum-assisted core needle biopsy. Ann Surg Oncol 2011;18:1364-71.
- Cusumano P, Polkowski WP, Liu H, et al. Percutaneous tissue acquisition: a treatment for breast cancer? Vacuum-assisted biopsy devices are not indicated for extended tissue removal. Eur J Cancer Prev 2008;17:323-30.
- Penco S, Rizzo S, Bozzini AC, et al. Stereotactic vacuum-assisted breast biopsy is not a therapeutic procedure even when all mammographically found calcifications are removed: analysis of 4,086 procedures. AJR Am J Roentgenol 2010;195:1255-60.
- He Q, Fan X, Guan Y, et al. Percutaneous excisional biopsy of impalpable breast lesions under ultrasound visualization. Breast 2008;17:666-70.
- Lee JM, Kaplan JB, Murray MP, et al. Complete excision of the MRI target lesion at MRI-guided vacuum-assisted biopsy of breast cancer. AJR Am J Roentgenol 2008;191:1198-202.
- Villa A, Tagliafico A, Chiesa F, et al. Atypical ductal hyperplasia diagnosed at 11-gauge vacuum-assisted breast biopsy performed on suspicious clustered microcalcifications: could patients without residual microcalcifications be managed conservatively? AJR Am J Roentgenol 2011;197:1012-8.
- Burbank F, Parker SH, Fogarty TJ. Stereotactic breast biopsy: improved tissue harvesting with the Mammotome. Am Surg 1996;62:738-44.
- Chun K, Velanovich V. Patient-perceived cosmesis and satisfaction after breast biopsy: comparison of stereotactic incisional, excisional, and wire-localized biopsy techniques. Surgery 2002;131:497-501.
- Steyaert L, Van Kerkhove F, Casselman JW. Sonographically guided vacuum-assisted breast biopsy using handheld mammotome. Recent Results Cancer Res 2009;173:43-95.
- Daniel BL, Freeman LJ, Pyzoha JM, et al. An MRI-compatible semiautomated vacuum-assisted breast biopsy system: initial feasibility study. J Magn Reson Imaging 2005;21:637-44.
- Luo HJ, Chen X, Tu G, et al. Therapeutic application of ultrasound-guided 8-gauge Mammotome system in presumed benign breast lesions. Breast J 2011;17:490-7.
- Killebrew LK, Oneson RH. Comparison of the diagnostic accuracy of a vacuum-assisted percutaneous intact specimen sampling device to a vacuum-assisted core needle sampling device for breast biopsy: initial experience. Breast J 2006;12:302-8.


