Enhancing breast projection in autologous reconstruction using the St Andrew’s coning technique and 3D volumetric analysis
Introduction
The number of patients undergoing both prophylactic and therapeutic mastectomy has grown exponentially in the last decade due to the increased availability of genetic testing and breast cancer surveillance (1-4). For post-mastectomy patients, autologous breast reconstruction has shown to significantly improve their psychosexual well-being and has subsequently become an integral part of the breast cancer treatment (5-7). Using advanced imaging modalities, such as computed tomographic angiography (CTA), as a routine preoperative planning tool, deep inferior epigastric artery perforator (DIEP) free flap reconstruction has become a safe, reliable surgical technique, associated with enhanced clinical outcomes and patient satisfaction (8-13). In contrast to the vast number of studies investigating the vascular anatomy and imaging of the abdominal wall, the number of studies exploring various surgical techniques to enhance the aesthetic outcome of DIEP flaps is substantially fewer.
The influence of several preoperative factors on the aesthetic outcome of DIEP flaps has been documented, such as adjuvant radiotherapy, the timing of reconstruction, the choice of donor site, and the role of appropriate symmetrization procedure to the contralateral breast. However, an individual surgeon’s ability to design a flap and manipulate it in three-dimension (3D) to fashion a patient-specific breast mound with appropriate shape and projection is ultimately one of the most important factors. To this effect, Blondeel et al. have outlined a “three-step” approach that can be applied systematically when shaping and insetting a DIEP flap in any clinical case: prepare the breast footprint, shape the conus, and cover the flap with appropriate skin envelope (14). Since then, a number of studies have published methods to improve the preparation of breast footprint and safe, reliable skin envelope by incorporating the breast aesthetic subunit principle and the “dual-plane” insetting techniques (15-20). In contrast, it is challenging to report in writing how a surgeon transforms a 2D flap into a 3D structure and only a small number of authors have described their technique of shaping the breast conus (14,21-23).
Blondeel et al. have outlined their “three-suture” technique for shaping DIEP flap, where the Scarpa’s fascia is first sutured to the pectoralis fascia acing the flap under the pectoralis tendon, the second suture holds the lateral edge of the flap to the lateral inframammary fold (IMF) in tension, and the last suture is placed medially to form a smooth medial cleavage (14). In bilateral reconstruction, Nahabedian describes a similar technique of ensuring appropriate flap projection with sutures at the medial and lateral edges, and additional superomedial, inferomedial or lateral sutures as required (21). However, in unilateral reconstruction, the author either “rolls” the flap into a cone or creates a lateral fold so that the zone 2 is placed under the zone 1 to provide projection (21). Using the conical folding technique in 126 unilateral reconstructions, Wang et al. demonstrated satisfactory projection and volume at 6-month follow-up (22).
Recently, Tomita et al. have exploited the growing accessibility and availability of imaging technologies, such as 3D photography, and 3D printing to assist preoperative planning and guide intraoperatively flap shaping and insetting in unilateral reconstructions (23). Using a commercial 3D scanner, the authors have scanned the breast region and created a mirrored image of the unaffected contralateral breast. This was 3D-printed as a mould and intraoperatively, the flap was placed inside it to trim and shape to fit. They report subjective assessment of satisfactory symmetrization and project at 2-month follow-up.
In recent times, reports using 3D photography, also known as 3D scanning or surface imaging, for volumetric analysis in breast reconstruction has been growing (24-37). Compared to the conventional imaging modalities, such as CT scans, 3D photography forgoes radiation exposure and a growing number of investigators have demonstrated its accuracy and reliability in breast application (26-28,32,35-37). Three types of 3D photography are most commonly utilized in medical application: laser imaging, structured light technique, and stereophotogrammetry. Based on passive stereophotogrammetry, VECTRA XR scanners (Canfield Scientific, Fairfield, NJ, USA) are arguably one of the most frequently investigated 3D scanners (35-37).
3D printing, also known as additive manufacturing or rapid prototyping, is a novel technology that can fabricate haptic biomodels of patient-specific anatomical structures using various imaging sources, such as 3D photography, CT scan, and magnetic resonance imaging (MRI) (38-40). In the last decade, 3D printers have become more affordable and convenient to use. In breast reconstruction, clinicians have used 3D printing to demonstrate breast volume differential both qualitatively and quantitatively for preoperative planning (41).
In the current study, we describe our St Andrew’s coning technique for the first time, where circular rounds of suture are placed within the cutaneous layer of DIEP flaps to create projection. We have used a 3D-printed model of the mirror image of the contralateral breast on the operating table to help determine desired flap projection prior to insetting the flap. We detail our suturing technique in a prospective case series of three delayed unilateral breast reconstructions where symmetrization to the contralateral breast was not indicated.
Methods
Patients
We describe the coning technique used on 1,000 consecutive patients, with the volumetric analyses detailed having been performed in a prospective case series of 3 female patients who underwent delayed unilateral autologous breast reconstruction with DIEP flaps at St Andrew’s Centre for Plastic Surgery and Burns, Chelmsford, UK. The mean age of the patients was 54 (range, 43 to 63) years. The inclusion criteria comprised unilateral reconstructions, in patients for who no contralateral symmetrisation procedure was planned, such as a reduction or augmentation mammoplasty. All procedures were performed by a single surgeon (VV).
3D photography
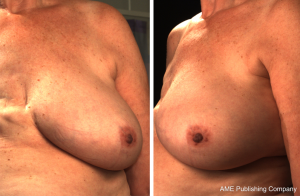
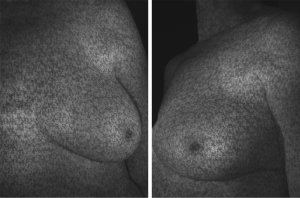
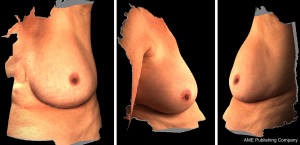
3D photography of the breast region was taken in all patients during the routine preoperative work-up using a commercial 3D scanner, VECTRA XR scanner (Canfield Scientific). Patients were asked to stand with their arms in varying positions and each scanning took approximately 1–2 seconds. Photographs were created in both standard format and pixelated format to highlight surface contour (see Figures 1 and 2). After waiting less than 5 minutes for image processing, a 3D image of the bilateral breasts was created (see Figure 3), able to manipulated in a multi-planar fashion as required, and exported from the proprietary software in a Standard Tessellation Language (STL) file format to be prepared for 3D printing.
3D printing
Firstly, the 3D image was cropped to isolate the contralateral breast and then, mirrored using the Magics software (Materialise NV, Leuven, Belgium). The file was exported and converted into a printer-friendly file using the MakerBot Desktop software (MakerBot Industries, New York, NY, USA). Subsequently, the mirrored images were 3D-printed using the MakerBot Z18 3D printer (MakerBot Industries), which took mean 10.5 hours per patient (range, 8.5–12 hours). The final products were immediately useful after removing from the printer’s build plate (see Figure 4).

Coning technique
The surgical coning technique began after completion of flap harvest, after disconnection of the vascular pedicle. The breast base is transcribed onto the skin surface of the flap (see Figure 5A), in order to plan the central focus of coning for maximum projection. The non-projected flap can be highlighted at this point (see Figure 5B).
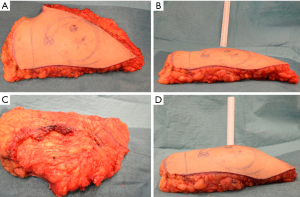
The flap under-surface is used for placement of the coning sutures. A multi-filament, absorbable suture is placed through the sub-Scarpa’s fat, and a continuous loose purse-string technique is used in this fashion to encircle the planned breast base, before tying the suture loosely (see Figure 5C). The sutures are placed superficially in order to avoid deep penetration which may occlude intra-flap vasculature. Two to three further such purse-string rings are created as needed, in increasing diameters from the initial purse-string, in order to create the desired amount of projection (see Figure 5D).
Results
In all cases, a demonstrable increase in central flap projection was achieved. The mean increase in central flap projection was 2.5 cm (range, 2–3.5 cm), achieving over 100% increases in central flap projection (see Figure 2).
There were no partial or complete flap failures, and no complications recorded attributed to the St Andrew’s coning technique, or otherwise. In fact, no complications in the authors preceeding 1,000 cases of using this technique were attributable to the coning technique described.
Discussion
We describe the St Andrew’s coning technique, where we supinate the DIEP flap after the flap harvest and place rounds of dissolvable sutures in the adipose tissue prior to the flap inset, in order to create an appropriate conus. We have individualized each flap to achieve optimal symmetrization by utilizing the 3D-printed haptic models of the mirrored image of the contralateral breast to adjust the suturing.
With increasing population, the rate of prevalence and incidence of breast cancer continues to rise and, in the United States alone, one in eight women will be affected by the disease in their lifetime (42). Furthermore, an increasing number of women are receiving both therapeutic and prophylactic mastectomy for breast cancer, boosted by the increased availability of genetic testing (1-4). Encouragingly, post-mastectomy breast reconstruction has demonstrated to significantly improve the psychosexual wellbeing of patients and, as a result, it has become an integral part of the comprehensive treatment of breast cancer (5-7). Unlike synthetic implants, autologous reconstruction with abdominal wall as donor site yields a more natural-appearing and a longer-lasting outcome, without encountering the conventional prosthesis-related complications (43-46). Compared to the earlier TRAM flaps, DIEP flaps can provide adequate volume replacement, without significantly compromising the donor site to ventral bulge or hernia (47,48). Advancement of modern imaging technologies, especially the establishment of CTA as a routine preoperative planning tool in DIEP flap reconstruction, has enabled surgeons to select appropriate perforators and flap designs, leading to improved clinical outcomes (8-13).
In contrast to the studies investigating the vascular anatomy and imaging of the abdominal wall (12,24,49-55), there is a relative paucity of studies exploring surgical techniques to enhance aesthetic outcome in DIEP flap reconstructions. Several preoperative considerations are considered critical in order to ensure aesthetically satisfactory result, such as adjuvant radiotherapy, the timing of reconstruction, choice of donor site, and symmetrization of the contralateral breast via augmentation or reduction mammoplasty depending on the patient preference (21). However, the aesthetic outcome of DIEP flaps ultimately relies on an individual surgeon’s technique and experience in flap shaping and insetting.
To this effect, Blondeel et al. have presented a systematic approach that reproducibly produces aesthetically-pleasing breast mound with DIEP flaps in any clinical situation: firstly, prepare the breast footprint; secondly, shape the conus; and lastly, cover the flap with suitable skin envelope (14). Creating the breast footprint and the skin envelope requires defining the IMF and replacing the mastectomy scar with aesthetic lines applying the breast aesthetic subunit principle (15). Initially, devised for planning nasal and facial reconstructive surgeries (56-59), aesthetic subunit principle has also demonstrated to improve the cosmetic outcome and patient satisfaction when applied in autologous breast reconstructions (16-18). Recently, in a direct comparison study, Gravvanis et al. have reported that insetting DIEP flaps as a single aesthetic subunit, where the inferior mastectomy skin flap is deepithelialized and overlaid by the free flap so that a new IMF is defined using the edge of the free flap, yields a more natural shape and a satisfactory scar pattern than flap insetting as a double subunit, where the IMF is preserved (19). Similarly, when the mastectomy skin envelope is compromised due to radiation or is lacking in volume due to the reconstruction being delayed, this can be overcome by insetting the DIEP flap in “dual-plane”, below and above the pectoral muscle in the upper and lower pole respectively (20).
In contrast, only a few techniques of shaping the breast conus have been reported to date and the evidence is limited to sporadic case series and descriptive accounts (14,21-23). As Blondeel et al. points out this is likely attributable to the fact that describing how to manipulate a 2D structure (i.e., DIEP flap) into a 3D form (i.e., conus) is difficult to achieve in writing (14). Furthermore, the ability to think and handle tissue in 3D is considered a key skill of a well-trained plastic surgeon and the significance of its discussion may be overlooked (14). Nonetheless, the shape and the projection of an ideal conus is patient-specific and is critical towards achieving an aesthetically pleasing outcome and ensuring a high quality of life. Blondeel et al. describe a “three-suture” technique where the first key suture connects the Scarpa’s fascia to the pectoralis fascia and positions the DIEP flap underneath the pectoralis tendon. The second suture is used to affix the lateral edge of the flap to the most lateral edge of the IMF in tension. The last one is placed in the medial edge of the IMF to create a smooth medial cleavage (14). Similarly, Nahabedian reports his technique of securing medial and lateral edges of the free flap to the sternal border and the lateral chest wall respectively, in bilateral breast reconstructions, with superomedial, inferomedial and lateral sutures (21). In unilateral reconstructions, Nahabedian uses the contralateral breast as a template and folds the free flap either in a conical fashion or creates a lateral fold where the zone 2 is placed under the zone 1 and secured with a lateral suture (21). In 2015, Wang et al. report a retrospective case series of 126 unilateral DIEP reconstructions where the flap is folded into a cone before insetting (22). Regardless of the shape in which the flaps were harvested—fusiform, semi-circle, or crescent—the authors have noted satisfactory breast projection and volume at 6-month follow-up. Interestingly, they have not reported patient satisfaction or quality of life data.
Recently, Tomita et al. have presented an algorithm incorporating the use of novel 3D scanning technology to guide surgeons shape patient-specific DIEP flaps in a prospective cohort of 11 delayed unilateral reconstructions (23). Preoperatively, the breast region is scanned with the patient sitting up, using a commercial 3D scanner, David Structured Light Scanner SLS-1 (David Vision Systems GmbH, Koblenz, Germany), from which the required flap volume is calculated. In addition, the scanned image of the contralateral breast is mirrored and 3D-printed as a mould. On the operating table, after the microvascular anastomosis, the flap is carefully placed inside the sterilized mould, from where it was trimmed to fit and the shape was secured with randomly-placed dissolvable sutures. The authors report good symmetrization and cosmetic effect at 2-month follow-up. One of the major limitations of this technique is that it is only applicable in unilateral reconstructions where the contralateral breast does not require a symmetrization procedure. Furthermore, the hard plastic mould does not account for postoperative swelling and facilitate “over-correction” of the volume of the reconstructed breast. Interestingly, the authors do not report the thickness of the breast mould, which may have led to further “under-correction” of the reconstruction. In comparison, the St Andrew’s coning sutures are readily reproducible and are flexible enough for the surgeon to tailor the technique to accommodate individual differences. In our series, none of our patients required a symmetrization procedure to the contralateral breast and we utilized the 3D-printed mirror image from 3D photography of the contralateral side to help surgeons guide placement of the St Andrew’s coning sutures.
3D photography captures the light reflected off a surface to construct a virtual 3D model (24). Originally used in automotive and aerospace industries, Galdino et al. first used the technology in plastic surgery to quantitatively assess breast symmetry (25). Similar to other medical imaging modalities, 3D surface imaging has advanced significantly in the last decade and is accurate, reliable, and simple to use (26-28). In clinical application for breast volumetric analysis and imaging, three 3D scanning techniques have been studied: laser imaging, structured light technique, and stereophotogrammetry. 3D laser imaging projects a particular pattern of laser (i.e., spot or stripe) and utilizes the triangulation calculation method to determine a 3D coordinate (28,60). Konica Minolta 3D scanner (Konica Minolta Inc., Tokyo, Japan), one of the most extensively studied clinical laser scanner, has been utilized in planning implant breast reconstructions (30). However, its accuracy and validity has not yet been reported. Likewise, structured light technique projects an organised pattern of light (i.e. stripe, grid, or dots) and captures the distortion in the light pattern using multiple calibrated cameras to derive at 3D surface data (27,31). Axis Three Torso System (Axis Three, Miami, FL, USA) is the most frequently reported structured light 3D scanner and has demonstrated its accuracy and reliability in simulating breast augmentation outcomes (32). However, it has not been studied in autologous breast reconstructions. Based on the human eye physiology, stereophotogrammetry utilizes a stereo pair of cameras to derive 3D data from the points of light intersection (28,33,34). Depending on the presence of additional light being directly projected on to the object, stereophotogrammetry can be classified into: active, passive, and hybrid. Lacking the additional lighting to resist interference from the ambient light, passive stereophotogrammetry requires high-quality cameras in a carefully controlled environment. However, as a result, passive stereophotogrammetry scanners such as, our VECTRA XR scanners (Canfield Scientific), can capture high-resolution 3D photographs at fast speed and is one of the most extensively investigated commercial scanner in breast application (24). Several studies have demonstrated high accuracy and reliability of the VECTRA devices (35-37). However, its widespread use is limited by its high cost, relatively slow image-processing speed, and lack of portability.
3D printing describes a technology where haptic biomodels are fabricated in a layer-by-layer fashion using CAD files derived from routine medical imaging sources, such as 3D photography, CT and MRI scans (38-40). In contrast to the current medical imaging techniques, clinicians are able to interact hands-on with the 3D-printed biomodels, which enables a superior understanding of visuospatial relationship between the patient-specific anatomical structures. In clinical application, 3D printing is useful for preoperative planning, building intraoperative guidance devices, education of patients and junior doctors, and designing customized prosthesis. To date, investigators have utilized routine preoperative CTA scans in breast reconstructions to 3D-print accurate models that enables both qualitative and quantitative appreciation of the breast volume differential (41). In the current case series, 3D-printed biomodels of mirrored image of the contralateral breast are brought into the operating theatre to help guide surgeons when performing the described St Andrew’s coning sutures.
One of the major limitations of our study was that none of the patients required secondary corrective surgery, such as reduction mammoplasty, augmentation or mastopexy, to the contralateral side. It may be useful in the future utilize the Canfield Scientific’s Breast Sculptor® software that reliably simulates breast procedure outcomes (37) in order to create the 3D image of an ideal reconstructed breast in each case that could be 3D-printed for use intraoperatively. Furthermore, assessment of outcome at long-term follow-up may have been useful to demonstrate clinical utility of the St Andrew’s coning suture technique. In order to demonstrate the accuracy and reliability of our 3D scanning technique, the current study needs to be replicated in a larger, comparative study.
Conclusions
The St Andrew’s coning technique is a useful aesthetic maneuver for achieving breast projection during DIEP flap breast reconstruction, with 3D imaging techniques able to assist in perioperative assessment of breast volume.
Acknowledgements
None.
Footnote
Conflicts of Interest: The authors have no conflicts of interest to declare.
Ethical Statement: The authors declare that the study obtained ethics approval for the use of 3D printing (Peninsula Health Human Research Ethics Committee, Reference/Approval number LRR/14/PH/33). Participants gave informed consent before taking part.
References
- Kurian AW, Lichtensztajn DY, Keegan TH, et al. Use of and mortality after bilateral mastectomy compared with other surgical treatments for breast cancer in California, 1998-2011. JAMA 2014;312:902-14. [Crossref] [PubMed]
- Kummerow KL, Du L, Penson DF, et al. Nationwide trends in mastectomy for early-stage breast cancer. JAMA Surg 2015;150:9-16. [Crossref] [PubMed]
- Tuttle TM, Habermann EB, Grund EH, et al. Increasing use of contralateral prophylactic mastectomy for breast cancer patients: a trend toward more aggressive surgical treatment. J Clin Oncol 2007;25:5203-9. [Crossref] [PubMed]
- Wong SM, Freedman RA, Sagara Y, et al. Growing Use of Contralateral Prophylactic Mastectomy Despite no Improvement in Long-term Survival for Invasive Breast Cancer. Ann Surg 2017;265:581-9. [Crossref] [PubMed]
- Shons AR, Mosiello G. Postmastectomy breast reconstruction: current techniques. Cancer Control 2001;8:419-26. [Crossref] [PubMed]
- Elder EE, Brandberg Y, Bjorklund T, et al. Quality of life and patient satisfaction in breast cancer patients after immediate breast reconstruction: a prospective study. Breast 2005;14:201-8. [Crossref] [PubMed]
- Ng SK, Hare RM, Kuang RJ, et al. Breast Reconstruction Post Mastectomy: Patient Satisfaction and Decision Making. Ann Plast Surg 2016;76:640-4. [Crossref] [PubMed]
- Masia J, Clavero JA, Larranaga JR, et al. Multidetector-row computed tomography in the planning of abdominal perforator flaps. J Plast Reconstr Aesthet Surg 2006;59:594-9. [Crossref] [PubMed]
- Rozen WM, Anavekar NS, Ashton MW, et al. Does the preoperative imaging of perforators with CT angiography improve operative outcomes in breast reconstruction? Microsurgery 2008;28:516-23. [Crossref] [PubMed]
- Gill PS, Hunt JP, Guerra AB, et al. A 10-year retrospective review of 758 DIEP flaps for breast reconstruction. Plast Reconstr Surg 2004;113:1153-60. [Crossref] [PubMed]
- Bui DT, Cordeiro PG, Hu QY, et al. Free flap reexploration: indications, treatment, and outcomes in 1193 free flaps. Plast Reconstr Surg 2007;119:2092-100. [Crossref] [PubMed]
- Masia J, Kosutic D, Clavero JA, et al. Preoperative computed tomographic angiogram for deep inferior epigastric artery perforator flap breast reconstruction. J Reconstr Microsurg 2010;26:21-8. [Crossref] [PubMed]
- Macadam SA, Zhong T, Weichman K, et al. Quality of Life and Patient-Reported Outcomes in Breast Cancer Survivors: A Multicenter Comparison of Four Abdominally Based Autologous Reconstruction Methods. Plast Reconstr Surg 2016;137:758-71. [Crossref] [PubMed]
- Blondeel PN, Hijjawi J, Depypere H, et al. Shaping the breast in aesthetic and reconstructive breast surgery: an easy three-step principle. Plast Reconstr Surg 2009;123:455-62. [Crossref] [PubMed]
- Restifo RJ. The "aesthetic subunit" principle in late TRAM flap breast reconstruction. Ann Plast Surg 1999;42:235-9. [Crossref] [PubMed]
- Spear SL, Davison SP. Aesthetic subunits of the breast. Plast Reconstr Surg 2003;112:440-7. [Crossref] [PubMed]
- Song AY, Fernstrom MH, Scott JA, et al. Assessment of TRAM aesthetics: the importance of subunit integration. Plast Reconstr Surg 2006;117:15-24. [Crossref] [PubMed]
- Pulzl P, Schoeller T, Wechselberger G. Respecting the aesthetic unit in autologous breast reconstruction improves the outcome. Plast Reconstr Surg 2006;117:1685-91; discussion 1692-3.
- Gravvanis A, Smith RW. Shaping the breast in secondary microsurgical breast reconstruction: single- vs. two-esthetic unit reconstruction. Microsurgery 2010;30:509-16. [Crossref] [PubMed]
- Gravvanis A, Samouris G, Galani E, et al. Dual plane diep flap inset: Optimizing esthetic outcome in delayed autologous breast reconstruction. Microsurgery 2015;35:432-40. [Crossref] [PubMed]
- Nahabedian MY. Achieving ideal breast aesthetics with autologous reconstruction. Gland Surg 2015;4:134-44. [PubMed]
- Wang T, He J, Xu H, et al. Achieving Symmetry in Unilateral DIEP Flap Breast Reconstruction: An Analysis of 126 Cases over 3 Years. Aesthetic Plast Surg 2015;39:63-8. [Crossref] [PubMed]
- Tomita K, Yano K, Hata Y, et al. DIEP Flap Breast Reconstruction Using 3-dimensional Surface Imaging and a Printed Mold. Plast Reconstr Surg Glob Open 2015;3:e316. [Crossref] [PubMed]
- Chae MP, Rozen WM, Spychal RT, et al. Breast volumetric analysis for aesthetic planning in breast reconstruction: a literature review of techniques. Gland Surg 2016;5:212-26. [PubMed]
- Galdino GM, Nahabedian M, Chiaramonte M, et al. Clinical applications of three-dimensional photography in breast surgery. Plast Reconstr Surg 2002;110:58-70. [Crossref] [PubMed]
- Tzou CH, Artner NM, Pona I, et al. Comparison of three-dimensional surface-imaging systems. J Plast Reconstr Aesthet Surg 2014;67:489-97. [Crossref] [PubMed]
- Olesen OV, Paulsen RR, Hojgaar L, et al. Motion tracking in narrow spaces: a structured light approach. Med Image Comput Comput Assist Interv 2010;13:253-60. [PubMed]
- Lane C, Harrell W Jr. Completing the 3-dimensional picture. Am J Orthod Dentofacial Orthop 2008;133:612-20. [Crossref] [PubMed]
- Thoma A, Veltri K, Khuthaila D, et al. Comparison of the deep inferior epigastric perforator flap and free transverse rectus abdominis myocutaneous flap in postmastectomy reconstruction: a cost-effectiveness analysis. Plast Reconstr Surg 2004;113:1650-61. [Crossref] [PubMed]
- Tepper OM, Karp NS, Small K, et al. Three-dimensional imaging provides valuable clinical data to aid in unilateral tissue expander-implant breast reconstruction. Breast J 2008;14:543-50. [Crossref] [PubMed]
- Geng J. Structured-light 3D surface imaging: a tutorial. Adv Opt Phot 2011;3:128-60. [Crossref]
- Mailey B, Freel A, Wong R, et al. Clinical accuracy and reproducibility of Portrait 3D Surgical Simulation Platform in breast augmentation. Aesthet Surg J 2013;33:84-92. [Crossref] [PubMed]
- Halazonetis DJ. Acquisition of 3-dimensional shapes from images. Am J Orthod Dentofacial Orthop 2001;119:556-60. [Crossref] [PubMed]
- Moyer HR, Carlson GW, Styblo TM, et al. Three-dimensional digital evaluation of breast symmetry after breast conservation therapy. J Am Coll Surg 2008;207:227-32. [Crossref] [PubMed]
- de Menezes M, Rosati R, Ferrario VF, et al. Accuracy and reproducibility of a 3-dimensional stereophotogrammetric imaging system. J Oral Maxillofac Surg 2010;68:2129-35. [Crossref] [PubMed]
- Rosati R, De Menezes M, Rossetti A, et al. Digital dental cast placement in 3-dimensional, full-face reconstruction: a technical evaluation. Am J Orthod Dentofacial Orthop 2010;138:84-8. [Crossref] [PubMed]
- Roostaeian J, Adams WP Jr. Three-Dimensional Imaging for Breast Augmentation: Is This Technology Providing Accurate Simulations? Aesthet Surg J 2014;34:857-75. [Crossref] [PubMed]
- Kamali P, Dean D, Skoracki R, et al. The Current Role of Three-Dimensional (3D) Printing in Plastic Surgery. Plast Reconstr Surg 2016. [Epub ahead of print]. [PubMed]
- Chae MP, Rozen WM, McMenamin PG, et al. Emerging Applications of Bedside 3D Printing in Plastic Surgery. Front Surg 2015;2:25. [Crossref] [PubMed]
- Gerstle TL, Ibrahim AM, Kim PS, et al. A plastic surgery application in evolution: three-dimensional printing. Plast Reconstr Surg 2014;133:446-51. [Crossref] [PubMed]
- Chae MP, Hunter-Smith DJ, Spychal RT, et al. 3D volumetric analysis for planning breast reconstructive surgery. Breast Cancer Res Treat 2014;146:457-60. [Crossref] [PubMed]
- DeSantis C, Ma J, Bryan L, et al. Breast cancer statistics, 2013. CA Cancer J Clin 2014;64:52-62. [Crossref] [PubMed]
- Kroll SS. Why autologous tissue? Clin Plast Surg 1998;25:135-43. [PubMed]
- Pomahac B, Recht A, May JW, et al. New trends in breast cancer management: is the era of immediate breast reconstruction changing? Ann Surg 2006;244:282-8. [Crossref] [PubMed]
- Saulis AS, Mustoe TA, Fine NA. A retrospective analysis of patient satisfaction with immediate postmastectomy breast reconstruction: comparison of three common procedures. Plast Reconstr Surg 2007;119:1669-76; discussion 1677-8.
- Brinkman JN, Timman R, Gopie JP, et al. Aesthetic outcome after implant and DIEP flap breast reconstruction: An exploratory, prospective comparison of 25 cases. J Plast Reconstr Aesthet Surg 2015;68:1018-9. [Crossref] [PubMed]
- Koshima I, Soeda S. Inferior epigastric artery skin flaps without rectus abdominis muscle. Br J Plast Surg 1989;42:645-8. [Crossref] [PubMed]
- Allen RJ, Treece P. Deep inferior epigastric perforator flap for breast reconstruction. Ann Plast Surg 1994;32:32-8. [Crossref] [PubMed]
- Moon HK, Taylor GI. The vascular anatomy of rectus abdominis musculocutaneous flaps based on the deep superior epigastric system. Plast Reconstr Surg 1988;82:815-32. [Crossref] [PubMed]
- Losken A, Fishman I, Denson DD, et al. An objective evaluation of breast symmetry and shape differences using 3-dimensional images. Ann Plast Surg 2005;55:571-5. [Crossref] [PubMed]
- Campaigne BN, Katch VL, Freedson P, et al. Measurement of breast volume in females: description of a reliable method. Ann Hum Biol 1979;6:363-7. [Crossref] [PubMed]
- Chang JB, Small KH, Choi M, et al. Three-dimensional surface imaging in plastic surgery: foundation, practical applications, and beyond. Plast Reconstr Surg 2015;135:1295-304. [Crossref] [PubMed]
- Losken A, Seify H, Denson DD, et al. Validating three-dimensional imaging of the breast. Ann Plast Surg 2005;54:471-6; discussion 477-8. [Crossref] [PubMed]
- Kim H, Mun GH, Wiraatmadja ES, et al. Preoperative magnetic resonance imaging-based breast volumetry for immediate breast reconstruction. Aesthetic Plast Surg 2015;39:369-76. [Crossref] [PubMed]
- Holm C, Mayr M, Hofter E, et al. Perfusion zones of the DIEP flap revisited: a clinical study. Plast Reconstr Surg 2006;117:37-43. [Crossref] [PubMed]
- Menick FJ. Artistry in aesthetic surgery. Aesthetic perception and the subunit principle. Clin Plast Surg 1987;14:723-35. [PubMed]
- Burget GC, Menick FJ. The subunit principle in nasal reconstruction. Plast Reconstr Surg 1985;76:239-47. [Crossref] [PubMed]
- Gonzalez-Ulloa M. Restoration of the face covering by means of selected skin in regional aesthetic units. Br J Plast Surg 1956;9:212-21. [Crossref] [PubMed]
- Warpeha RL. Resurfacing the burned face. Clin Plast Surg 1981;8:255-67. [PubMed]
- Hill DL, Berg DC, Raso VJ, et al. Evaluation of a laser scanner for surface topography. Stud Health Technol Inform 2002;88:90-4. [PubMed]


