Microsurgical dissection of facial nerve in parotidectomy: a discussion of techniques and long-term results
Introduction
Parotidectomy, a surgical procedure to treat benign and malignant diseases of the parotid gland, has well-documented post-operative complications (1,2). Major complications include facial nerve paresis or paralysis, and minor complications include salivary fistula, auriculotemporal syndrome (gustatory sweating or Frey syndrome), great auricular nerve anesthesia, and hemorrhage, infection, seroma formation (3,4). The major complications are often a result of damage to the facial nerve which may be accompanied by permanent disfigurement and a significant negative effect on the patient’s quality of life (4,5). Surgical technique is one of the primary factors contributing to post-operative complication rates and there is a continuous endeavor by surgeons to minimize the risk of complications resulting from parotidectomy (3,6,7). Facial nerve branches are of small size and often difficult to identify even when loupe magnification is used. As a result partial or complete severance of the nerve branches can occur during surgery. Studies have demonstrated that incidences of temporary facial nerve paralysis or paresis following parotidectomy range from 9% to 100%. Permanent paralysis or weakness occurs in 0% to 29% of cases (3,8-10). Moreover, after more extensive operations, including total parotidectomy, temporary paresis and paralysis are even more frequent. To reduce these risks, we propose a number of microsurgical best practices for parotidectomy, and in this study, we will analyze the long-term results we have achieved utilizing these techniques.
Methods
Patient selection and characteristics
This study was a retrospective case analysis of 109 patients who underwent parotidectomy performed by the authors between January 1994 and December 2014 at Chang Gung Memorial Hospital and China Medical University Hospital. The institutional review board of the hospital approved the study (CMUH No: DMR016-IRB-862). The procedures were planned based on clinical findings and the results of fine needle biopsies, with additional imaging in the form of ultrasound scans, CT scans of the head neck with medium contrast and MRIs. Depending on the pathology, size and location of the tumors, superficial or total conservative parotidectomies were performed.
The patient-related data collected included age, gender, histological diagnoses, procedure type, facial nerve function preoperatively and postoperatively, and complications with timings. Facial nerve function was graded using the standard House-Brackmann (HB) method. Patients were assessed on the second day, 1 week and 3 months after the treatment.
Of the total 109 patients, 45 were males and 64 were females. The average age of the patient cohort was 46.2 years with a range of 6 to 74 years.
Surgical procedure
The skin incision started at the preauricular region and continued to the internal margin of the tragus. Once the incision reached the ear lobe, it continued posteriorly for 1 to 2 cm on the mastoid, and ran down on the inferior border of the mandible for 4–5 cm. Next, the skin flap was elevated at the level of the superficial plane of the parotid fascia. Following this, the dissection extended to the inferior border of the parotid under the microscope, until the marginal mandibular branch of the facial nerve was identified at a level, where became more superficial and crossed the facial artery and vein. Consequently, the dissection continued in a retrograde fashion under magnification aiming to isolate the nerve branches until their origin from the main trunk. The great auricular nerves as well as the superficial aponeurotic muscle system were always preserved. The entire dissection took place on a plane superficial to the facial nerve trunk and branches, and was carried out using microsurgical scissors and microforceps. Once the main trunks with its branches were isolated, the dissection could proceed on the deep plane removing the whole gland in case a total parotidectomy was required. During the procedure, bipolar diathermy and small hemostatic clips were used for hemostasis. Nerve branches were found without the use of a nerve stimulator. At the conclusion of the surgery, the fascial planes were sutured, a suction drain was inserted, and the skin incision was closed using no resorbable sutures.
Results
A total of 109 surgical procedures were performed. Seventy-eight patients received superficial parotidectomies and 31 patients underwent total conservative parotidectomy. The average operation time in the superficial parotidectomy group was 3 hours, ranging from 2 hours 15 minutes to 4 hours 30 minutes. In the total parotidectomy group, the average operation time was 3 hours 45 minutes, ranging from 2 hours 45 minutes to 7 hours 45 minutes.
In the entire patient population, follow-up period ranged from 3 months to 16 years with a median of 32 months. The number of post-operative complication was 33 with a rate of 30%. In the superficial parotidectomy cohort (78 patients), the number of complications was 17 (21%). In the total parotidectomy cohort (31 patients), the number of complications was 16 (51%). In the superficial parotidectomy cohort 7 patients presented temporary facial nerve palsy, 5 had salivary fistula formation and five patients developed Frey’s syndrome. Post-operative complications in the total parotidectomy patient cohort included temporary facial nerve palsy in 12 patients, salivary fistula in one patient and development of Frey’s syndrome in 4 patients. Data are summarized in Table 1.
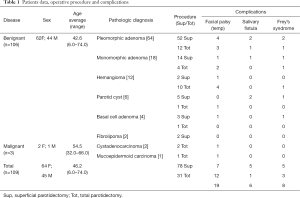
Full table
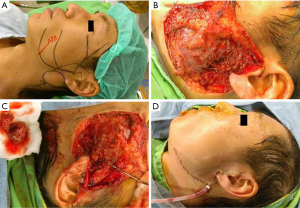
In patients who demonstrated temporary facial nerve palsy, seven patients had buccal nerve branch damage and six patients had marginal nerve branch damage. Four patients had symptoms from both the buccal and marginal branches and two patients from the frontal branch. These patients had moderate dysfunction using the HB criteria—grossly obvious but no disfiguring difference between the two sides of the face, noticeable but no severe synkinesis, contracture, and/or hemifacial spasm. This was accompanied by loss of sensation or numbness as well as some incontinence of the lower lip. These complications resolved in all 19 patients between 14 days and 3 months post-operative. There were no cases of permanent facial nerve palsy. Clinical pictures are showed in Figures 1-4.
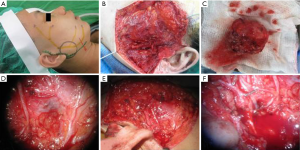
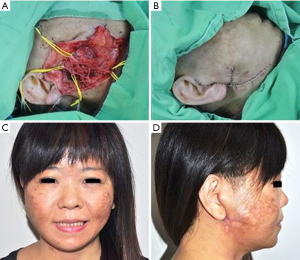
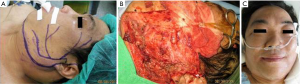
The tumor pathology showed pleomorphic adenoma in 64 cases (58%), Warthin tumor in 18 cases (16%), hemangioma in 12 cases (11%), parotid cyst in 6 cases (5%), basal cell adenoma in 4 patients (4%) and fibrolipoma in 2 cases (2%). There were also three cases of malignant disease: two cystadenocarcinoma and one mucoepidermoid carcinoma.
Discussion
Preservation of the facial nerve remains one of the most important and challenging aspects of parotid gland surgery. Minimizing the incidence and severity of post-operative facial nerve damage is of paramount concern to the surgeon. In prior studies, Henney et al. (3) have described the incidence rates of post-operative temporary facial paralysis after initial parotid surgery to be 42%, of which 78% recovered within 3 months. Marshall et al. (11) have reported that permanent facial paralysis occurred in 1.9% in their series while Mehle et al. (9) 3.9% of cases. In this study, the incidence of temporary facial paralysis was 17% with no reported cases of permanent facial paralysis.
In general, complications following parotid gland surgery are grossly dependent on the aggressiveness and the dimensions of the tumor as well as its relation with the facial nerve. Ward (12), Woods (13), Aljamo (14), Mehle (9), and Valentini (15) have reported that superficial parotidectomy and/or total parotidectomy with preservation of the facial nerve is the gold standard for excision of parotid tumors. However, in their studies, they report a number of post-operative complications related to facial nerve injury. To minimize these complications, we introduce the use of microsurgical techniques for the dissection of the facial nerve.
With the assistance of the microscope, we were able to accurately locate all facial nerve branches thus enabling their preservation. Although, even in conventional dissection of facial nerve with naked eye or loupes the nerve branches appear grossly intact, the tiny vessels accompanying the nerve branches may be injured, resulting in temporary functional problems such as weakness in mouth closure and oral incontinence. The benefits of dissection under the microscope are particularly apparent in cases with severe fibrosis around the parotid gland, such as in recurrent parotid tumors, where the absence of distinct tissue planes makes difficult to distinguish the nerve branches from the surrounding tissue. Compression of the nerve branches may also cause nerve atrophy making identification and preservation of the nerve without magnification extremely difficult (16). The microsurgical dissection can also prove beneficial in paediatric patients where the branches are naturally smaller. Additionally, with the use of microsurgical dissection, small nutrient vessels around the branches of facial nerve can also be preserved. This may offer improved recovery time after surgery, especially for cases with resection of both superficial and deep lobes, in which the nutrient vessels around the nerves can be easily damaged. Finally, Ussmueller (7) and Hahn (17) suggested the use of diathermy scissors and bipolar electrosurgical device to minimize the postoperative bleeding following parotidectomy. In our series, we found that the additional use of microsurgical instruments under magnification allows surgeons to achieve an accurate hemostasis avoiding inadvertent cauterization of tissue in the operating field and unnecessary traction on the nerve branches. Although we found a high rate of temporary facial nerve palsy especially after the dissection in hemangiomas, 5 complications in 12 patients (41%), we suggest utilizing the described techniques in those circumstances. The hemangiomas excision along the facial nerve results in challenging dissection in most of the cases, and only by adopting fine and distinct surgical methods we were able to avoid major complications and minimize side effects.
Another important aspect of our microsurgical technique was the use of retrograde dissection of the facial nerve. As reported by O’Regan B (18) and Scarpini (19), retrograde parotidectomy permits a more conservative surgical approach than anterograde parotidectomy, reducing the extent of dissection and the extent of normal parotid gland sacrificed. Recently, Mahmmood (20) reported low morbidity rates with a temporary facial nerve weakness of 26.6%. This study suggested to perform a retrograde approach using the buccal branch as a guide to identify the nerve trunk and the rest of the branches. Lai et al. (21) used the marginal mandibular nerve as the landmark in their 54 superficial parotidectomies and the rate of transient paralysis was 22.2%. Mahmmood (20) and K. Anjum (22) studies utilized the buccal branch as a guide in the retrograde technique parotidectomy and reported respectively 26.6% and 46.0% of temporary facial palsy. Although Lai (21) found in his study lesser complications, he reported also a main major complication as permanent facial nerve paralysis (1.4%). In our 78 cases of superficial parotidectomy only 9% reported temporary facial palsy, and none permanent palsy. The average operation time was 3 hours for superficial parotidectomies and 3 hours 45 minutes, for total. Disadvantages of this method are the longer operative time and the increased cost for the microsurgical equipment. However, in our opinion these are outweighed by the reduced rate of complications and lower risk of temporary or permanent nerve injury. This microsurgical method allows easier identification of nerve branches and trunk, thus can add potential benefits especially in cases impacted by fibrosis, tumor size, age of patient. A limit of this study can be represented by the significant large period and the multicentricity, although some of the surgeons were trained in both hospitals. Furthermore, the HB scale, used for the measurement of facial nerve involvement, can have a significant inter-observer variability during a long period study.
Conclusions
Our data confirms the low morbidity associated with superficial parotidectomy and total conservative parotidectomy using microsurgical techniques. In a consecutive series of 109 cases and a long-term follow-up period, no permanent injury to the facial nerve was reported, whilst 7% of cases sustained temporary nerve injury.
Further comparative studies with conventional techniques are necessary but, in our opinion, the use of retrograde dissection of the facial nerve branches under microscope during parotidectomy, may represent a useful tool in improving accuracy and minimizing local tissue trauma that can affect nerve recovery. This is particularly true in situations such as tumor recurrence, tissue fibrosis or in case of sizeable tumors around the facial nerve branches. We believe that the decreased risk of facial nerve post-operative symptoms outweigh the disadvantage of increased operative time of this procedure.
Acknowledgements
None.
Footnote
Conflicts of Interest: The authors have no conflicts of interest to declare.
Ethical Statement: The institutional review board of the hospital approved the study (CMUH No: DMR016-IRB-862) and written informed consent was obtained from all patients.
References
- Mohammed F, Asaria J, Payne RJ, et al. Retrospective review of 242 consecutive patients treated surgically for parotid gland tumours. J Otolaryngol Head Neck Surg 2008;37:340-6. [PubMed]
- Nouraei SA, Ismail Y, Ferguson MS, et al. Analysis of complications following surgical treatment of benign parotid disease. ANZ J Surg 2008;78:134-8. [Crossref] [PubMed]
- Henney SE, Brown R, Phillips D. Parotidectomy: the timing of post-operative complications. Eur Arch Otorhinolaryngol 2010;267:131-5. [Crossref] [PubMed]
- Patel N, Har-El G, Rosenfeld R. Quality of life after great auricular nerve sacrifice during parotidectomy. Arch Otolaryngol Head Neck Surg 2001;127:884-8. [PubMed]
- Nitzan D, Kronenberg J, Horowitz Z, et al. Quality of life following parotidectomy for malignant and benign disease. Plast Reconstr Surg 2004;114:1060-7. [Crossref] [PubMed]
- Watanabe Y, Ishikawa M, Shojaku H, et al. Facial nerve palsy as a complication of parotid gland surgery and its prevention. Acta Otolaryngol Suppl 1993;504:137-9. [Crossref] [PubMed]
- Ussmueller JO, Jaehne M, Neumann BG. The use of diathermy scissors in parotid gland surgery. Arch Otolaryngol Head Neck Surg 2004;130:187-9. [Crossref] [PubMed]
- Harney M, Walsh P, Conlon B, et al. Parotid gland surgery: a retrospective review of 108 cases. J Laryngol Otol 2002;116:285-7. [Crossref] [PubMed]
- Mehle ME, Kraus DH, Wood BG, et al. Facial nerve morbidity following parotid surgery for benign disease: the Cleveland Clinic Foundation experience. Laryngoscope 1993;103:386-8. [Crossref] [PubMed]
- Guntinas-Lichius O, Gabriel B, Klussmann JP. Risk of facial palsy and severe Frey's syndrome after conservative parotidectomy for benign disease: analysis of 610 operations. Acta Otolaryngol 2006;126:1104-9. [Crossref] [PubMed]
- Marshall AH, Quraishi SM, Bradley PJ. Patients' perspectives on the short- and long-term outcomes following surgery for benign parotid neoplasms. J Laryngol Otol 2003;117:624-9. [Crossref] [PubMed]
- Ward CM. Injury of the facial nerve during surgery of the parotid gland. Br J Surg 1975;62:401-3. [Crossref] [PubMed]
- Woods JE. Parotidectomy versus limited resection for benign parotid masses. Am J Surg 1985;149:749-50. [Crossref] [PubMed]
- Alajmo E, Polli G, De Meester W. Total parotidectomy--a routine treatment for parotid gland swellings? J Laryngol Otol 1989;103:181-6. [Crossref] [PubMed]
- Valentini V, Fabiani F, Perugini M, et al. Surgical techniques in the treatment of pleomorphic adenoma of the parotid gland: our experience and review of literature. J Craniofac Surg 2001;12:565-8. [Crossref] [PubMed]
- Ciudad P, Yeo MS, Sapountzis S, et al. Microsurgical debulking procedure after free lymph node flap transfer. Microsurgery 2014;34:670-1. [Crossref] [PubMed]
- Hahn CH, Sørensen CH. LigaSure small jaws versus cold knife dissection in superficial parotidectomy. Eur Arch Otorhinolaryngol 2013;270:1489-92. [Crossref] [PubMed]
- O’Regan B, Bharadwaj G, Bhopal S, et al. Facial nerve morbidity after retrograde nerve dissection in parotid surgery for benign disease: a 10-year prospective observational study of 136 cases. Br J Oral Maxillofac Surg 2007;45:101-7. [Crossref] [PubMed]
- Scarpini M, Amore Bonapasta S, Ruperto M, et al. Retrograde parotidectomy for pleomorphic adenoma of the parotid gland: a conservative and effective approach. J Craniofac Surg 2009;20:967-9. [Crossref] [PubMed]
- Mahmmood VH. Buccal branch as a guide for superficial parotidectomy. J Craniofac Surg 2012;23:e447-9. [Crossref] [PubMed]
- Lai YT, Liang Q, Jia XH, et al. Tumor recurrence and complications of parotidectomy using the marginal mandibular branch as a landmark during the retrograde technique. J Craniofac Surg 2015;26:e193-5. [Crossref] [PubMed]
- Anjum K, Revington PJ, Irvine GH. Superficial parotidectomy: antegrade compared with modified retrograde dissections of the facial nerve. Br J Oral Maxillofac Surg 2008;46:433-4. [Crossref] [PubMed]


