
Predictive value of the pretreatment serum sialic acid/total protein ratio for bone metastases in newly diagnosed prostate cancer patients: development of a nomogram model
Highlight box
Key findings
• This study found that pretreatment serum sialic acid/total protein (SA/TP) levels may serve as valuable biomarkers for predicting both the likelihood of prostate cancer (PCa) and the associated risk of bone metastases. And a nomogram for predicting the risk of bone metastases in PCa patients based on SA/TP was developed and validated.
What is known and what is new?
• Bone metastasis is an important and lethal disease in advanced PCa. Currently, there is a lack of effective predictive tools.
• This study introduces the pretreatment SA/TP level and, based on it, presents a nomogram that integrates multiple predictive factors.
What is the implication, and what should change now?
• This novel nomogram can be regarded as a useful tool for assessing the risk of bone metastases in PCa patients and offers a reference for clinical decision-making. This promotes the early detection and treatment of bone metastases in PCa patients.
Introduction
Prostate cancer (PCa) is a prevalent malignant neoplasm of the male reproductive system, characterized by a high incidence and mortality rate, and is becoming a significant public health challenge globally. According to projections from the American Cancer Society, the incidence of PCa was expected to rank first among malignant tumors in males in the United States by 2024, with its mortality rate anticipated to be the second highest (1). In East Asia, the age-standardized incidence rate (ASIR) for PCa was 15.3 per 100,000 in 2022, which contrasts sharply with the ASIR of 82.8 per 100,000 in Northern Europe. Furthermore, East Asia also had a comparatively low age-standardized death rate (ASDR), with 3.8 deaths per 100,000 (2,3). Nevertheless, as economic conditions improve, dietary habits change, and medical care advances, both the incidence and mortality rates associated with PCa have shown a discernible upward trend in China (4-6). PCa frequently manifests insidiously, resulting in a considerable proportion of patients being diagnosed with advanced-stage tumors accompanied by metastasis. Bones are the primary site for distant metastases in PCa. Patients with bone metastases often endure severe complications like bone pain, spinal cord compression, and pathological fractures, resulting in significantly reduced survival time compared to those without bone involvement (7). Currently, there is a lack of specific treatments for PCa patients with bone metastases (8). Therefore, it is essential to identify PCa and its bone metastases early and monitor their progress for a longer period of time. Prostate-specific antigen (PSA) is the primary tumor marker for PCa in clinical practice, essential for early detection. However, its associated costs are substantial, and the waiting period can be lengthy. Furthermore, there is insufficient evidence to substantiate the existence of a PSA threshold that demonstrates both high sensitivity and specificity for diagnosing bone metastases in PCa patients (9). While emission computerized tomography (ECT) is among the recommended standard diagnostic modalities for detecting bone metastases, it also has several disadvantages, such as high price, low specificity, and radiological hazards (10).
Abnormal glycosylation plays a crucial role in tumor growth, invasion, metastasis, and immune evasion and is recognized as one of the key cancer biomarkers (11). Sialic acid (SA) is located predominantly at the methylated non-reducing terminals of extracellular membrane glycoproteins and glycolipids, contributing to various cellular functions such as cell surface receptor formation. During the progression of malignancy, abnormal glycosylation on membrane proteins and lipids coupled with increased sialyltransferase activity results in increased sialylation at the cell surface. This process leads to the release of aberrantly sialylated glycoconjugates into the circulation, significantly elevating SA levels (12). Achalli et al. conducted studies on healthy individuals, patients with potentially malignant oral diseases, and those with oral cancer, and found that the SA levels in serum and saliva gradually increased among these groups, suggesting that SA is related to the degree of malignant transformation of tumors. Another study on patients with colon cancer also confirmed that the SA levels were significantly higher in colon cancer patients compared to the healthy control group (13,14). Furthermore, the correlation between SA levels and the diagnosis, treatment, and prognosis of other malignant tumors, such as lung and breast cancers, has also been confirmed by several studies (15,16). Serum total protein (TP) serves as a widely utilized indicator for assessing nutritional status, and a reduction in its level is detrimental to disease recovery. Patients with malignant tumors often experience varying degrees of malnutrition, and advanced cases may even progress to cachexia (17). Furthermore, systemic inflammatory response syndrome induced by tumors inhibits albumin synthesis. Consequently, owing to these combined factors, TP levels in patients with advanced tumors decline sharply (18).
Obviously, the SA/TP ratio can comprehensively reflect the malignancy degree of tumors and the nutritional status within the body. Previous research has demonstrated that SA/TP measurement can serve as an auxiliary tool for diagnosing and monitoring childhood malignant tumors and oral cancer (19,20). Therefore, this study retrospectively analyzed clinical data from patients with benign prostatic hyperplasia (BPH) and PCa to evaluate the diagnostic significance of pretreatment SA/TP levels concerning PCa and its bone metastases. We present this article in accordance with the TRIPOD reporting checklist (available at https://gs.amegroups.com/article/view/10.21037/gs-2025-39/rc).
Methods
Participants
A retrospective review was conducted to collect the medical records of 1499 patients diagnosed with PCa or BPH who underwent biopsy or postoperative pathological evaluation at the Urology Department, Qilu Hospital of Shandong University, from November 2014 to July 2021. The inclusion criteria for participants were as follows: (I) diagnosis of PCa or BPH following a biopsy or postoperative pathological examination; (II) absence of any prior cancer diagnoses; (III) availability of complete clinical data necessary for the study, including preoperative hematological assessments, biopsy or postoperative pathology results; and (IV) no previous administration of radiotherapy, chemotherapy or endocrine therapy as adjuvant treatment. A person involved in any one of the following criteria was excluded: (I) coexisting primary malignant tumors; (II) concurrent with acute or chronic prostatitis or other active infections; (III) pathologically confirmed PCa but not adenocarcinoma; (IV) having a documented history of hematological disorders or utilized anticoagulant or thrombogenic medications within the 2 weeks preceding presentation; and (V) patients with incomplete clinical data. Following the established inclusion and exclusion criteria, a thorough data screening was performed on the collected cases. A total of 641 patients were ultimately included in this study, comprising 478 individuals with PCa and 163 with BPH. The study was conducted in accordance with the Declaration of Helsinki and its subsequent amendments. The study was approved by Medical Ethics Committee of Qilu Hospital of Shandong University (No. KYLL-202208-044-1) and individual consent for this retrospective analysis was waived.
Data collection
Clinical data were extracted from the electronic medical record database of Qilu Hospital of Shandong University, encompassing patients’ age at diagnosis, alkaline phosphatase (AKP), lactate dehydrogenase (LDH), fibrinogen (FIB), and PSA levels, pathological characteristics [including pathological grade and tumor stage (T stage)], ECT examination results and smoking history. The pathological grade was assessed according to the Gleason score (GS), while the pathological tumor (pT) stage was determined using the tumor-node-metastasis (TNM) 2021. Bone metastases evaluation was conducted on the basis of ECT findings. Compared with both the contralateral side and adjacent normal tissue, a significantly elevated concentration of radioactive substances in a specific region was indicative of a metastatic lesion.
Analysis of hematology indices
Each patient who had not undergone any clinical intervention had 5 mL of venous blood drawn in the morning following a 12-hour fasting period. The blood sample must be collected in a test tube containing an anticoagulant and serum separation gel and allowed to clot at room temperature. Subsequently, the sample was centrifuged at 2,000 rpm for 10 minutes. The concentrations of SA and TP in the separated plasma were detected via a Roche Cobas 8000 automatic analyzer (Roche, Mannheim, Germany). The normal concentration range for SA was between 45.60 and 75.40 mg/dL, while that for TP was from 60.0 to 85.0 g/L. Additionally, FIB levels were measured by collecting a 3.5 mL venous blood sample into a test tube with 3.80% sodium citrate. The normal FIB concentration range was established as between 2.00 and 4.00 g/L.
Statistical analysis
Statistical analysis was performed utilizing the Statistical Package for Social Sciences version 26.0 (SPSS Inc., Chicago, IL, USA) and GraphPad Prism 9.5 software (GraphPad Software Inc., San Diego, CA, USA). Continuous variables were presented as the means ± standard deviations or medians (quartiles), contingent upon the results of normality tests. Data exhibiting a normal distribution were subjected to Student’s t-test, whereas non-normally distributed data were analyzed using the Mann-Whitney U test or Kruskal-Wallis H test. Categorical variables were expressed as numbers (percentages) and analyzed with the Chi-squared test. Receiver operating characteristic curves (ROC) were generated to assess the diagnostic utility of SA/TP, and the area under the curve (AUC) and Youden index were computed to ascertain the optimal cut-off value for SA/TP. Subgroups were delineated based on this optimal cut-off value, allowing comparisons of relevant clinical parameters between them. The correlations between various parameters and PCa, as well as its bone metastases status, were assessed through univariate and multivariate logistic regression analysis. Using the “createDataPartition” function in R software (version 4.3.2), some PCa patients were partitioned into training and validation sets at a 7:3 ratio. A nomogram was constructed using the independent variables (P<0.05) identified in the multivariate analysis of the training set via R software. The calibration curves, ROC curves, and decision curve analysis (DCA) curves for the training and validation sets, which were plotted using R software with the rms, Hmisc, pROC, and rmda packages, were used to evaluate the model’s performance and clinical applicability.
Results
Study population baseline characteristics
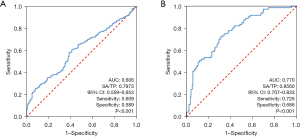
Following a comprehensive screening process, a total of 641 patients were enrolled in this study based on their fulfillment of the inclusion criteria. Of these participants, 478 were diagnosed with PCa, while the remaining 163 were diagnosed with BPH. According to the results of the ECT examination, among the PCa patients, 73 exhibited bone metastases, 169 showed no evidence of bone metastases, 89 were suspected of having bone metastases, and 147 either did not undergo ECT or had lost results (Table 1). The median ages of the PCa and BPH patients were 70.00 (64.00, 76.00) and 69.00 (62.00, 75.00) years, respectively (P=0.08). The median SA/TP level in the PCa cohort was found to be significantly elevated at 0.8280 (0.7461, 0.9451) compared to that in the BPH group at 0.7781 (0.7274, 0.8689) (P<0.001) (Table S1, Figure S1).
Table 1
| Parameters | Number (%) | SA/TP levels | P value |
|---|---|---|---|
| Age (years) | 0.32 | ||
| ≤70 | 246 (51.46) | 0.8205 (0.7370, 0.9525) | |
| >70 | 232 (48.54) | 0.8348 (0.7560, 0.9379) | |
| LDH (U/L) | <0.001 | ||
| ≤194 | 244 (51.05) | 0.8132 (0.7389, 0.8938) | |
| >194 | 234 (48.95) | 0.8572 (0.7598, 1.0171) | |
| AKP (U/L) | <0.001 | ||
| ≤73 | 244 (51.05) | 0.7888 (0.7240, 0.8558) | |
| >73 | 234 (48.95) | 0.8866 (0.7966, 1.0590) | |
| FIB (g/L) | <0.001 | ||
| ≤3.15 | 239 (50.00) | 0.7696 (0.7139, 0.8205) | |
| >3.15 | 239 (50.00) | 0.9258 (0.8333, 1.0999) | |
| PSA (ng/mL) | <0.001 | ||
| ≤39.71 | 239 (50.00) | 0.8003 (0.7235, 0.8647) | |
| >39.71 | 239 (50.00) | 0.8742 (0.7756, 1.0348) | |
| Smoking history | |||
| Yes | 166 (34.73) | 0.8730 (0.7864, 1.0088) | <0.001 |
| No | 307 (64.22) | 0.8139 (0.7341, 0.9068) | |
| Missing information | 5 (1.05) | – | |
| Pathological grade | 0.001 | ||
| Low (GS ≤7) | 203 (42.47) | 0.8124 (0.7358, 0.8864) | |
| High (GS >7) | 259 (54.18) | 0.8528 (0.7562, 0.9847) | |
| Missing information | 16 (3.35) | – | |
| pT stage | 0.43 | ||
| pT2 | 342 (71.55) | 0.8232 (0.7421, 0.9357) | |
| pT3–4 | 117 (24.48) | 0.8302 (0.7547, 0.9601) | |
| Biopsy not performed | 19 (3.97) | – | |
| ECT | <0.001† | ||
| Without bone metastases | 169 (35.36) | 0.8095 (0.7190, 0.8987) | <0.001‡ |
| Bone metastases | 73 (15.27) | 0.9720 (0.8420, 1.1332) | |
| Suspicion | 89 (18.62) | 0.8495 (0.7674, 1.0178) | |
| ECT not performed | 147 (30.75) | – |
Data are presented as n (%) or median (quartile). †, without bone metastases vs. suspicion vs. bone metastases; ‡, without bone metastases vs. bone metastases. AKP, alkaline phosphatase; ECT, emission computerized tomography; FIB, fibrinogen; GS, Gleason score; LDH, lactate dehydrogenase; PCa, prostate cancer; PSA, prostate-specific antigen; pT, pathological tumor; SA/TP, sialic acid/total protein.
Correlations between SA/TP levels and clinical characteristics of PCa patients
To further assess the correlations between pretreatment SA/TP levels and clinical characteristics of PCa patients, a total of 478 PCa patients were stratified into distinct subgroups based on the median values or pathological feature types of the study parameters (Table 1). It can be obviously seen that there were significant statistical differences in SA/TP levels in the AKP, LDH, FIB, and PSA subgroups (all P<0.001), indicating a strong association between pretreatment SA/TP levels and these biomarkers. Notably, patients with a history of smoking presented significantly elevated SA/TP levels compared to non-smokers, demonstrating a statistically significant difference (P<0.001) (Table 1). Despite the lack of a statistically significant difference in SA/TP levels across age subgroups (P=0.32), interestingly, the median SA/TP value for elderly patients surpassed that of younger individuals [0.8205 (0.7370, 0.9525) vs. 0.8348 (0.7560, 0.9379)] (Table 1). The cohort of 478 PCa patients was divided into four groups on the basis of ECT results. Obviously, the median SA/TP in the bone metastases subgroup was 0.9720 (0.8420, 1.1332), which was significantly greater than that in patients without bone metastases [0.8095 (0.7190, 0.8987)] or with suspected bone metastases [0.8495 (0.7674, 1.0178)] (Figure S1). Regarding the pT stage, an increase in the pT stage correlated with a rise in the SA/TP ratio, but this difference was not statistically significant (P=0.43). In addition, when employing the Gleason system to assess patients’ pathological grades, SA/TP levels were significantly elevated in patients diagnosed with high-grade cancer (GS >7) compared to those diagnosed with low-grade cancer (GS ≤7) (P<0.001) (Table 1).
Predictive potential of pretreatment SA/TP levels for PCa and bone metastases
We evaluated the predictive value of pretreatment SA/TP levels for PCa using ROC curves, finding an AUC of 0.606 [95% confidence interval (CI): 0.559–0.653] with an optimal cutoff of 0.7973, resulting in 60.9% sensitivity and 58.9% specificity (Figure 1A). SA/TP levels above this threshold were linked to higher PCa risk. For patients with and without bone metastases, the ROC curves showed an AUC of 0.770 (95% CI: 0.707–0.833). We further analyzed the ROC curve to determine the optimal threshold for predicting the risk of bone metastases, which was established at 0.8560, with sensitivity and specificity values of 72.6% and 68.6%, respectively (Figure 1B). Based on the aforementioned thresholds, the study cohort was stratified into two groups according to SA/TP levels (≤0.7973 vs. >0.7973). The findings indicated that patients with SA/TP >0.7379 were more likely to demonstrate increased age (P=0.01), elevated levels of AKP, LDH, FIB, and PSA (all P<0.001), as well as a history of smoking (P=0.006) (Table S2). Subsequently, we categorized the PCa patients included in this study into two groups based on their SA/TP levels (≤0.8560 vs. >0.8560). Table S3 showed that SA/TP levels were significantly linked to high tumor grade (P<0.001) and correlated with AKP, LDH, FIB, PSA, and smoking history, but not with pT stage (P=0.61).
Univariate and multivariate analysis of the relationships between pretreatment SA/TP levels and PCa and bone metastases
According to univariate analysis, the SA/TP levels exhibited significant correlations with the risk of PCa and bone metastases (all P<0.001), which persisted in the multivariate analysis (all P<0.05). Furthermore, elevated levels of AKP, LDH, FIB, and PSA were also significantly associated with PCa and bone metastases (all P<0.05), whereas the GS was strongly correlated with bone metastases [hazard ratio (HR) =3.497, 95% CI: 1.878–6.513, P<0.001]. Age and smoking history did not reveal statistically significant differences in predicting PCa or bone metastases (Tables 2,3). In subsequent multivariate analyses, the PSA and pretreatment SA/TP levels remained independent predictors of PCa (PSA: HR =1.215, 95% CI: 1.160–1.273, P<0.001; SA/TP: HR =1.704, 95% CI: 1.016–2.860, P=0.04) (Table 2). Among patients with PCa, high AKP and PSA levels, an elevated GS, along with increased SA/TP levels continued to serve as independent predictors for bone metastases (AKP: HR =1.030, 95% CI: 1.016–1.043, P<0.001; PSA: HR =1.004, 95% CI: 1.002–1.007, P<0.001; GS: HR =3.748, 95% CI: 1.444–9.733, P=0.007; SA/TP: HR =2.567, 95% CI: 1.051–6.271, P=0.04).
Table 2
| Variables | Univariate regression | Multivariate regression | |||
|---|---|---|---|---|---|
| HR (95% CI) | P value | HR (95% CI) | P value | ||
| Age (years) | 1.018 (0.997–1.040) | 0.09 | – | – | |
| AKP (U/L) | 1.016 (1.008–1.024) | <0.001 | 1.004 (0.996–1.013) | 0.32 | |
| LDH (U/L) | 1.005 (1.001–1.009) | 0.01 | 0.997 (0.991–1.004) | 0.42 | |
| FIB (g/L) | 1.555 (1.217–1.987) | <0.001 | 0.952 (0.696–1.300) | 0.76 | |
| PSA (ng/mL) | 1.210 (1.157–1.266) | <0.001 | 1.215 (1.160–1.273) | <0.001 | |
| Smoking history (yes vs. no) | 1.033 (0.711–1.502) | 0.86 | – | – | |
| SA/TP (>0.7973 vs. ≤0.7973) | 2.230 (1.552–3.203) | <0.001 | 1.704 (1.016–2.860) | 0.04 | |
AKP, alkaline phosphatase; CI, confidence interval; FIB, fibrinogen; HR, hazard ratio; LDH, lactate dehydrogenase; PCa, prostate cancer; PSA, prostate-specific antigen; SA/TP, sialic acid/total protein.
Table 3
| Variables | Univariate regression | Multivariate regression | |||
|---|---|---|---|---|---|
| HR (95% CI) | P value | HR (95% CI) | P value | ||
| Age (years) | 0.992 (0.957–1.027) | 0.63 | – | – | |
| AKP (U/L) | 1.035 (1.023–1.047) | <0.001 | 1.030 (1.016–1.043) | <0.001 | |
| LDH (U/L) | 1.015 (1.009–1.022) | <0.001 | 1.004 (0.996–1.012) | 0.30 | |
| FIB (g/L) | 1.506 (1.159–1.957) | 0.002 | 0.809 (0.532–1.230) | 0.32 | |
| PSA (ng/mL) | 1.006 (1.004–1.008) | <0.001 | 1.004 (1.002–1.007) | <0.001 | |
| Smoking history (yes vs. no) | 1.404 (0.794–2.481) | 0.24 | – | – | |
| GS (≤7 vs. >7) | 3.497 (1.878–6.513) | <0.001 | 3.748 (1.444–9.733) | 0.007 | |
| SA/TP (>0.8560 vs. ≤0.8560) | 5.800 (3.156–10.657) | <0.001 | 2.567 (1.051–6.271) | 0.04 | |
AKP, alkaline phosphatase; CI, confidence interval; FIB, fibrinogen; GS, Gleason score; HR, hazard ratio; LDH, lactate dehydrogenase; PCa, prostate cancer; PSA, prostate-specific antigen; SA/TP, sialic acid/total protein.
Development of a nomogram model for bone metastases
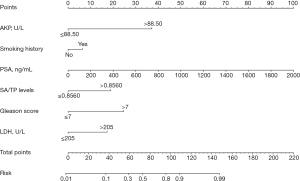
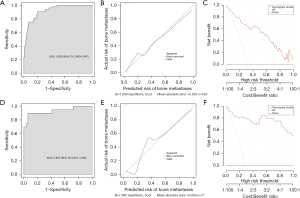
The ‘createDatePartition’ function in R software was used to stratify a cohort of 242 patients into training and validation sets at a 7:3 ratio (Table 4). Logistic regression analysis was conducted on the training set data, incorporating independent risk factors (P<0.05) identified through multivariate stepwise regression analysis alongside a history of smoking, to establish a predictive model for the risk of bone metastases. The variables AKP, LDH, and SA/TP were categorized based on their optimal cutoff values (Table 5, Figure 2). The ROC curve for the training set revealed an AUC of 0.920 (95% CI: 0.880–0.961), indicating robust predictive performance of the model. Calibration curve assessment demonstrated that the predicted outcomes closely aligned with ideal expectations, while the DCA curve illustrated that this model could provide significant net clinical benefits to patients (Figure 3A-3C). The ROC curve, calibration curve, and DCA curve of the validation set further confirmed that the model exhibited robust predictive performance (Figure 3D-3F).
Table 4
| Parameters | Total (n=242) | Training set (n=170) | Validation set (n=72) | P value |
|---|---|---|---|---|
| Age (years) | 0.56 | |||
| ≤80 | 225 (92.98) | 157 (92.35) | 68 (94.44) | |
| >80 | 17 (7.02) | 13 (7.65) | 4 (5.56) | |
| AKP (U/L) | 0.43 | |||
| ≤88.50 | 166 (68.60) | 114 (67.06) | 52 (72.22) | |
| >88.50 | 76 (31.4) | 56 (32.94) | 20 (27.78) | |
| LDH (U/L) | 0.40 | |||
| ≤205 | 151 (62.40) | 109 (64.12) | 42 (58.33) | |
| >205 | 91 (37.60) | 61 (35.88) | 30 (41.67) | |
| FIB (g/L) | 0.46 | |||
| ≤3.60 | 177 (73.14) | 122 (71.76) | 55 (76.39) | |
| >3.60 | 65 (26.86) | 48 (28.24) | 17 (23.61) | |
| SA/TP levels | 0.12 | |||
| ≤0.8560 | 136 (56.20) | 90 (52.94) | 46 (63.89) | |
| >0.8560 | 106 (43.80) | 80 (47.06) | 26 (36.11) | |
| GS† | 0.31 | |||
| Low (GS ≤7) | 103 (42.92) | 69 (40.83) | 34 (47.89) | |
| High (GS >7) | 137 (57.08) | 100 (59.17) | 37 (52.11) | |
| Smoking history | 0.19 | |||
| No | 160 (66.12) | 108 (63.53) | 52 (72.22) | |
| Yes | 82 (33.88) | 62 (36.47) | 20 (27.78) | |
| PSA (ng/mL) | 54.86 (16.13, 158.35) | 54.59 (18.57, 152.62) | 58.86 (14.56, 184.70) | 0.85 |
Data are presented as n (%) or median (quartile). †, two cases of missing data were not included. AKP, alkaline phosphatase; FIB, fibrinogen; GS, Gleason score; LDH, lactate dehydrogenase; PSA, prostate-specific antigen; SA/TP, sialic acid/total protein.
Table 5
| Variables | Univariate regression | Multivariate regression | |||
|---|---|---|---|---|---|
| HR (95% CI) | P value | HR (95% CI) | P value | ||
| Age (≤80 vs. >80 years) | 2.006 (0.640–6.289) | 0.23 | – | – | |
| AKP (≤88.50 vs. >88.50 U/L) | 19.423 (8.569–44.026) | <0.001 | 10.037 (3.675–27.409) | <0.001 | |
| LDH (≤205 vs. >205 U/L) | 5.245 (2.607–10.553) | <0.001 | 2.755 (1.041–7.293) | 0.04 | |
| FIB (≤3.60 vs. >3.60 g/L) | 8.289 (3.914–17.552) | <0.001 | – | – | |
| PSA (ng/mL) | 1.005 (1.002–1.007) | <0.001 | 1.003 (1.001–1.006) | 0.01 | |
| Smoking history (yes vs. no) | 2.167 (1.113–4.219) | 0.02 | – | – | |
| GS (≤7 vs. >7) | 3.818 (1.791–8.140) | <0.001 | 4.741 (1.543–14.567) | 0.007 | |
| SA/TP (>0.8560 vs. ≤0.8560) | 6.833 (3.230–14.457) | <0.001 | 3.359 (1.278–8.826) | 0.01 | |
AKP, alkaline phosphatase; CI, confidence interval; FIB, fibrinogen; GS, Gleason score; HR, hazard ratio; LDH, lactate dehydrogenase; PCa, prostate cancer; PSA, prostate-specific antigen; SA/TP, sialic acid/total protein.
Discussion
The complexity, high incidence rate, and propensity for metastases make PCa a significant threat to male health. According to statistics, in 2022, PCa ranked second in terms of the global incidence of male cancers and was the fifth leading cause of male cancer-related mortality (3,21). Currently, PSA remains the most widely utilized screening biomarker for PCa in clinical practice. However, its application for population-based screening has been subject to ongoing debate. Pinsky et al. extended the follow-up period of the Prostate, Lung, Colorectal, and Ovarian (PLCO) Cancer Screening Trial to 15 years and found no significant differences in PCa mortality between the intervention group utilizing PSA screening and the control group. Conversely, findings from the European Randomized Study of Screening for Prostate Cancer (ERSPC), conducted across eight European nations, indicated that as follow-up duration increased, the effect of PSA screening on reducing PCa mortality became more pronounced—resulting in a relative reduction of 20% (22-25). While PSA plays a crucial role in the early diagnosis of PCa, it is not without drawbacks, such as substantial detection burden and elevated false-positive rates. Metastatic diseases associated with advanced PCa represent the primary cause of cancer-related mortality. It is reported that the 5-year overall survival rate for patients with localized PCa can reach as high as 99%, whereas this rate significantly decreases to merely 30% for those with metastatic PCa (1,8). The most prevalent sites of metastasis in advanced cases are local lymph nodes, followed by bone. Furthermore, over 80% of patients exhibiting distant metastatic disease present with bone metastases (25,26). Compared with other diagnostic modalities, ECT has been utilized for diagnosing bone metastases in PCa patients because of its high sensitivity, ease of operation, and minimal discomfort. However, it is not without limitations, such as low specificity and difficulty in implementation within primary healthcare settings (27,28).
Currently, there are several treatment modalities available for localized PCa, including active surveillance, surgical intervention (radical prostatectomy with or without pelvic lymph node dissection), external beam radiation therapy, and androgen deprivation therapy (ADT). In general, patients with localized PCa who undergo radical prostatectomy or chemotherapy can achieve outcomes that meet the criteria for cure (21,25,29). Although most PCa patients exhibit slow progression, a significant proportion of patients still face the risk of progressing to moderate or high-risk localized, locally advanced, or metastatic cancer. For those diagnosed with metastatic PCa, reducing circulating androgen levels through chemical castration serves as the cornerstone of systemic treatment (21,29). In recent years, the advent of various novel pharmacological agents has led to substantial changes in the management paradigm for metastatic PCa. Research indicated that in metastatic hormone-sensitive PCa (mHSPC) patients, combining docetaxel with innovative hormonal therapies can markedly enhance overall survival rates (30,31). Nevertheless, there remains a lack of effective treatment options for patients with PCa accompanied by bone metastases. Therefore, the early detection of localized PCa and its associated risk of bone metastases can significantly contribute to prolonging patients’ life expectancy. Previous studies have demonstrated that one of the most prevalent aberrant glycosylation alterations in cancer is the overexpression of SA. SA shields cancer cells from immune surveillance by obscuring tumor antigen sites, thereby diminishing anti-tumor immunity and complementing alternative activation. Furthermore, SA interacts with receptors present on both innate and adaptive immune cells [such as natural killer (NK) and T cells], which are part of the SA-binding immunoglobulin-like lectins (Siglecs) family, thereby facilitating immune suppression. Additionally, SA serves as a ligand for selectins, which mediate the adhesion of cancer cells to target sites, consequently enhancing the dissemination and metastasis of tumor cells (16,32-34). A recent study has indicated that micronucleus formation may also play a role in the metastasis and progression of PCa. This process can enhance the invasive and metastatic capabilities of prostate tumor cells by inducing alterations in their phenotypes and functional characteristics (35). TP serves as a widely recognized biomarker for assessing an individual’s nutritional status and the severity of various diseases. Cancer is a prevalent cause of reduced serum TP levels. Patients with cancer frequently experience malnutrition and, in severe cases, cachexia. Studies indicated that over 50% of individuals with advanced-stage cancer developed cachexia (36,37).
On the basis of these findings, we inferred that in patients with PCa and bone metastases, SA levels were significantly elevated while TP levels were markedly decreased compared with those with BPH, resulting in increased pretreatment SA/TP. The results of this study further substantiated this hypothesis. This study examined the levels of SA/TP, along with other pertinent laboratory indicators and clinical characteristics, in a cohort of 641 patients diagnosed with BPH and PCa. Our findings revealed that pretreatment SA/TP levels were significantly higher in PCa patients compared to those with BPH, which was similar to the results of Vajaria et al., who reported that the serum and saliva total SA (TSA)/TP ratios of patients with oral precancer and oral cancer were significantly higher (20). Patients diagnosed with PCa were subsequently stratified by their age and LDH, AKP, FIB, and PSA levels. The results of the subgroup analysis indicated a significant correlation between pretreatment SA/TP levels and LDH, AKP, FIB, and PSA levels. However, no statistically significant correlation was found between SA/TP levels and age. Notably, as the pathological grade escalated, there was a corresponding increase in SA/TP levels, and there was a significant correlation between the two indicators. Although the median pretreatment SA/TP level was elevated in patients with advanced tumors (pT3–4) compared to those with early-stage tumors (pT2), no meaningful correlation was detected between SA/TP levels and pathological stage (P=0.43). Previous research indicated that smoking was linked to aggressive characteristics of PCa and a poor prognosis. Kenfield et al. reported that the recurrence rate of PCa was significantly higher among smokers (38,39). Our study similarly demonstrated that patients with a history of smoking presented markedly elevated SA/TP levels relative to non-smokers (P<0.001). In accordance with the presence or absence of ECT examination and its results, PCa patients were categorized into four distinct groups: no bone metastases, suspected bone metastases, bone metastases, and no ECT. In the first three groups, the SA/TP levels gradually increased. Then, ROC curves were generated independently to predict PCa and its associated bone metastases based on the pretreatment SA/TP levels. According to the Youden index, the optimal SA/TP cut-off value for differentiating BPH from PCa was 0.7973 (P<0.001), while the optimal cut-off value for distinguishing between patients without and with bone metastases was established at 0.8560 (P<0.001). It can be seen that the increase in pretreatment SA/TP levels was of good sensitivity and specificity for the diagnosis of PCa and its bone metastases. Additionally, subgroup analyses based on the above optimal value further confirmed that SA/TP was significantly associated with LDH, AKP, FIB, PSA levels, smoking history, and pathological grade. Furthermore, both univariate and multivariate regression analyses indicated that the SA/TP ratio was independently correlated with PCa and its bone metastases. Consequently, elevated SA/TP levels hold substantial significance in diagnosing PCa and its bone metastases and can serve as a valuable diagnostic marker in clinical practice. To create a predictive model for assessing bone metastases risk in PCa patients, we randomly allocated 242 PCa patients into a training set and a validation set at a ratio of 7:3. Through univariate and multivariate regression analysis, we ultimately constructed a nomogram model incorporating factors such as AKP, LDH, PSA, SA/TP, smoking history, and pathological grade. We subsequently evaluated the model’s performance using ROC curves, calibration plots, and DCA curves across both sets, and the results showed that it had good predictive performance, accuracy, and clinical effectiveness. Therefore, this model can serve as a reliable tool for evaluating the risk of bone metastases among PCa patients in clinical settings and provide a reference for clinical decision-making.
Nevertheless, there were still some limitations worth considering in our research. Firstly, it was a single-center retrospective analysis with all sample data sourced from Qilu Hospital of Shandong University, which introduced a degree of selection bias. Secondly, the average incidence of obesity among adults in Europe and America is significantly higher than that in East Asian countries. Moreover, compared with the high-fat and high-protein Western diet pattern, the East Asian population mainly adheres to a vegetarian diet. These ethnic and dietary differences can affect the components of plasma proteins, protein metabolism, and inflammatory responses, thereby influencing the SA/TP level (40-43). The participants of this study were exclusively recruited from the Chinese ethnicity, thereby limiting the generalizability of the findings. Lastly, given the sensitivity and specificity concerns associated with ECT examinations, there may be instances of data misjudgment. Consequently, to more robustly validate our findings, further large-scale prospective studies are warranted in the future.
Conclusions
This study demonstrated a significant correlation between pretreatment SA/TP levels and the risk of PCa as well as its subsequent bone metastases. These findings indicated that pretreatment SA/TP levels might serve as a valuable biomarker for predicting both the likelihood of PCa and the associated risk of bone metastases. Furthermore, a predictive model based on pretreatment SA/TP levels could effectively assess the risk of bone metastases in patients with PCa.
Acknowledgments
None.
Footnote
Reporting Checklist: The authors have completed the TRIPOD reporting checklist. Available at https://gs.amegroups.com/article/view/10.21037/gs-2025-39/rc
Data Sharing Statement: Available at https://gs.amegroups.com/article/view/10.21037/gs-2025-39/dss
Peer Review File: Available at https://gs.amegroups.com/article/view/10.21037/gs-2025-39/prf
Funding: This study was supported by
Conflicts of Interest: All authors have completed the ICMJE uniform disclosure form (available at https://gs.amegroups.com/article/view/10.21037/gs-2025-39/coif). The authors have no conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved. The study was conducted in accordance with the Declaration of Helsinki and its subsequent amendments. The study was approved by Medical Ethics Committee of Qilu Hospital of Shandong University (No. KYLL-202208-044-1) and individual consent for this retrospective analysis was waived.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Siegel RL, Giaquinto AN, Jemal A. Cancer statistics, 2024. CA Cancer J Clin 2024;74:12-49. [Crossref] [PubMed]
- Bernard B, Muralidhar V, Chen YH, et al. Impact of ethnicity on the outcome of men with metastatic, hormone-sensitive prostate cancer. Cancer 2017;123:1536-44. [Crossref] [PubMed]
- Bray F, Laversanne M, Sung H, et al. Global cancer statistics 2022: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J Clin 2024;74:229-63. [Crossref] [PubMed]
- Kaiser A, Haskins C, Siddiqui MM, et al. The evolving role of diet in prostate cancer risk and progression. Curr Opin Oncol 2019;31:222-9. [Crossref] [PubMed]
- Liu J, Dong L, Zhu Y, et al. Prostate cancer treatment - China's perspective. Cancer Lett 2022;550:215927. [Crossref] [PubMed]
- Sun J, Tian T, Wang N, et al. Pretreatment level of serum sialic acid predicts both qualitative and quantitative bone metastases of prostate cancer. Front Endocrinol (Lausanne) 2024;15:1338420. [Crossref] [PubMed]
- Tsuzuki S, Park SH, Eber MR, et al. Skeletal complications in cancer patients with bone metastases. Int J Urol 2016;23:825-32. [Crossref] [PubMed]
- Zhang X. Interactions between cancer cells and bone microenvironment promote bone metastasis in prostate cancer. Cancer Commun (Lond) 2019;39:76. [Crossref] [PubMed]
- Singh OP, Yogi V, Redhu P, et al. Role of serum prostate-specific antigen as predictor for bone metastases in newly diagnosed prostate cancer. J Cancer Res Ther 2019;15:S39-41. [Crossref] [PubMed]
- Chow KM, So WZ, Lee HJ, et al. Head-to-head Comparison of the Diagnostic Accuracy of Prostate-specific Membrane Antigen Positron Emission Tomography and Conventional Imaging Modalities for Initial Staging of Intermediate- to High-risk Prostate Cancer: A Systematic Review and Meta-analysis. Eur Urol 2023;84:36-48. [Crossref] [PubMed]
- Hodgson K, Orozco-Moreno M, Goode EA, et al. Sialic acid blockade inhibits the metastatic spread of prostate cancer to bone. EBioMedicine 2024;104:105163. [Crossref] [PubMed]
- Zhang Z, Wuhrer M, Holst S. Serum sialylation changes in cancer. Glycoconj J 2018;35:139-60. [Crossref] [PubMed]
- Achalli S, Madi M, Babu SG, et al. Sialic acid as a biomarker of oral potentially malignant disorders and oral cancer. Indian J Dent Res 2017;28:395-9. [Crossref] [PubMed]
- İliklerden ÜH, Peksen C, Kalayci T, et al. Evaluation of preoperative and postoperative total serum sialic acid levels in patients with colon cancer. Ann Ital Chir 2020;91:649-7.
- Hu W, Ge W, Xia P, et al. Diagnostic Potential of Serum Glycome Analysis in Lung Cancer: A Glycopattern Study. J Proteome Res 2024;23:500-9. [Crossref] [PubMed]
- Liang L, Shen Y, Zhang J, et al. Identification of breast cancer through spectroscopic analysis of cell-membrane sialic acid expression. Anal Chim Acta 2018;1033:148-55. [Crossref] [PubMed]
- Castro MJ, Jiménez JM, López M, et al. Impact of Preoperative Total Proteins and Glycated Hemoglobin on Recurrences after Early Colorectal Cancer. Nutrients 2021;13:711. [Crossref] [PubMed]
- Hsu CC, Chou WC, Hung YS, et al. Predictive Value of Albumin and Neutrophil-to-Lymphocyte Ratio Score for Treatment Completeness and Safety Profiles in Patients With Head and Neck Cancer Receiving Definitive Concurrent Chemoradiotherapy. In Vivo 2022;36:2875-83. [Crossref] [PubMed]
- Romppanen J, Petäjä J, Heikinheimo M, et al. Total and lipid-bound serum sialic acid in children with malignancy or infections. Anticancer Res 1998;18:2793-7.
- Vajaria BN, Patel KR, Begum R, et al. Evaluation of serum and salivary total sialic acid and α-l-fucosidase in patients with oral precancerous conditions and oral cancer. Oral Surg Oral Med Oral Pathol Oral Radiol 2013;115:764-71. [Crossref] [PubMed]
- Wasim S, Lee SY, Kim J. Complexities of Prostate Cancer. Int J Mol Sci 2022;23:14257. [Crossref] [PubMed]
- Hugosson J, Roobol MJ, Månsson M, et al. A 16-yr Follow-up of the European Randomized study of Screening for Prostate Cancer. Eur Urol 2019;76:43-51. [Crossref] [PubMed]
- Obiora D, Orikogbo O, Davies BJ, et al. Controversies in prostate cancer screening. Urol Oncol 2025;43:49-53. [Crossref] [PubMed]
- Pinsky PF, Prorok PC, Yu K, et al. Extended mortality results for prostate cancer screening in the PLCO trial with median follow-up of 15 years. Cancer 2017;123:592-9. [Crossref] [PubMed]
- Rebello RJ, Oing C, Knudsen KE, et al. Prostate cancer. Nat Rev Dis Primers 2021;7:9. [Crossref] [PubMed]
- Berish RB, Ali AN, Telmer PG, et al. Translational models of prostate cancer bone metastasis. Nat Rev Urol 2018;15:403-21. [Crossref] [PubMed]
- Beheshti M, Langsteger W, Fogelman I. Prostate cancer: role of SPECT and PET in imaging bone metastases. Semin Nucl Med 2009;39:396-407. [Crossref] [PubMed]
- Langsteger W, Rezaee A, Pirich C, et al. (18)F-NaF-PET/CT and (99m)Tc-MDP Bone Scintigraphy in the Detection of Bone Metastases in Prostate Cancer. Semin Nucl Med 2016;46:491-501. [Crossref] [PubMed]
- Teo MY, Rathkopf DE, Kantoff P. Treatment of Advanced Prostate Cancer. Annu Rev Med 2019;70:479-99. [Crossref] [PubMed]
- Achard V, Putora PM, Omlin A, et al. Metastatic Prostate Cancer: Treatment Options. Oncology 2022;100:48-59. [Crossref] [PubMed]
- Yamada Y, Beltran H. The treatment landscape of metastatic prostate cancer. Cancer Lett 2021;519:20-9. [Crossref] [PubMed]
- Boelaars K, Rodriguez E, Huinen ZR, et al. Pancreatic cancer-associated fibroblasts modulate macrophage differentiation via sialic acid-Siglec interactions. Commun Biol 2024;7:430. [Crossref] [PubMed]
- Cheng B, Xie R, Dong L, et al. Metabolic Remodeling of Cell-Surface Sialic Acids: Principles, Applications, and Recent Advances. Chembiochem 2016;17:11-27. [Crossref] [PubMed]
- Nagasundaram M, Horstkorte R, Gnanapragassam VS. Sialic Acid Metabolic Engineering of Breast Cancer Cells Interferes with Adhesion and Migration. Molecules 2020;25:2632. [Crossref] [PubMed]
- Popęda M, Kowalski K, Wenta T, et al. Emerin mislocalization during chromatin bridge resolution can drive prostate cancer cell invasiveness in a collagen-rich microenvironment. Exp Mol Med 2024;56:2016-32. [Crossref] [PubMed]
- Cong M, Song C, Xu H, et al. The patient-generated subjective global assessment is a promising screening tool for cancer cachexia. BMJ Support Palliat Care 2022;12:e39-46. [Crossref] [PubMed]
- Liu XY, Zhang X, Ruan GT, et al. One-Year Mortality in Patients with Cancer Cachexia: Association with Albumin and Total Protein. Cancer Manag Res 2021;13:6775-83. [Crossref] [PubMed]
- Brookman-May SD, Campi R, Henríquez JDS, et al. Latest Evidence on the Impact of Smoking, Sports, and Sexual Activity as Modifiable Lifestyle Risk Factors for Prostate Cancer Incidence, Recurrence, and Progression: A Systematic Review of the Literature by the European Association of Urology Section of Oncological Urology (ESOU). Eur Urol Focus 2019;5:756-87. [Crossref] [PubMed]
- Kenfield SA, Stampfer MJ, Chan JM, et al. Smoking and prostate cancer survival and recurrence. JAMA 2011;305:2548-55. [Crossref] [PubMed]
- Guillet C, Masgrau A, Walrand S, et al. Impaired protein metabolism: interlinks between obesity, insulin resistance and inflammation. Obes Rev 2012;13:51-7. [Crossref] [PubMed]
- Vankrunkelsven W, Derde S, Gunst J, et al. Obesity attenuates inflammation, protein catabolism, dyslipidaemia, and muscle weakness during sepsis, independent of leptin. J Cachexia Sarcopenia Muscle 2022;13:418-33. [Crossref] [PubMed]
- Ahmed B, Konje JC. The epidemiology of obesity in reproduction. Best Pract Res Clin Obstet Gynaecol 2023;89:102342. [Crossref] [PubMed]
- Hou Y, Wang SF, Zhou K, et al. Comparison and recommendation of dietary patterns based on nutrients for Eastern and Western patients with inflammatory bowel disease. Front Nutr 2022;9:1066252. [Crossref] [PubMed]


