
Symphony of rebirth: a retrospective comparative cohort study on heterologous breast reconstruction after radiotherapy
Highlight box
Key findings
• Implant-based breast reconstruction after radiotherapy is feasible.
• No significant increase in early complications was observed in irradiated patients.
• Aesthetic outcomes were comparable between irradiated and non-irradiated groups.
What is known and what is new?
• Radiotherapy is often seen as a risk factor for implant reconstruction. Previous studies reported higher complication rates in irradiated patients.
• This study shows that, with proper planning, reconstruction after radiotherapy is safe and effective.
What is the implication, and what should change now?
• Prior radiotherapy should not be considered an absolute contraindication.
• Implant reconstruction can be offered with confidence in selected cases.
• Surgical decisions should be based on patient-specific factors, not solely on radiotherapy history.
Introduction
Breast cancer is the most frequently diagnosed cancer and the leading cause of cancer-related death among women worldwide, with epidemiological data indicating that one in eight women will develop the disease during their lifetime (1). For early-stage breast cancer, the current standard of care consists of breast-conserving surgery followed by adjuvant radiotherapy, which offers oncologic safety comparable to mastectomy while preserving breast contour and improving quality of life (2). Despite its efficacy, this treatment paradigm is associated with a risk of loco-regional recurrence, which occurs in approximately 7% to 15% of cases (3-5). In such scenarios, salvage mastectomy is often required, and the subsequent choice of reconstructive strategy becomes particularly complex, especially in the setting of previously irradiated tissues.
Radiotherapy induces profound alterations in the microvascular and structural integrity of soft tissues, leading to fibrosis, decreased vascularity, and impaired wound healing capacity (6-9). These changes significantly affect surgical planning and outcomes in breast reconstruction, particularly in implant-based procedures, which are more susceptible to complications in irradiated fields. While autologous reconstruction is often considered the preferred approach in such patients due to its reliance on well-vascularized tissue, it is not always feasible or accepted by the patient due to longer operative times, donor site morbidity, and more complex recovery. As a result, prosthetic reconstruction remains widely used, especially in high-volume centers where evolving surgical techniques have improved the feasibility and safety of heterologous reconstruction even in the post-radiotherapy setting (8-11).
However, current evidence regarding the safety, efficacy, and aesthetic outcomes of implant-based reconstruction after radiotherapy remains limited and heterogeneous. Much of the literature includes small sample sizes, lacks comparison with non-irradiated cohorts, or focuses on highly selected populations. Moreover, the impact of key variables such as implant plane selection (prepectoral vs. subpectoral), use of mesh reinforcement, and the role of direct-to-implant versus staged reconstruction in previously irradiated patients remains insufficiently clarified. In this context, recent reviews have emphasized the need to reconsider reconstructive protocols for irradiated patients. Broyles et al. highlighted the increasing complexity of preoperative radiotherapy and advocated for the development of new paradigms in reconstructive planning to mitigate its effects (12). Silverstein and Momeni explored the ongoing debate between breast-conserving therapy with radiotherapy and mastectomy followed by reconstruction, emphasizing the importance of individualized treatment choices based on tumor biology, patient expectations, and reconstructive feasibility (13). Furthermore, Maita et al. outlined technical considerations for reconstruction in patients undergoing neoadjuvant or adjuvant radiotherapy, including the importance of flap vascularity, timing of reconstruction, and management of irradiated tissues (14).
Given these considerations, further studies comparing outcomes between irradiated and non-irradiated patients undergoing similar reconstructive approaches are essential to guide clinical decision-making. In particular, there is a lack of data focusing specifically on heterologous reconstruction using expanders and implants in patients who undergo salvage mastectomy following breast-conserving surgery and radiotherapy.
The aim of this study was to evaluate the safety, short-term complication rates, and aesthetic outcomes of implant-based breast reconstruction in patients with prior radiotherapy (PRS group) compared to those without radiotherapy exposure (NPRS group), treated with similar surgical techniques. By focusing on a homogeneous surgical protocol and a consistent evaluation framework, this retrospective cohort analysis aims to provide additional evidence on the feasibility of heterologous reconstruction in a high-risk population, and to inform future prospective research in this domain. We present this article in accordance with the STROBE reporting checklist (available at https://gs.amegroups.com/article/view/10.21037/gs-2024-552/rc).
Methods
This retrospective study was conducted at the Breast Unit of the “Spedali Riuniti” of Livorno, part of the Azienda USL Toscana Nord Ovest, located in Livorno, Italy. The study was conducted in accordance with the Declaration of Helsinki and its subsequent amendments. Ethics approval was not required as this was a retrospective analysis of fully anonymized data obtained from operative records and clinical charts, without direct patient contact or identifiable information. All patients provided informed consent for the surgical procedures and for the use of anonymized data for research purposes. Informed consent for the publication of results was also obtained.
Each patient provided comprehensive informed consent, including detailed information about the heterologous reconstruction procedure, potential risks, and expected benefits. No off-label procedures were used, and ethical approval was not required. All patients treated between January 2020 and December 2023 with conservative mastectomy and heterologous reconstruction were enrolled. Data were divided into two groups: patients who had undergone radiotherapy (PRS) and those who had not (NPRS), and were subsequently compared. Data were retrospectively collected by analyzing operative reports (O4C software) and patient charts (C7 software).
The inclusion criteria for the PRS group comprised patients who had previously undergone quadrantectomy followed by radiotherapy for breast carcinoma and subsequently developed a local recurrence. The NPRS group included all patients who underwent mastectomy with heterologous reconstruction without any prior history of breast radiotherapy. Patients subsequently underwent homolateral nipple-sparing mastectomy (NSM) or skin-sparing mastectomy (SSM), with the choice of procedure determined based on the lesion’s location, potential nipple involvement, and breast volume. Reconstruction was performed either immediately, in a single stage, or delayed, with implant placement in either the subpectoral or prepectoral space. Preoperative risk factors, including age, smoking status, body mass index (BMI), and diabetes, were recorded (Figure 1).
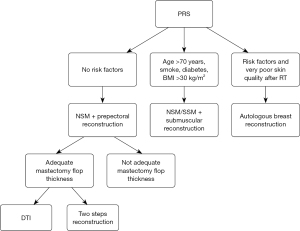
In patients with a history of prior radiotherapy (PRS group), the reconstructive strategy was determined according to a decision-making algorithm based on clinical risk factors and intraoperative findings, aiming to tailor the surgical approach to individual patient profiles. In the absence of significant risk factors, such as age under 70 years, non-smoking status, absence of diabetes, and BMI below 30 kg/m2, a NSM followed by prepectoral implant-based reconstruction was performed. Within this subgroup, the choice between direct-to-implant (DTI) reconstruction and a two-stage approach with tissue expander placement was guided by intraoperative assessment of mastectomy flap thickness and vascularity: adequate flap characteristics allowed for immediate DTI reconstruction, whereas inadequate flaps required a staged procedure. In the presence of risk factors (age over 70 years, smoking, diabetes, or BMI greater than 30), the implant was placed in a submuscular pocket, with NSM or SSM selected based on tumor location and nipple involvement. In patients presenting with multiple risk factors and severely compromised skin quality following radiotherapy, autologous breast reconstruction was recommended as a safer and more reliable alternative. This algorithmic approach enabled individualized planning, optimizing both surgical safety and aesthetic outcomes (Figure 1).
All patients in the PRS group received a standardized adjuvant radiotherapy regimen consisting of 50 Gy delivered in 25 fractions. The prepectoral approach was consistently reinforced with a synthetic surgical mesh (Ti-Loop®) in all cases to support the skin envelope and minimize complications. Only patients who had completed the reconstructive course, either through a DTI procedure or with a prosthetic implant following expander removal (TE), were included in the study.
Postoperative parameters, including pain, asthenia, postoperative nausea and vomiting (PONV), and complications such as minor and major infections, bleeding, and wound dehiscence, were carefully assessed.
The aesthetic appearance and overall patient satisfaction were evaluated by a plastic surgeon who was independent of the operative team and did not participate in the surgical procedures, in order to minimize assessment bias.
This study was specifically designed to evaluate early postoperative outcomes occurring within 120 days after surgery. Long-term complications, such as capsular contracture, implant rupture, or delayed implant loss, were not assessed and fall outside the scope of this analysis.
Statistical analysis
The statistical evaluation focused on comparing postoperative outcomes and complication rates between the PRS and NPRS groups. Categorical variables, including surgical procedures and complications, were compared using standard contingency table analysis. For rare events or small sample subsets, appropriate exact methods were employed. Continuous variables, such as age and BMI, were described using median and range, without application of inferential testing due to the limited size of the irradiated cohort. Statistical significance was considered for P values <0.05. The results of the analysis are reported in Tables 1-4.
Table 1
| Characteristic | Total (n=175) | PRS (n=15) | NPRS (n=160) | P value |
|---|---|---|---|---|
| Age, years | 63 [42–87] | 62 [52–87] | 64 [42–84] | – |
| BMI, kg/m2 | 28 [25–32] | 29 [26–31] | 28 [25–32] | – |
| Smokers | 24 (13.7) | 4 (26.7) | 20 (12.5) | 0.28 |
| Diabetics | 5 (2.9) | 1 (6.7) | 4 (2.5) | 0.94 |
| NSM | 108 (61.7) | 11 (73.3) | 97 (60.6) | 0.49 |
| SSM | 67 (38.3) | 4 (26.7) | 63 (39.4) | 0.49 |
| Prepectoral TE | 102 (58.3) | 4 (26.7) | 98 (61.3) | 0.02 |
| Subpectoral TE | 58 (33.1) | 10 (66.7) | 48 (30.0) | 0.009 |
| DTI | 15 (8.6) | 1 (6.7) | 14 (8.8) | >0.99 |
| Lipofilling | 22 (12.6) | 6 (40.0) | 16 (10.0) | 0.003 |
Data are presented as median [range] or n (%). BMI, body mass index; DTI, direct-to-implant; NSM, nipple-sparing mastectomy; NPRS, non-irradiated patient group; PRS, previously irradiated patient group; SSM, skin-sparing mastectomy; TE, tissue expander.
Table 2
| Outcome | Total (n=175) | PRS (n=15) | NPRS (n=160) | P value |
|---|---|---|---|---|
| Discharged on POD 1 | 132 (75.4) | 12 (80.0) | 131 (81.9) | >0.99 |
| Discharged on POD 2 | 23 (13.1) | 2 (13.3) | 21 (13.1) | >0.99 |
| Discharged on POD 3+ | 10 (5.7) | 1 (6.7) | 9 (5.6) | >0.99 |
| Asthenia | 16 (9.1) | 1 (6.7) | 15 (9.4) | >0.99 |
| Pain | 8 (4.6) | 1 (6.7) | 7 (4.4) | >0.99 |
| PONV | 10 (5.7) | 1 (6.7) | 9 (5.6) | >0.99 |
Data are presented as n (%). NPRS, non-irradiated patient group; PRS, previously irradiated patient group; POD, postoperative day; PONV, postoperative nausea and vomiting.
Table 3
| Complication | Total (n=175) | PRS (n=15) | NPRS (n=160) | P value |
|---|---|---|---|---|
| Infection | 3 (1.7) | 1 (6.7) | 15 (9.4) | >0.99 |
| Bleeding | 9 (5.1) | 0 (0.0) | 9 (5.6) | 0.74 |
| Wound dehiscence | 3 (1.7) | 1 (6.7) | 9 (5.6) | >0.99 |
Data are presented as n (%).
Table 4
| Aesthetic score | Total (n=175) | PRS (n=15) | NPRS (n=160) | P value |
|---|---|---|---|---|
| Score 3 | 20 (11.4) | 1 (6.7) | 19 (11.9) | 0.86 |
| Score 4 | 89 (50.9) | 9 (60.0) | 80 (50.0) | 0.64 |
| Score 5 | 66 (37.7) | 5 (33.3) | 61 (38.1) | 0.93 |
Data are presented as n (%). NPRS, non-irradiated patient group; PRS, previously irradiated patient group.
Results
The study included 175 patients. A total of 15 patients (9%) had received pre-operative radiotherapy before surgery (PRS group), whereas 160 patients (91%) had not (NPRS group). The median age in the PRS group was 62 years (range, 52–87 years), with a median BMI of 29 kg/m2 (range, 26–31 kg/m2). In the NPRS group, the median age was 64 years (range, 42–84 years) and the median BMI was 28 kg/m2 (range, 25–32 kg/m2). The two groups were comparable in demographic characteristics, except for a higher proportion of smokers in PRS (4/15, 26.7%) than in NPRS (20/160, 12.5%; P=0.28); diabetes prevalence was similar (PRS 1/15, 6.7% vs. NPRS 4/160, 2.5%; P=0.94).
Regarding implant positioning, subpectoral tissue expanders were significantly more frequent in PRS (10/15, 66.7%) than in NPRS (48/160, 30.0%; P=0.009), whereas prepectoral expanders predominated in NPRS (98/160, 61.3%) compared with PRS (4/15, 26.7%; P=0.02). NSM and SSM were chosen in both groups without statistically significant differences (NSM 73.3% vs. 60.6%; SSM 26.7% vs. 39.4%; both P=0.49). Direct-to-implant (DTI) procedures were comparable (PRS 1/15, 6.7% vs. NPRS 14/160, 8.8%; P>0.99). Lipofilling was more frequent in PRS (6/15, 40.0%) than in NPRS (16/160, 10.0%; P=0.003) (Table 1).
Surgical procedures
In the NPRS group, 97 patients (60.6%) underwent NSM and 63 patients (39.4%) SSM. Among these, 98 reconstructions (61.3%) were performed in the prepectoral space and 48 reconstructions (30.0%) in the subpectoral space; in addition, 14 DTI procedures (8.8%) were recorded.
In the PRS group, 11 patients (73.3%) underwent NSM and 4 patients (26.7%) SSM. Among these, 4 reconstructions (26.7%) were prepectoral and 10 reconstructions (66.7%) subpectoral; 1 DTI procedure (6.7%) was performed. A statistically significant difference therefore emerged in implant positioning, with a higher prevalence of subpectoral reconstruction in PRS.
Discharge, complications and viability
In the NPRS group, 131/160 patients (81.9%) were discharged on postoperative day (POD) 1, 21/160 (13.1%) on POD 2 and 8/160 (5.0%) on POD 3 or later. Nine postoperative hematomas (5.6%) were observed, all managed conservatively.
In the PRS group, 12/15 patients (80.0%) were discharged on POD 1, 2/15 (13.3%) on POD 2 and 1/15 (6.7%) on POD 3. The POD 2 delays were due to asthenia in one case and persistent pain in another; the POD 3 discharge followed prolonged PONV (Table 2).
Infections were classified as major or minor. In NPRS, one major infection occurred three months after SSM during adjuvant chemotherapy and required explantation, subpectoral re-implantation, and placement of a local antibiotic carrier (Stimulan®). Six further minor infections were successfully managed with oral antibiotics. In PRS, one major infection required explantation of the tissue expander and immediate prosthetic replacement after four days of intravenous antibiotics.
There were nine cases of minor wound dehiscence in NPRS: three were treated with negative-pressure wound therapy (NPWT) and six with standard dressings. One wound dehiscence occurred in PRS (an 87-year-old smoker after DTI) and was managed by outpatient excision of ischemic tissue and resuturing. Postoperative bleeding occurred in nine NPRS patients (5.6%) and in none of the PRS patients (Table 3).
Aesthetic functional outcomes
A plastic surgeon independent of the operative team scored aesthetic outcomes on a 1-to-5 scale. In PRS (n=15), 1 patient (6.7%) scored 3, 9 patients (60.0%) scored 4 and 5 patients (33.3%) scored 5. In NPRS (n=160), 19 patients (11.9%) scored 3, 80 patients (50.0%) scored 4 and 61 patients (38.1%) scored 5 (Table 4).
Statistical analyses
Apart from implant positioning and lipofilling (both P<0.05), no significant differences were found between PRS and NPRS. Discharge timing (POD 1, POD 2, POD 3+), asthenia, pain and PONV were comparable (P>0.99for each). Complications—infection (P>0.99), bleeding (P=0.74) and wound dehiscence (P>0.99)—and aesthetic outcomes (score 3: P=0.86, score 4: P=0.64, score 5: P=0.93) likewise showed no statistically significant differences between the groups.
Discussion
Breast reconstruction following mastectomy represents a cornerstone in the therapeutic journey and the reconstitution of body image for individuals diagnosed with breast carcinoma. This process extends beyond a mere surgical intervention, embodying a pathway toward the restoration of physical and psychological wholeness, which is crucial for patient welfare. The decision-making process leading to implant-based reconstruction in our study was based on thorough consultations between patient and surgeon, highlighting the importance of a customized and well-informed approach achieved through a comprehensive evaluation of patient needs, expectations, and prior clinical history, including exposure to radiotherapy. The choice of implant plane (prepectoral versus submuscular) may also influence the occurrence of specific complications. In our experience, submuscular placement was preferred in irradiated patients due to the increased fragility and reduced vascularity of the mastectomy flaps, which may predispose to wound dehiscence or delayed healing. Furthermore, the autologous reconstruction option was discussed with patients, focusing on both its advantages and possible complications.
Our investigation compared patients with and without previous radiotherapy who underwent heterologous breast reconstruction with prosthetic implants. Statistical analyses revealed no significant differences in the postoperative course or complication rates between the two groups, suggesting that prior radiotherapy did not markedly impact the outcomes for these patients. Specifically, no statistically significant differences were observed regarding the timing of hospital discharge between the PRS and NPRS groups. Similarly, there were no significant differences in bleeding rates, infection rates, pain, or PONV. Aesthetic outcomes, evaluated by an independent plastic surgeon, demonstrated that in the PRS group, 86.7% of patients achieved scores of 4 or 5. Thus, despite the known challenges posed by prior irradiation, the majority of patients exhibited satisfactory cosmetic results.
The scientific literature corroborates that tissues previously subjected to radiotherapy present unique challenges due to alterations in vascularization, elasticity, and healing capacity (10-14). While radiotherapy is a critical modality for breast carcinoma treatment, it can adversely impact postoperative outcomes by increasing the risks of capsular contracture, infection, impaired wound healing, and suboptimal aesthetic results (13-19). Despite these challenges, prosthetic reconstruction remains a widely adopted choice due to its less invasive nature compared with autologous options, alongside evolving surgical techniques aimed at improving outcomes even in irradiated fields (14,20). Our data from Table 1 indicated that subpectoral tissue expander placement was preferred in the PRS group (66.7%), while prepectoral reconstruction was generally favored in the NPRS group (64.4%). This choice reflects intraoperative findings, as mastectomy flaps in irradiated patients were often more fragile and thinner, making submuscular positioning more appropriate.
It is important to contextualize these findings within the broader spectrum of reconstructive options. While the latissimus dorsi flap provides a reliable option for breast reconstruction, it carries drawbacks such as increased postoperative pain and functional impairments. Misra et al. demonstrated that patients undergoing latissimus dorsi reconstruction may experience more postoperative pain compared to other techniques (21-25). Additionally, long-term reductions in muscle strength and torso mobility have been documented (21-25). By opting for implant-based reconstruction, particularly in irradiated patients, our approach aims to minimize postoperative discomfort and preserve functional capacity (26).
El-Sabawi et al. conducted a review on satisfaction rates following implant-based reconstruction in radiotherapy-treated patients, reporting positive aesthetic outcomes (“good” or better) ranging from 36% to 100%, with most studies indicating rates between 70% and 90%. Patient satisfaction rates varied widely from 41% to 90% (26).
Nonetheless, radiotherapy remains a significant complicating factor for reconstruction. A recent study published in the British Journal of Surgery reported higher complication rates, including capsular contracture, in patients who had received radiotherapy compared to non-irradiated patients (27,28). Additionally, the need for revision surgery is significantly increased in this population. Another study published in the Journal of the National Cancer Institute emphasized that, despite these complications, implant-based reconstruction remains a viable option when careful patient selection and meticulous surgical planning are employed (29,30). These findings underscore the need for a personalized, multidisciplinary approach, emphasizing clear communication with patients regarding their surgical options and potential risks.
Limitations and future directions
A limitation of our study is the relatively small sample size of the radiotherapy group, which nevertheless reflects the total cohort of patients treated at our institution between January 2020 and December 2023 for ipsilateral breast cancer recurrence following breast-conserving surgery. The low number of cases requiring salvage mastectomy after radiotherapy is consistent with previous literature, where recurrence-related mastectomies remain uncommon events even over extended periods (7,30,31). As a retrospective study, our analysis is subject to the inherent limitations of this design, including potential selection bias and variability in follow-up documentation. Furthermore, the current study focuses exclusively on short-term postoperative outcomes. Longer follow-up periods are necessary to evaluate late complications such as capsular contracture, implant failure, or the need for revision surgery, which are particularly relevant in the setting of post-radiotherapy reconstruction.
Future research should focus on prospective, multicenter studies with larger sample sizes and standardized follow-up protocols. The inclusion of patient-reported outcome measures and objective aesthetic assessments will also be essential to better evaluate the long-term safety, satisfaction, and effectiveness of implant-based reconstruction in previously irradiated patients.
Conclusions
Our study confirms that heterologous breast reconstruction using prosthetic implants can be performed safely and effectively in patients irrespective of prior exposure to radiotherapy, with a low incidence of postoperative complications. While radiotherapy introduces significant technical and biological challenges, our findings underscore the pivotal role of a tailored, patient-centered approach, supported by clear and continuous communication between the surgical team and the patient, in optimizing both aesthetic and functional outcomes. These results reinforce the value of a skilled and experienced multidisciplinary team, as well as the integration of advanced surgical techniques, in enhancing postoperative recovery, quality of life, and psychosocial well-being. Breast reconstruction represents a fundamental component of the therapeutic continuum for patients undergoing mastectomy, and with meticulous planning and execution, the obstacles posed by radiotherapy can be effectively mitigated to achieve satisfactory long-term results.
Acknowledgments
None.
Footnote
Reporting Checklist: The authors have completed the STROBE reporting checklist. Available at https://gs.amegroups.com/article/view/10.21037/gs-2024-552/rc
Data Sharing Statement: Available at https://gs.amegroups.com/article/view/10.21037/gs-2024-552/dss
Peer Review File: Available at https://gs.amegroups.com/article/view/10.21037/gs-2024-552/prf
Funding: None.
Conflicts of Interest: All authors have completed the ICMJE uniform disclosure form (available at https://gs.amegroups.com/article/view/10.21037/gs-2024-552/coif). I.S. serves as an unpaid editorial board member for Gland Surgery from September 2023 to August 2025. W.M.R. serves as an unpaid editorial board member for Gland Surgery from March 2023 to February 2028. The other authors have no conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved. The study was conducted in accordance with the Declaration of Helsinki and its subsequent amendments. Ethics approval was not required as this was a retrospective analysis of fully anonymized data obtained from operative records and clinical charts, without direct patient contact or identifiable information. All patients provided informed consent for the surgical procedures and for the use of anonymized data for research purposes. Informed consent for the publication of results was also obtained.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Arnold M, Morgan E, Rumgay H, et al. Current and future burden of breast cancer: Global statistics for 2020 and 2040. Breast 2022;66:15-23. [Crossref] [PubMed]
- André C, Holsti C, Svenner A, et al. Recurrence and survival after standard versus oncoplastic breast-conserving surgery for breast cancer. BJS Open 2021;5:zraa013. [Crossref] [PubMed]
- Braunstein LZ, Niemierko A, Shenouda MN, et al. Outcome following local-regional recurrence in women with early-stage breast cancer: impact of biologic subtype. Breast J 2015;21:161-7. [Crossref] [PubMed]
- Freedman GM, Fowble BL. Local recurrence after mastectomy or breast-conserving surgery and radiation. Oncology (Williston Park) 2000;14:1561-81; discussion 1581-2, 1582-4.
- Miles RC, Gullerud RE, Lohse CM, et al. Local recurrence after breast-conserving surgery: multivariable analysis of risk factors and the impact of young age. Ann Surg Oncol 2012;19:1153-9. [Crossref] [PubMed]
- Barry M, Kell MR. Radiotherapy and breast reconstruction: a meta-analysis. Breast Cancer Res Treat 2011;127:15-22. [Crossref] [PubMed]
- Murphy BL, Boughey JC, Hieken TJ. Nipple-sparing Mastectomy for the Management of Recurrent Breast Cancer. Clin Breast Cancer 2017;17:e209-e213.
- Pacchioni L, Sapino G, Lusetti IL, et al. Sub-Muscular Direct-to-Implant Immediate Breast Reconstruction in Previously Irradiated Patients Avoiding the Use of ADM: A Preliminary Study. J Clin Med 2022;11:5856. [Crossref] [PubMed]
- Schaverien MV, Macmillan RD, McCulley SJ. Is immediate autologous breast reconstruction with postoperative radiotherapy good practice?: a systematic review of the literature. J Plast Reconstr Aesthet Surg 2013;66:1637-51. [Crossref] [PubMed]
- Casella D, Fusario D, Cassetti D, et al. Controlateral Symmetrisation in SRM for Breast Cancer: Now or Then? Immediate versus Delayed Symmetrisation in a Two-Stage Breast Reconstruction. Curr Oncol 2022;29:9391-400. [Crossref] [PubMed]
- Ochoa O, Chrysopoulo MT. Preoperative Assessment of the Breast Reconstruction Patient. Clin Plast Surg 2023;50:201-10. [Crossref] [PubMed]
- Broyles JM, Bazan JG, Park KU. Navigating the complexities of preoperative radiotherapy in breast reconstruction: a new paradigm? Gland Surg 2025;14:272-5. [Crossref] [PubMed]
- Silverstein ML, Momeni A. A plastic surgery perspective on the choice between breast conserving surgery with radiotherapy versus mastectomy and reconstruction. Gland Surg 2024;13:449-51. [Crossref] [PubMed]
- Maita KC, Torres-Guzman RA, Avila FR, et al. Technical consideration for breast reconstruction in patients requiring neoadjuvant or adjuvant radiotherapy: a narrative review. Ann Transl Med 2023;11:417. [Crossref] [PubMed]
- Quinn TT, Miller GS, Rostek M, et al. Prosthetic breast reconstruction: indications and update. Gland Surg 2016;5:174-86. [Crossref] [PubMed]
- Rozen WM, Ashton MW. Radiotherapy and breast reconstruction: oncology, cosmesis and complications. Gland Surg 2012;1:119-27. [Crossref] [PubMed]
- Cuomo R. Submuscular and Pre-Pectoral ADM Assisted Immediate Breast Reconstruction: A Literature Review. Medicina (Kaunas) 2020;56:256. [Crossref] [PubMed]
- Cuomo R. The State of the Art about Etiopathogenetic Models on Breast Implant Associated-Anaplastic Large Cell Lymphoma (BIA-ALCL): A Narrative Review. J Clin Med 2021;10:2082. [Crossref] [PubMed]
- Pagliara D, Grieco F, Rampazzo S, et al. Prevention of Breast Cancer-Related Lymphedema: An Up-to-Date Systematic Review of Different Surgical Approaches. J Clin Med 2024;13:555. [Crossref] [PubMed]
- Susini P, Marcaccini G, Giardino FR, et al. Selective Capsulotomies and Partial Capsulectomy in Implant-Based Breast Reconstruction Revision Surgery. Breast J 2024;2024:9097040. [Crossref] [PubMed]
- Misra A, Chester D, Park A. A comparison of postoperative pain between DIEP and extended latissimus dorsi flaps in breast reconstruction. Plast Reconstr Surg 2006;117:1108-12. [Crossref] [PubMed]
- Casella D, Palumbo P, Sandroni S, et al. Positive ROS (Reactive Oxygen Species) Modulator Engineered Device Support Skin Treatment in Locally Advanced Breast Cancer (LABC) Enhancing Patient Quality of Life. J Clin Med 2021;11:126. [Crossref] [PubMed]
- Sisti A, Sadeghi P, Cuomo R, et al. Pre-Pectoral One-Stage Breast Reconstruction with Anterior Coverage Using Superior Anterior Biological Acellular Dermal Matrix (ADM) and Inferior Anterior Dermal Sling Support. Medicina (Kaunas) 2022;58:992. [Crossref] [PubMed]
- Mataro I, Cuomo R, La Padula S. Breast Reconstruction: Developing a Volumetric Outcome Algorithm. Aesthetic Plast Surg 2024;48:4063-4. [Crossref] [PubMed]
- Eyjolfsdottir H, Haraldsdottir B, Ragnarsdottir M, et al. A Prospective Analysis on Functional Outcomes Following Extended Latissimus Dorsi Flap Breast Reconstruction. Scand J Surg 2017;106:152-7. [Crossref] [PubMed]
- El-Sabawi B, Ho AL, Sosin M, et al. Patient-centered outcomes of breast reconstruction in the setting of post-mastectomy radiotherapy: A comprehensive review of the literature. J Plast Reconstr Aesthet Surg 2017;70:768-80. [Crossref] [PubMed]
- de Boniface J, Coudé Adam H, Frisell A, et al. Long-term outcomes of implant-based immediate breast reconstruction with and without radiotherapy: a population-based study. Br J Surg 2022;109:1107-15. [Crossref] [PubMed]
- Jagsi R, Momoh AO, Qi J, et al. Impact of Radiotherapy on Complications and Patient-Reported Outcomes After Breast Reconstruction. J Natl Cancer Inst 2018;110:157-65. [Crossref] [PubMed]
- Momoh AO, Ahmed R, Kelley BP, et al. A systematic review of complications of implant-based breast reconstruction with prereconstruction and postreconstruction radiotherapy. Ann Surg Oncol 2014;21:118-24. [Crossref] [PubMed]
- Barellini L, Miccoli S, Daicampi B, et al. Video-assisted submuscular breast reconstruction: evaluating a novel technique in high-risk patients. Gland Surg 2025;14:172-8. [Crossref] [PubMed]
- ElSherif A, Armanyous S, Al-Hilli Z, et al. Mastectomy options for the treatment of ipsilateral breast cancer recurrence after lumpectomy. Am J Surg 2022;223:447-51. [Crossref] [PubMed]


