
Risk factors for postoperative outcomes in laparoscopic pancreaticoduodenectomy: a retrospective cohort study from 2015 to 2023
Highlight box
Key findings
• Significant risk factors for postoperative complications and intensive care unit (ICU) admission after laparoscopic pancreaticoduodenectomy (LPD) included lower preoperative bilirubin level, higher intraoperative blood loss, blood transfusion, revascularization, and chronic pancreatitis.
• The most common postoperative complications were delayed gastric emptying, biliary fistula, and pancreatic fistula.
• Intraoperative factors such as surgery duration, blood loss, and transfusion volume were significantly associated with a longer hospital stay.
What is known and what is new?
• LPD is associated with lower morbidity and faster recovery compared to traditional open pancreaticoduodenectomy. However, postoperative complications and prolonged hospital stays remain significant concerns.
• This study identified certain intraoperative and preoperative factors, including blood loss, transfusions, and revascularization, as key contributors to complications and longer hospital stays, offering new insights into the factors influencing outcomes in patients treated with LPD for pancreatic tumors.
What is the implication, and what should change now?
• Managing intraoperative factors, including minimizing blood loss and transfusion requirements, and optimizing surgical techniques to avoid revascularization may reduce the risk of complications and shorten the length of hospital stays.
• Future studies should refine predictive models for ICU admission and complications, focusing on improving the accuracy of risk stratification tools. Additionally, surgical teams should prioritize strategies that minimize blood loss and prevent unnecessary transfusions during LPD to enhance patient recovery and reduce postoperative morbidity.
Introduction
Study background
Pancreatic tumors are among the most malignant tumors (1,2). Pancreatic cancer (especially pancreatic ductal adenocarcinoma) is associated with the worst prognosis among all the common solid malignancies and is expected to surpass colorectal cancer (second only to lung cancer) as the leading cause of cancer-related death by 2040 (3-5). The preferred treatment option for pancreatic tumors, if conditions allow, is surgical resection. Due to its complexity and involvement of multiple anastomoses, pancreaticoduodenectomy (PD) is a challenging form of abdominal surgery and the most difficult procedure for a single tumor among abdominal surgeries (6). Complications after PD pose grave concerns for clinicians and patients alike. At the early stage, abdominal bleeding and gastrointestinal bleeding are frequently observed, and if the bleeding is serious, patients require a second operation (7). In the early to midterm postoperative period, if the anastomosis is poorly performed, digestive fluids leaking into the abdominal cavity may cause corrosion of internal organs, infection, and even rupture of blood vessels, which necessitates reoperation for anastomosis and hemostasis. Pancreatic fistula and biliary fistula are two major types of post-PD leakage complication (8). At a later stage, patients may develop pancreatic dysfunction, diabetes, or dyspepsia due to insufficient pancreatic secretion. Given the various complications and poor outcomes associated with PD, the surgical approach has been improved in recent years. Laparoscopic pancreaticoduodenectomy (LPD) is a laparoscopic surgery for peripelvic tumors and pancreatic tumors. After being refined over the years, LPD can now provide the same postoperative antitumor effect as that of open surgery, with less intraoperative bleeding and better recovery (9). Indeed, it has also been confirmed that LPD is associated with a significant reduction in intraoperative bleeding compared with open PD (OPD) (10-13). Previous studies have tended to focus on the differences in postoperative outcomes between LPD and OPD (13-17). However, the current knowledge gaps are as follows. Within LPD, the preoperative and intraoperative factors that influence postoperative outcomes have not been extensively examined. LPD can still be improved considerably in terms of postoperative complications, reoperation, intensive care unit (ICU) admission, and length of postoperative hospital stay (18,19). Knowledge of how to predict the occurrence of these outcomes is still limited.
Study objective
In this study, we conducted a retrospective analysis of data from patients with pancreatic tumors who underwent LPD at a single center. Our aim was to identify the risk factors for postoperative complications, prolonged postoperative hospital stays, and admission to the ICU. Subsequently, we sought to construct prognostic models with satisfactory performance. Our findings included a number of correlations that have not been previously reported. We present this article in accordance with the STROBE reporting checklist (available at https://gs.amegroups.com/article/view/10.21037/gs-2025-63/rc).
Methods
Study design and participants
The study was conducted in accordance with the Declaration of Helsinki and its subsequent amendments. The ethical approval of this study was obtained from The Second Hospital of Hebei Medical University (ethical approval number: 2025-R013) and informed consent was taken from all the patients.
This retrospective study included 199 patients newly diagnosed with pancreatic tumor who underwent LPD between 2015 and 2023. The inclusion criteria were as follows: (I) age between 18 and 85 years; (II) eligible for partial pancreatectomy via LPD; and (III) informed consent provided prior to surgery. Meanwhile, the exclusion criteria were as follows: (I) presence of distant metastasis; (II) ASA scores >3; and (III) participation in other clinical trials for pancreatic tumors within 6 months. All patients were diagnosed with pancreatic tumors through preoperative computed tomography (CT) imaging or histological analysis. Criteria for surgery were according to the National Comprehensive Cancer Network (NCCN) guidelines.
Variables
We documented baseline variables, intraoperative variables, and postoperative outcome variables. Baseline characteristics included age, gender, height, weight, chief complaints, clinical diagnosis, pre-existing comorbidities (such as hypertension, coronary heart disease, diabetes mellitus, chronic pancreatitis, and hepatitis), history of abdominal surgery, and whether patients received neoadjuvant chemotherapy. Intraoperative variables included surgical approach (pylorus-preserving or not), intraoperative bleeding volume, necessity of blood transfusion and its volume, operation duration, presence of pancreatic-jejunal or pancreatic-gastric anastomosis, biliary anastomosis, and revascularization (if applicable). Postoperative outcomes included complications, length of postoperative hospitalization, requirement for second operation, perioperative mortality, ICU admission, and ICU stay duration.
Data measurements
Data measurements were obtained through clinical records and laboratory data. Baseline clinical data, including age, gender, weight, preoperative comorbidities, and laboratory markers [e.g., total bilirubin, carbohydrate antigen 19-9 (CA19-9), and carbohydrate antigen 125 (CA125)], were extracted from electronic medical records. Intraoperative details, such as surgical time, bleeding volume, and transfusion volume were recorded from surgical records. Postoperative complications, such as pancreatic fistula, biliary fistula, abdominal infection, and lung infection were documented as yes or no based on the hospital medical record system or follow-up visits. Besides, pancreatic fistula was further labeled as Grade A, B or C according to the standard of International Study Group on Pancreatic Surgery (ISGPS). The time of measurement was 90 days. The length of postoperative hospitalization was calculated from the day of surgery to discharge, with a range from 6 to 87 days and the median is 15 days.
Bias
Selection bias could have arisen due to the single-center, retrospective nature of the study, which might have limited the generalizability of the findings to other populations. Information bias was also possible, as some variation in data recording and follow-up could exist due to differences in how the clinical data and outcomes were documented by different healthcare providers. Recall bias could have affected the self-reported information from patients, especially regarding prior treatments or comorbidities. To minimize these biases, objective clinical data from medical records and imaging were prioritized.
Study size
A total of 199 patients were included in the study. The sample size was based on available patient data from a single institution. Although the sample size was relatively small, it allowed for detailed data collection and provided comprehensive insights into the factors influencing postoperative outcomes.
Statistical methods
The SPSS (V25.0) software was used for statistical analysis. Firstly, descriptive statistics were used to summarize the categorical and continuous variables. Categorical variables, such as gender, postoperative complications, and ICU admission, are presented as frequencies and percentages. Continuous variables, such as age, blood loss, and surgical duration, are presented as the mean and SD or as quartiles (the minimum, Q1, median, Q3, and the maximum) when the data distribution was skewed. To compare two groups, t-tests or Mann-Whitney tests were used for continuous variables, while Chi-squared tests were used for categorical data. A P value of less than 0.05 was considered statistically significant. Pearson correlation analysis was used to assess linear relationships between continuous variables. Logistic regression models were used to predict postoperative complications and ICU admission, but none of the models achieved satisfactory performance (accuracy ≥80% for both the actual negative and positive groups). For some dimensions, the total sample size was less than 199 due to missing (blank) data in the hospital records, we use multiple imputation method to process the missing data. Perform multiple imputation using the Mice package in R language, with a set number of 5 imputations. Use predictive mean matching (PMI) method for continuous variables and logistic regression (Logreg) method for categorical variables. After completion of interpolation, by drawing histograms and boxplots of key variables before and after interpolation, the distribution characteristics of the data can be visually compared to preliminarily evaluate that interpolation did not significantly change the data structure.
Results
Patient demographics and intraoperative data
A total of 199 patients were enrolled in this study, and the baseline clinical characteristics are summarized in Tables 1,2. All cases were pancreatic cancers/tumors, without biliary tumors. Of the 199 patients, 101 (50.75%) were male and 98 (49.25%) were female. The mean age of the cohort was 60.3 (SD 9.8) years. Prior to undergoing surgical intervention, 60 (30.15%) patients received biliary drainage.
Table 1
| Features | Frequency | Percent (%) |
|---|---|---|
| Gender | ||
| Male | 101 | 50.75 |
| Female | 98 | 49.25 |
| Preoperative biliary drainage | ||
| No | 139 | 69.85 |
| Yes | 60 | 30.15 |
| Biliary drainage method | ||
| PTCD | 48 | 80.00 |
| ENBD | 9 | 15.00 |
| ENBD + PTCD | 2 | 3.33 |
| Cholecystocentesis | 1 | 1.67 |
| Comorbid diseases | ||
| Yes | 104 | 52.26 |
| No | 95 | 47.74 |
| Hypertension | ||
| No | 112 | 56.28 |
| Yes | 87 | 43.72 |
| Coronary heart disease | ||
| No | 183 | 91.96 |
| Yes | 16 | 8.04 |
| Diabetes mellitus | ||
| No | 148 | 74.37 |
| Yes | 51 | 25.63 |
| Chronic pancreatitis | ||
| No | 187 | 93.97 |
| Yes | 12 | 6.03 |
| Hepatitis | ||
| No | 195 | 97.99 |
| Yes | 4 | 2.01 |
| History of abdominal surgery | ||
| No | 171 | 85.93 |
| Yes | 28 | 14.07 |
| Neoadjuvant chemotherapy | ||
| No | 195 | 97.99 |
| Yes | 4 | 2.01 |
The data in this table do not include censored data. ENBD, endoscopic nasobiliary drainage; PTCD, percutaneous transhepatic cholangial drainage.
Table 2
| Features | Mean | SD | Min | Q1 | Median | Q3 | Max |
|---|---|---|---|---|---|---|---|
| Age (years) | 60.3 | 9.8 | – | – | – | – | – |
| Height (m) | 1.66 | 0.079 | – | – | – | – | – |
| Weight (kg) | 63.6 | 11.0 | – | – | – | – | – |
| CA19-9 (IU/mL) | – | – | 0.6 | 46.1 | 149 | 492.8 | 1,001.0 |
| CA125 (IU/mL) | – | – | 1.7 | 10.4 | 15.4 | 22.2 | 275.0 |
| Bilirubin (μmol/L) | – | – | 0 | 12.16 | 47.4 | 131.9 | 369.0 |
CA125, carbohydrate antigen 125; CA19-9, carbohydrate antigen 19-9; SD, standard deviation.
Regarding comorbidities, 104 (52.26%) patients had at least one pre-existing condition, with the most common being hypertension (43.72%), diabetes mellitus (25.63%), coronary heart disease (8.04%), and hepatitis (2.01%). A history of abdominal surgery was reported by 28 patients (14.07%). Only 2.01% of patients received neoadjuvant chemotherapy before surgery. The median of carbohydrate antigen 19-9 (CA19-9) (IU/mL) is 149, with a first quartile (Q1) of 0.6 and a third quartile (Q3) of 492.8. The median of carbohydrate antigen 125 (CA125) (IU/mL) is 15.4, Q1 is 10.4, and Q3 is 22.2. The median preoperative bilirubin (µ mol/L) level was 47.4, Q1 was 12.16, and Q3 was 131.9.
The intraoperative data are presented in Tables 3,4. A total of 7 (3.52%) patients underwent LPD with pylorus preservation. Intraoperative conversion occurred in 12 (6.03%) patients. The majority of patients (95.98%) underwent the Child method for gastrointestinal anastomosis, involving PD with pancreatic-jejunal anastomosis. Only eight patients underwent pancreatic-gastric anastomosis. Additionally, revascularization was performed in 28 (14.07%)patients.
Table 3
| Features | Frequency | Percent (%) |
|---|---|---|
| Pylorus preservation | ||
| No | 192 | 96.48 |
| Yes | 7 | 3.52 |
| Intraoperative change of surgical method | ||
| No | 187 | 93.97 |
| Yes | 12 | 6.03 |
| Gastrointestinal anastomosis | ||
| Child | 191 | 95.98 |
| Pancreatic-gastric | 8 | 4.02 |
| Stenting in pancreatic-enteric anastomosis | ||
| Yes | 160 | 80.40 |
| No | 39 | 19.60 |
| Stenting in biliary anastomosis | ||
| No | 188 | 94.47 |
| Yes | 11 | 5.53 |
| Revascularization | ||
| No | 171 | 85.93 |
| Yes | 28 | 14.07 |
The data in this table do not include censored data.
Table 4
| Features | Mean | SD | Min | Q1 | Median | Q3 | Max |
|---|---|---|---|---|---|---|---|
| Duration of surgery (min) | 430.8 | 120.2 | – | – | – | – | – |
| Intraoperative bleeding (mL) | – | – | 50 | 200 | 500 | 850 | 7,000 |
| Total amount of blood transfusion (mL) | – | – | 0 | 0 | 400 | 600 | 3,250 |
SD, standard deviation.
The mean duration of the surgical procedure was 430.8 (SD 120.2) minutes. The median intraoperative blood loss was 500 mL (Q1 =200 mL, Q3 =850 mL). A total of 122 (61.3%) patients received an intraoperative blood transfusion, with a median transfusion volume of 400 mL (Q1 =0 mL, Q3 =600 mL).
Postoperative outcomes
The intraoperative conditions are presented in Figure 1, while the postoperative outcomes (30 days after operation) are summarized in Tables 5,6. The most common postoperative complications included pancreatic fistula (9/199, 4.52%), biliary fistula (10/199, 5.03%), delayed gastric emptying (17/199, 8.54%), gastrointestinal fistula (2/199, 1.01%), postoperative gastrointestinal bleeding (11/199, 5.53%), abdominal infection (9/199, 4.52%), and lung infection (5/199, 2.51%).
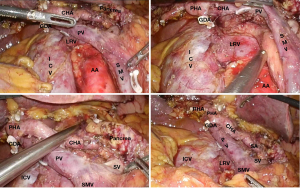
Table 5
| Features | Frequency | Percent (%) |
|---|---|---|
| Pancreatic fistula | ||
| No | 190 | 95.48 |
| A | 2 | 1.01 |
| B | 2 | 1.01 |
| C | 5 | 2.51 |
| Biliary fistula | ||
| No | 189 | 94.97 |
| Yes | 10 | 5.03 |
| Gastric emptying obstruction | ||
| No | 182 | 91.46 |
| Yes | 17 | 8.54 |
| Gastrointestinal fistula | ||
| No | 197 | 98.99 |
| Yes | 2 | 1.01 |
| Postoperative gastrointestinal bleeding | ||
| No | 188 | 94.47 |
| Yes | 11 | 5.53 |
| Abdominal infection | ||
| No | 190 | 95.48 |
| Yes | 9 | 4.52 |
| Lung infection | ||
| No | 194 | 97.49 |
| Yes | 5 | 2.51 |
| Second operation | ||
| No | 188 | 94.47 |
| Yes | 11 | 5.53 |
| Perioperative death | ||
| No | 197 | 98.99 |
| Yes | 2 | 1.01 |
| ICU admission | ||
| No | 160 | 80.40 |
| Yes | 39 | 19.60 |
ICU, intensive care unit.
Table 6
| Features | Mean | SD | Min | Q1 | Median | Q3 | Max |
|---|---|---|---|---|---|---|---|
| Number of lymph nodes | 11.4 | 6.8 | – | – | – | – | – |
| Number of positive lymph nodes | – | – | 0 | 0.0 | 0.0 | 1.0 | 18.0 |
| Tumor long diameter (cm) | – | – | 1 | 3 | 3.5 | 4 | 13.5 |
| Tumor volume (cm3) | – | – | 0.21 | 9 | 17.5 | 33.4 | 405.0 |
| ICU stay (days) | – | – | 0 | 0 | 0 | 0 | 24 |
| Postoperative hospital stays (days) | – | – | 6 | 12 | 15 | 21 | 87 |
ICU, intensive care unit; SD, standard deviation.
Among these complications, the most concerning postoperative outcomes in terms of frequency were pancreatic fistula, biliary fistula, delayed gastric emptying, and gastrointestinal bleeding. Importantly, no instances of postoperative incision infection, celiac fistula, anastomotic ulcer, intestinal obstruction, renal failure, liver failure, heart failure, myocardial ischemia, acute respiratory distress syndrome, or systemic inflammatory response syndrome, were reported.
Eleven (5.53%) patients required a second operation, and there were two perioperative deaths. During the postoperative period, 39 (19.60%) patients were admitted to the ICU, with a median stay of 0 day. The median tumor volume was 17.5 (Q1 =9, Q3 =33.4) cm3, and the median longest diameter of 3.5 (Q1 =3, Q3 =4) cm. The median length of hospitalization was 15 (Q1 =12, Q3 =21) days.
Risk factors for postoperative complications and ICU admission
First, we focused on preoperative and intraoperative risk factors that could potentially influence postoperative complications (Table 7). The analysis revealed that biliary fistula was associated with a lower preoperative bilirubin level (33.7 vs. 68.3 µmol/L). Similarly, patients who required a second operation also had lower preoperative bilirubin levels (33.5 vs. 68.8 µmol/L).
Table 7
| Factors | Complication or outcome | N | Mean | SD | Min | Q1 | Median | Q3 | Max |
|---|---|---|---|---|---|---|---|---|---|
| Preoperative bilirubin (μmol/L) | Biliary fistula** | 10 | – | – | 29.5 | 30.4 | 33.7 | 40.5 | 44.7 |
| No biliary fistula | 189 | – | – | 0 | 13.1 | 68.3 | 136.0 | 369.0 | |
| Preoperative bilirubin (μmol/L) | Second operation** | 11 | – | – | 27.6 | 28.9 | 33.5 | 44.5 | 53.9 |
| No second operation | 188 | – | – | 0 | 13.0 | 68.8 | 136.2 | 369.0 | |
| Intraoperative bleeding (mL) | ICU admission** | 39 | – | – | 100 | 400 | 1,000 | 1,750 | 7,000 |
| No ICU admission | 160 | – | – | 50 | 200 | 400 | 800 | 5,000 | |
| Total amount of blood transfusion (mL) | ICU admission** | 39 | – | – | 0 | 400 | 600 | 1,200 | 3,250 |
| No ICU admission | 160 | – | – | 0 | 0 | 300 | 600 | 1,680 | |
| Duration of surgery (min) | ICU admission** | 39 | 504.71 | 145.79 | – | – | – | – | – |
| No ICU admission | 160 | 412.78 | 108.25 | – | – | – | – | – | |
| Duration of surgery (min) | Gastrointestinal bleeding* | 10 | 336.50 | 117.758 | – | – | – | – | – |
| No bleeding | 189 | 435.91 | 120.162 | – | – | – | – | – |
*, P<0.05; **, P<0.01. ICU, intensive care unit; SD, standard deviation.
Additionally, intraoperative bleeding volume was positively correlated with ICU admission (1,000 vs. 400 mL). As anticipated, the ICU-admission group also received a significantly higher volume of blood transfusions (600 vs. 300 mL). A longer operation time was positively associated with ICU admission (504.71 vs. 412.78 minutes) but was negatively correlated with postoperative gastrointestinal bleeding (336.50 vs. 435.91 minutes).
Several key categorical variables were identified as being associated with postoperative complications or outcomes (Table 8). Intraoperative blood transfusion was found to be positively associated with abdominal infection (9/113 vs. 0/77). Furthermore, revascularization during surgery was correlated to a higher likelihood of abdominal infection (4/24 vs. 5/166). Notably, patients with chronic pancreatitis had a significantly higher incidence of postoperative ICU admission (6/7 vs. 33/153). Similarly, there were a greater proportion of ICU admissions in patients requiring revascularization (10/18 vs. 29/142).
Table 8
| Factor | Complication or outcome | No (n) | Yes (n) | Chi-squared | P value |
|---|---|---|---|---|---|
| Intraoperative blood transfusion | Abdominal infection | 4.364 | 0.04 | ||
| No | 77 | 0 | |||
| Yes | 113 | 9 | |||
| Revascularization | Abdominal infection | 4.802 | 0.03 | ||
| No | 166 | 5 | |||
| Yes | 24 | 4 | |||
| Chronic pancreatitis | ICU admission | 4.552 | 0.03 | ||
| No | 153 | 33 | |||
| Yes | 7 | 6 | |||
| Revascularization | ICU admission | 4.247 | 0.04 | ||
| No | 142 | 29 | |||
| Yes | 18 | 10 | |||
| Gastrointestinal anastomosis | Delayed gastric emptying | 6.856 | 0.009 | ||
| Child | 178 | 14 | |||
| Pancreatic-gastric | 4 | 3 |
ICU, intensive care unit.
Regarding the type of gastrointestinal anastomosis, patients who underwent the pancreatic-gastric anastomosis method had a higher incidence of delayed gastric emptying (3/4 vs. 14/178).
Finally, we employed various logistic regression models to predict ICU admission based on the available clinical and surgical factors. Unfortunately, none of the models achieved satisfactory performance, with accuracies below 80% for both the actual negative and positive groups. This suggests that other, yet unidentified factors may play a role in determining ICU admission, warranting further investigation into additional predictors beyond the currently available clinical and surgical data.
Factors associated with length of postoperative hospitalization
Among all continuous and categorical variables, we found that the length of postoperative hospitalization was closely associated with three intraoperative factors (Table 9): the duration of surgery (r=0.173; P=0.01), the volume of blood transfusion (r=0.175; P=0.01), and the intraoperative bleeding volume (r=0.148; P=0.04). However, no correlation was found between postoperative stay and other variables.
Table 9
| Features | Pearson R | P value |
|---|---|---|
| Duration of surgery (min) | 0.173 | 0.01* |
| Total volume of blood transfusion (mL) | 0.175 | 0.01* |
| Intraoperative bleeding volume (mL) | 0.148 | 0.04* |
| Weight (kg) | 0.094 | 0.19 |
| Tumor volume (cm3) | −0.07 | 0.33 |
| Height (m) | −0.069 | 0.34 |
| CA125 (IU/mL) | −0.061 | 0.40 |
| Bilirubin (μmol/L) | −0.026 | 0.71 |
| Age (years) | 0.019 | 0.80 |
| CA19-9 (IU/mL) | 0.01 | 0.89 |
*, P<0.05. CA125, carbohydrate antigen 125; CA19-9, carbohydrate antigen 19-9.
Discussion
The principal findings of this study are as follows. After LPD, patients with biliary fistula and a second operation had a lower level of preoperative bilirubin. Lung or abdominal infection was associated with a high level of intraoperative bleeding. The risk factors of ICU admission included intraoperative bleeding, amount of blood transfusion, duration of surgery, chronic pancreatitis history, and revascularization. Finally, the length of postoperative hospitalization was correlated with duration of surgery, amount of blood transfusion, and intraoperative bleeding.
In terms of postoperative complications, there is a consensus that LPD has fewer complications (such as lung infection, incision infection, and pancreatic fistula) as compared to OPD (20-22). In comparison with OPD, LPD involves less blood loss, faster recovery of gastrointestinal function, and shorter recovery time. LPD has less bleeding compared with OPD; the small amount of bleeding under laparoscopy may affect the brightness and clarity of the operative field, and the operator should be particularly careful in maintaining a clear operative field.
Postoperative pancreatic fistula (POPF) remains a critical complication following LPD. Factors such as vascular reconstruction, soft pancreatic texture, pancreatic duct diameter ≤3 mm, low preoperative serum prealbumin levels, and elevated C-reactive protein on postoperative day 7 have been identified as independent risk factors for POPF (23,24). Preoperative biliary drainage (PBD) can significantly increase operative time, hospital stay, and morbidity and should be managed carefully in the perioperative period (25). Laparoscopic approaches tend to have longer operative times, which can be associated with increased complications such as longer length of hospital stay, and higher major morbidity rates as compared to open surgery. However, these drawbacks are often balanced by improved recovery outcomes, such as less blood loss and shorter length of hospital stay (26-28). The learning curve plays a critical role in operative outcomes for LPD. According to a retrospective study in Korea, it was suggested that the LPD technique stabilizes after approximately 44 cases, marking the point at which the surgeon’s skills and technique result in more predictable and successful outcomes (29).
In this study, we found that intraoperative bleeding may increase the risk of lung or abdominal infection. This is not surprising, as increased surgical bleeding can indeed raise the risk of postoperative infection. A previous study has shown a reduction in intraoperative bleeding with LPD compared to OPD and a concomitant reduction in lung infection (30). In addition, those patients with greater blood loss require a greater volume of intraoperative blood transfusion, and we found that those with postoperative infections did indeed have a higher volume of intraoperative blood transfusion. Therefore, the exact cause of postoperative infections, which could be due to both direct blood loss and blood transfusion, needs to be confirmed in subsequent research. Patients admitted to the ICU had greater intraoperative blood loss, which may be explained by the following: (I) a greater amount of intraoperative bleeding in the LPD indicates that the tumor is more complex and thus more difficult to resect, and as a result, there is a higher likelihood of serious postoperative complications; (II) intraoperative blood transfusion is associated with a higher likelihood of postoperative infectious disease and allergies, which may induce a higher inflammatory response; (III) higher intraoperative blood loss is associated with a longer operation duration (our study found that patients with a longer operative time are more likely to be admitted to the ICU postoperatively), which suggests that intraoperative transfusions may cause more intraoperative infections due to the higher operative difficulty and longer operative times, ultimately leading to deterioration in the postoperative period (12).
Another interesting finding was that a low bilirubin level was associated with a higher risk of biliary fistula and second operation, which has rarely been reported. One study found preoperative total bilirubin >200 µmol/L to be an independent risk factor for perioperative calculation of blood loss (31). However, in general, percutaneous transhepatic biliary drainage should be performed preoperatively in patients with serum bilirubin above 200 µmol/L (30). In our study, bilirubin levels were 33.7 and 68.3 µmol/L in biliary fistula and non-biliary fistula groups, respectively, both of which were considerably lower than 200 µmol/L. Normally, bilirubin levels are elevated in patients with pancreatic tumors. A 2023 study reported a 6.4% incidence of biliary fistula after PD, and one of the risk factors included a higher preoperative bilirubin level (8). For the first time, we found a positive correlation between low preoperative bilirubin and postoperative biliary fistulae in PD, but this result needs to be validated in a larger sample of data. Moreover, the preoperative bilirubin was lower in the second-operation group, but again, this should be confirmed is future research due to the small sample size. However, this also suggests that if preoperative bilirubin is low, it should also be monitored closely in the postoperative period, and all potential factors related to secondary surgery should be considered.
Furthermore, we examined the features influencing the length of postoperative hospitalization, and three key factors were identified: duration of surgery, amount of blood transfusion, and intraoperative bleeding. Often, typical postoperative complications (e.g., pancreatic and biliary fistulas) prolong the postoperative hospital length of stay (8,32-34). Previously, it has been reported that the mean length of hospital stay is prolonged in older adult patients (9). Our results do not support the effect of age on the duration of postoperative hospitalization. Rather, age was associated with three highly correlated intraoperative factors (duration of surgery is naturally correlated with the volume of blood transfusion and intraoperative bleeding). This intriguing result suggests that it is possible to shorten the length of postoperative hospital length of stay simply by optimizing the LPD procedure, with this being less related to the patients’ clinical characteristics. Conversely, patients should be expected to require a longer hospital stay if they lose a large amount of blood intraoperatively and if the duration of surgery is particularly long.
It should be noted that a shortcoming of this study is that our dataset underperformed both in predicting ICU admission by classification and postoperative length of stay by regression, and there is a need to add other preoperative/intraoperative dimensions and increase the number of patient samples. In addition, many common complications did not occur (or the positive samples were very few) because the sample size was small. In particular, intraoperative bleeding was associated with an increased risk of infection, ICU admission, and prolonged hospital stay. The novel results that low preoperative bilirubin levels may predict biliary fistula and a need for second operations require further validation. However, the study’s small sample size, retrospective design, and inclusion of only a single center limit its ability to establish causality or offer generalizability. Although the identified risk factors are likely applicable to other high-risk surgeries, their impact may vary across different settings or patient populations. The novel associations, especially those regarding bilirubin levels, need to be replicated in larger, multicenter studies before they can be applied more broadly. Thus, caution is needed in translating these findings to other institutions or clinical contexts.
Conclusions
For patients with a high volume of intraoperative bleeding and blood transfusion in LPD, preventative measures against lung and abdominal infections should be enacted well in advance. Moreover, early warning measures for ICU admission are necessary in the postoperative period, especially for patients with chronic pancreatitis, those with a high volume of intraoperative bleeding or intraoperative blood transfusion, and those who have a long duration of surgery who are expected endure longer postoperative hospitalization.
Acknowledgments
None.
Footnote
Reporting Checklist: The authors have completed the STROBE reporting checklists. Available at https://gs.amegroups.com/article/view/10.21037/gs-2025-63/rc
Data Sharing Statement: Available at https://gs.amegroups.com/article/view/10.21037/gs-2025-63/dss
Peer Review File: Available at https://gs.amegroups.com/article/view/10.21037/gs-2025-63/prf
Funding: None.
Conflicts of Interest: All authors have completed the ICMJE uniform disclosure form (available at https://gs.amegroups.com/article/view/10.21037/gs-2025-63/coif). The authors have no conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved. The study was conducted in accordance with the Declaration of Helsinki and its subsequent amendments. The ethical approval of this study was obtained from The Second Hospital of Hebei Medical University (ethical approval number: 2025-R013). All participants signed the informed consent prior to recruitment.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Visani M, Acquaviva G, De Leo A, et al. Molecular alterations in pancreatic tumors. World J Gastroenterol 2021;27:2710-26. [Crossref] [PubMed]
- Howe JR, Merchant NB, Conrad C, et al. The North American Neuroendocrine Tumor Society Consensus Paper on the Surgical Management of Pancreatic Neuroendocrine Tumors. Pancreas 2020;49:1-33. [Crossref] [PubMed]
- Grossberg AJ, Chu LC, Deig CR, et al. Multidisciplinary standards of care and recent progress in pancreatic ductal adenocarcinoma. CA Cancer J Clin 2020;70:375-403. [Crossref] [PubMed]
- Halbrook CJ, Lyssiotis CA, Pasca di Magliano M, et al. Pancreatic cancer: Advances and challenges. Cell 2023;186:1729-54. [Crossref] [PubMed]
- Felsenstein M, Hruban RH, Wood LD. New Developments in the Molecular Mechanisms of Pancreatic Tumorigenesis. Adv Anat Pathol 2018;25:131-42. [Crossref] [PubMed]
- Beger HG, Mayer B, Poch B. Duodenum-Preserving Pancreatic Head Resection for Benign and Premalignant Tumors-a Systematic Review and Meta-analysis of Surgery-Associated Morbidity. J Gastrointest Surg 2023;27:2611-27. [Crossref] [PubMed]
- Vasudevan SA, Ha TN, Zhu H, et al. Pancreaticoduodenectomy for the treatment of pancreatic neoplasms in children: A Pediatric Surgical Oncology Research Collaborative study. Pediatr Blood Cancer 2020;67:e28425. [Crossref] [PubMed]
- Wang R, Jiang P, Chen Q, et al. Pancreatic fistula and biliary fistula after laparoscopic pancreaticoduodenectomy: 500 patients at a single institution. J Minim Access Surg 2023;19:28-34. [Crossref] [PubMed]
- Hendi M, Mou Y, Lu C, et al. Laparoscopic pancreaticodoudenectomy: An excellent approach in elderly patients, a multicenter, comparative study. Medicine (Baltimore) 2020;99:e22175. [Crossref] [PubMed]
- Zhang H, Lan X, Peng B, et al. Is total laparoscopic pancreaticoduodenectomy superior to open procedure? A meta-analysis. World J Gastroenterol 2019;25:5711-31. [Crossref] [PubMed]
- Liu M, Ji S, Xu W, et al. Laparoscopic pancreaticoduodenectomy: are the best times coming? World J Surg Oncol 2019;17:81. [Crossref] [PubMed]
- Qin R, Kendrick ML, Wolfgang CL, et al. International expert consensus on laparoscopic pancreaticoduodenectomy. Hepatobiliary Surg Nutr 2020;9:464-83. [Crossref] [PubMed]
- Yan Y, Hua Y, Chang C, et al. Laparoscopic versus open pancreaticoduodenectomy for pancreatic and periampullary tumor: A meta-analysis of randomized controlled trials and non-randomized comparative studies. Front Oncol 2022;12:1093395. [Crossref] [PubMed]
- Xu S, Deng X, Wang S, et al. Short‑ and long‑term outcomes after laparoscopic and open pancreaticoduodenectomy for elderly patients: a propensity score‑matched study. BMC Geriatr 2024;24:462. [Crossref] [PubMed]
- Wang M, Qin T, Zhang H, et al. Laparoscopic versus open surgery for perihilar cholangiocarcinoma: a multicenter propensity score analysis of short- term outcomes. BMC Cancer 2023;23:394. [Crossref] [PubMed]
- Perri G, van Hilst J, Li S, et al. Teaching modern pancreatic surgery: close relationship between centralization, innovation, and dissemination of care. BJS Open 2023;7:zrad081. [Crossref] [PubMed]
- Wang M, Pan S, Qin T, et al. Short-Term Outcomes Following Laparoscopic vs Open Pancreaticoduodenectomy in Patients With Pancreatic Ductal Adenocarcinoma: A Randomized Clinical Trial. JAMA Surg 2023;158:1245-53. [Crossref] [PubMed]
- Chao YJ, Lu WH, Liao TK, et al. Feasibility of simultaneous development of laparoscopic and robotic pancreaticoduodenectomy. Sci Rep 2023;13:6190. [Crossref] [PubMed]
- Chen YX, Du L, Wang LN, et al. Effects of Dexmedetomidine on Systemic Inflammation and Postoperative Complications in Laparoscopic Pancreaticoduodenectomy: A Double-blind Randomized Controlled Trial. World J Surg 2023;47:500-9. [Crossref] [PubMed]
- Nassour I, Wang SC, Christie A, et al. Minimally Invasive Versus Open Pancreaticoduodenectomy: A Propensity-matched Study From a National Cohort of Patients. Ann Surg 2018;268:151-7. [Crossref] [PubMed]
- de Rooij T, Lu MZ, Steen MW, et al. Minimally Invasive Versus Open Pancreaticoduodenectomy: Systematic Review and Meta-analysis of Comparative Cohort and Registry Studies. Ann Surg 2016;264:257-67. [Crossref] [PubMed]
- Poves I, Burdío F, Morató O, et al. Comparison of Perioperative Outcomes Between Laparoscopic and Open Approach for Pancreaticoduodenectomy: The PADULAP Randomized Controlled Trial. Ann Surg 2018;268:731-9. [Crossref] [PubMed]
- Zhang S, Yadav DK, Wang G, et al. Causes and predictors of unplanned reoperations within 30 days post laparoscopic pancreaticoduodenectomy: a comprehensive analysis. Front Oncol 2024;14:1464450. [Crossref] [PubMed]
- Chang JH, Kakati RT, Wehrle C, et al. Incidence of clinically relevant postoperative pancreatic fistula in patients undergoing open and minimally invasive pancreaticoduodenectomy: a population-based study. J Minim Invasive Surg 2024;27:95-108. [Crossref] [PubMed]
- Neelma , Rashid A, Waqas M, et al. Initial experience of pancreaticoduodenectomy in a newly developed hepato-pancreato-biliary unit serving in a lower-middle-income country. J Cancer Allied Spec 2024;10:575. [Crossref] [PubMed]
- Emmen AMLH, Jones LR, Wei K, et al. Impact of patient age on outcome of minimally invasive versus open pancreaticoduodenectomy: a propensity score matched study. HPB (Oxford) 2025;27:102-10. [Crossref] [PubMed]
- Gong S, Li S, Liang Y, et al. External versus internal pancreatic duct drainage for early efficacy after laparoscopic pancreaticoduodenectomy in the early stages of the low-flow center learning curve: a retrospective comparative study. Gland Surg 2024;13:2068-77. [Crossref] [PubMed]
- Valukas CS, Zaza NM, Vitello D, et al. A Comparative Analysis of Open Versus Minimally Invasive Pancreatoduodenectomies. J Surg Oncol 2025;131:816-26. [Crossref] [PubMed]
- Kim HJ, Cho CK. Analysis of the learning curve for laparoscopic pancreaticoduodenectomy based on a single surgeon's experience: a retrospective observational study. Ann Surg Treat Res 2024;107:27-34. [Crossref] [PubMed]
- Tang YC, Liu QQ, He YG, et al. Laparoscopic pancreaticoduodenectomy: a retrospective study of 200 cases and the optimization of the single-center learning curve. Transl Cancer Res 2021;10:3436-47. [Crossref] [PubMed]
- Zhu L, Yang Y, Cheng H, et al. The role of preoperative biliary drainage on postoperative outcome after pancreaticoduodenectomy in patients with obstructive jaundice. Gland Surg 2023;12:593-608. [Crossref] [PubMed]
- Yu C, Lin YM, Xian GZ. Hemoglobin loss method calculates blood loss during pancreaticoduodenectomy and predicts bleeding-related risk factors. World J Gastrointest Surg 2024;16:419-28. [Crossref] [PubMed]
- Tang W, Qiu JG, Li GZ, et al. Clinical application of "Double R" anastomosis technique in laparoscopic pancreaticoduodenectomy procedure. Medicine (Baltimore) 2021;100:e26204. [Crossref] [PubMed]
- Sheng J, Yang Y, Tang N, et al. Development and validation of a nomogram based on preoperative factors for predicting clinically relevant postoperative pancreatic fistula following pancreaticoduodenectomy. Gland Surg 2025;14:37-47. [Crossref] [PubMed]
(English Language Editor: J. Gray)


