Can magnetic resonance imaging obviate the need for biopsy for microcalcifications?
Introduction
Breast cancer screening with mammography (MG) has increased in recent years and has been accompanied by an expected increase in the rate of additional examinations for suspected malignancy (1). The American College of Radiology’s Breast Imaging Reporting and Data System (BI-RADS) is widely used to evaluate MG findings, including microcalcifications (1). The appearance of microcalcifications without a visible, associated mass on MG presents a diagnostic dilemma. If it is not possible to identify lesions with ultrasound, stereotactic vacuum-assisted breast biopsy (SVAB) is usually the next step. However, SVAB is not performed in all cases: some patients do not wish to undergo biopsy, doctors sometimes judge that SVAB is not necessary, and SVAB simply cannot be performed at some centers and facilities. Dynamic contrasted-enhanced magnetic resonance imaging (MRI) is performed in many of the cases in which SVAB is undesirable or impossible. If MRI does not suggest malignancy, SVAB is omitted and short-interval follow-up is often provided.
Although MRI can be diagnostically helpful (2), malignancies are sometimes detected during follow-up after a negative scan. In the current study, we sought to elucidate whether some MRI findings could be used avoid SVAB.
Methods
Patients
This retrospective study was approved by our hospital’s ethics committee (approval 26-59). The patients provided informed consent for this study.
Patients with BI-RADS 3, 4, and 5 microcalcifications detected with MG were analyzed from April 2012 to September 2014. The patients additionally underwent contrast-enhanced MRI of the breasts. The exclusion criteria for our study were asthma, contrast media allergy, decreased renal function, and metal implants. All patients with enhancing lesions in the region of the microcalcifications underwent SVAB. Non-enhancing lesions were biopsied immediately or followed-up, depending on the patient’s preferences.
MG
Bilateral digital MG was performed, including routine craniocaudal and mediolateral oblique views of the breasts. The digital mammograms were independently double-read using BI-RADS assessment categories by two breast surgeons with 12–24 years of experience. If different BI-RADS assessment categories were assigned by the readers, consensus was reached by discussion. Microcalcifications were classified according to BI-RADS descriptors for mammographic features including calcification morphology (punctate, amorphous, pleomorphic, or linear) and distribution (diffuse, regional, clustered, segmental, or linear).
Ultrasound
Bilateral whole-breast ultrasound was routinely performed with knowledge of the clinical and mammographic findings prior to MRI and SVAB. Ultrasound was performed by one of two breast surgeons with 12–24 years of experience. Lesions for which an ultrasound-guided core needle biopsy was possible were excluded from our study.
MRI procedure
In premenopausal women, we generally performed MRI within the second week of the menstrual cycle. MRI examinations were performed with the patients in the prone position. The instrument was a 3-Tesla system (MAGNETOM Verio 3T®; SIEMENS, Munich, Germany) with double breast-surface coils. Our imaging protocol includes a localizing sequence followed by transverse fast-spin echo T2-weighted imaging [repetition time:echo time (TR/TE), 4,130/61; matrix, 272×320] with fat suppression and the following parameters: a field-of-view of 34 cm, a section thickness of 3 mm, and an inter-slice gap of 0.6 mm. This is followed by a dynamic study consisting of serial imaging of a 3-dimensional transverse T1-weighted sequence (TR/TE, 4.11/1.53; matrix, 296×448) with fat suppression and the following parameters: a field-of-view of 34 cm and a section thickness of 1.2 mm. Gadopentetate dimeglumine (Magnevist®; Bayer, Osaka, Japan) was administered as a bolus intravenous injection (2 mL/s) at a dose of 0.2 mL/kg body weight, followed by a 35-mL saline flush. For the dynamic study, we acquired one pre-contrast and four contrast-enhanced T1-weighted scans; the scan time was 45 s per scan.
MRI interpretation
Two radiologists with 10–20 years of experience and knowledge of MG findings interpreted the MRI examinations and assigned each of them a BI-RADS category. We classified the results as either malignant suspicious (BI-RADS category 4 or higher) or benign suspicious (BI-RADS category 3 or lower). The category 3 and lower examinations were further subclassified as either non-enhancing or non-specific enhancing. We did not perform kinetic curve assessments. The malignant-suspicious lesions were as follows: linear-ductal and segmental enhancement patterns in terms of their distribution, and clumped-clustered ring enhancement and branching-ductal pattern in terms of their internal enhancement patterns. Non-specific enhancing described the following situations: focal enhancement and non-specific regional enhancement.
SVAB
We performed SVAB with the patient in a lateral decubitus position on a digital stereotactic table (Amulet®; FUJIFILM, Tokyo, Japan) with a vacuum-assisted biopsy device using 11-gauge probes (Mammotome®; DEVICOR MEDICAL JAPAN, Tokyo, Japan). SVAB was performed by one of two breast surgeons, each of whom had 7–12 years of experience with SVAB. Specimen radiography was performed for all cases. If microcalcifications were observed in the specimen, the biopsy was considered successful. Clips were placed through the 11-gauge probe to identify the SVAB site for subsequent surgical excision.
Histologic diagnosis
Histologic diagnoses were determined by two pathologists, each with 7–12 years of experience. The histologic findings were classified into two groups: malignant and benign. Malignant lesions included invasive carcinoma and ductal carcinoma in situ (DCIS). We considered atypical ductal hyperplasia (ADH) to be a high-risk lesion, for which the associated presence of carcinoma can be underestimated with SVAB. If SVAB yielded ADH, we performed surgical excision and reclassified the case as malignant if any neoplasia were found. If a benign lesion was found via SVAB, the patient was scheduled for repeat MG of the ipsilateral breast at 6 months.
Results
Patients
A total 87 patients were enrolled in this study. Their mean age was 51 years (age range, 25–76 years). Forty-seven of the patients were premenopausal and 40 of the patients were postmenopausal.
Histological results
Histological analysis showed invasive ductal carcinoma in 15 patients (17.2%), non-invasive ductal carcinoma in 13 patients (14.9%), and benign lesions in 59 patients (67.8%) (Table 1). The benign lesions included seven ADHs and one atypical lobular hyperplasia.
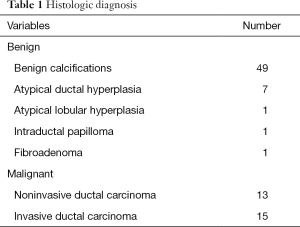
Full table
Mammographic findings
The mammographic findings of microcalcifications are shown in Table 2. There were 73 cases with category 3 calcifications, eight with category 4 calcifications, and six with category 5 calcifications. The positive predictive values (PPV) for the detection of malignancy were 27.2% (17/73) for category 3 microcalcifications, 62.5% (5/8) for category 4 microcalcifications, and 100% (6/6) for category 5 microcalcifications.
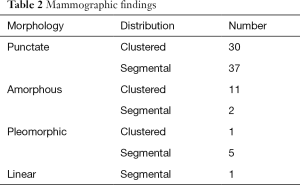
Full table
MRI findings
MRI findings are shown in Table 3. In 19 patients (21.8%), there was no enhancement in the corresponding area of microcalcifications. Non-specific enhancement was observed in 41 patients (47.1%), while malignant-suspicious enhancement was observed in 27 patients (31.0%). The no enhancement group did not include any malignant lesions, while the malignant-suspicious enhancement group included 19 (70.3%) malignant lesions and the non-specific enhancement group included nine (21.9%) malignant lesions. Therefore, the overall PPV and negative predictive value (NPV) of MRI were 70.3% (19/27) and 85.0% (51/60), respectively. However, the NPV of MRI was 100% (19/19) in the group with no enhancement.
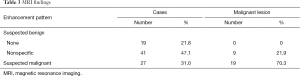
Full table
All lesions observed on MRI had also been seen on MG; MRI did not reveal any additional, incidental lesions in our study cohort.
PPV and NPV according to BI-RADS MG category
PPV and NPV according to BI-RADS MG category are shown in Table 4. In BI-RADS MG category 3, there were 57 benign-suspicious lesions on MRI, of which eight were malignant (NPV of MRI: 85.9%). Furthermore, 16 lesions were malignant suspicious on MRI, of which nine were malignant (PPV of MRI: 56.2%). In BI-RADS MG category 4, there were three benign-suspicious lesions on MRI, of which one was malignant (NPV of MRI: 66.6%). Moreover, five lesions were malignant suspicious on MRI, of which four were malignant (PPV of MRI: 80.0%). In BI-RADS MG category 5, all lesions were both malignant suspicious and actually malignant (PPV of MRI: 100%).

Full table
Discussion
The malignant-suspicious enhancement group included 19 (70.3%) malignant lesions and the non-specific-enhancement group included nine (21.9%) malignant lesions. In the overall cohort, the PPV and NPV of MRI were 70.3% (19/27) and 85.0% (51/60), respectively, and sensitivity and specificity were 67.8% (19/28) and 86.4% (51/59), respectively, which generally suggests promising utility for MRI. However, the false-negative rate was quite high in the benign-suspicious category (about 20%), preventing the omission of SVAB in this group.
In BI-RADS MG category 3, the false-negative rate of benign-suspicious lesions on MRI was 15.0%, which is too high to omit biopsy. In BI-RADS MG category 4, the false-negative rate for benign-suspicious lesions on MRI was 33.3%, also precluding the omission of SVAB. A case of BI-RADS MG category 4 was malignant suspicious on MRI; however, the biopsy revealed mastopathy. In this case, MG had shown punctate-segmental microcalcification and MRI had revealed a segmental enhancement pattern. On histology, many secretory calcifications were noted, with no evidence of atypia. In BI-RADS MG category 5, all lesions were both malignant suspicious and actually malignant. Despite these findings, no malignancy was found in lesions that did not show enhancement on MRI, indicating that the absence of enhancement has a high NPV.
BI-RADS 4 and 5 calcifications on MG must be biopsied, even if the lesion is non-enhancing on MRI. However, BI-RADS 3 calcifications on MG present a problem. Many cases were seen in which observation had been selected because MRI findings did not suggest clear malignancy. For BI-RADS 3 calcifications on MG, the false-negative rate of benign-suspicious lesions was 15.0% on MRI. It may be possible to omit SVAB for microcalcifications if there is no enhancement on MRI; however, any kind of enhancement indicates the need for biopsy in cases of BI-RADS 3 calcifications on MG.
Barreau et al. performed a systematic review and meta-analysis of the utility of MRI for evaluating mammographic microcalcifications (3). Their analysis revealed pooled sensitivity and specificity values of 87% and 81% for all lesions, 57% and 32% for BI-RADS 3 lesions, 92% and 82% for BI-RADS 4 lesions, and 95% and 66% for BI-RADS 5 lesions. They concluded that breast MRI is not recommended for the diagnosis of malignancy in BI-RADS 3 and 5 mammographic microcalcifications, but can be considered for BI-RADS 4 mammographic microcalcifications. The presence or absence of enhancement is the preferable diagnostic criterion to rule out malignancy in mammographic microcalcifications at breast MRI. The results of our study also show the disadvantages of MRI for BI-RADS 3 calcifications on MG. On the other hand, other researchers have suggested that MRI has utility for non-calcified lesions, and have reported the following pooled diagnostic parameters: 99% sensitivity, 89% specificity, 56% PPV 56%, and 100% NPV (4).
Of course, it is necessary to judge carefully whether the site of microcalcifications on MG matches with an enhancing lesion on MRI. If we judged that there was no enhancement at a site of mammographic microcalcifications, it was because there was no enhancement in a region with much wider margins than those that had been identified on MG. Only in these cases did we feel that biopsy could be precluded.
Several studies have suggested that the evaluation of the kinetic curve in dynamic contrast-enhanced MRI is useful (5,6). We did not evaluate the kinetic curve, as we felt that it was too operator-dependent, and that region of interest (ROI) placement would not be accurate. The rapid-washout pattern, which was reported to be a sign of malignancy, was often associated with benign findings; the opposite pattern was also seen often (5). Furthermore, because the corresponding portion of microcalcification on MG was not certain, the enhanced lesion in which the ROI must be set was not clear.
MRI was provided to essentially all of the patients at our hospital with BI-RADS 3, 4, and 5 microcalcifications. However, patients who met the following exclusion criteria did not receive MRI: asthma, contrast media allergy, decreased renal function, and metal implants. In actuality, 270 patients with microcalcifications visited our hospital, of whom 202 received MRI and 68 were followed-up without MRI.
For the 68 patients who did not undergo MRI, follow-up consisted of MG and palpitation every half year, and was provided to each patient. We assumed that further investigations were performed if there was any change of findings during follow-up. None of the patients was diagnosed with malignant disease during the follow-up period (mean duration of follow-up, 23.1 months). However, some of the patients may have been diagnosed with malignant disease afterwards. Therefore, the extent to which our results underestimate the true rate of malignant disease is not certain.
Of the 202 patients who received MRI, 87 were enrolled in this study and 115 were followed-up with MG and palpation every half year. None of the 115 patients was diagnosed with malignant disease during the follow-up period (mean duration of follow-up, 17.1 months). At our facilities, patients who do not undergo biopsy are followed-up for 10 years. Similarly, patients who undergo biopsy without malignant results are also followed-up for 10 years, with follow-up MG performed every half year.
In our study, 23.2% of the lesions that were BI-RADS category 3 on MG turned out to be malignant. This percentage was higher than expected. Furthermore, the ratio of invasive carcinomas to noninvasive cases was high. The study cohort consisted of patients who were referred to our medical center, at which SVAB was actively performed. This may have resulted in more malignancies being discovered. Moreover, selection bias was present: this study only enrolled patients who received MRI and SVAB. Therefore, patients who participated in this study might not have been representative of the usual screening population. Accordingly, the incidence of malignancy that we observed in category 3 may differ from usual findings, and the proportion of invasive cancers was high.
This study has several limitations. The study population was small and the study design was retrospective. A larger study population and additional outcome data are needed to confirm our results. In addition, we did not perform a cost analysis of MRI or SVAB.
If we perform a biopsy when the amount of calcification is elevated in MG, the prognosis may not be shortened in comparison with active searching for the malignancy using SVAB. Rather, the side effects of SVAB may be more serious, including greater pain and bleeding after SVAB. However, it is necessary for a patient to understand the false-negative rate of MRI. Rapid diagnosis may allow partial resection in some patients, whereas delayed diagnosis can necessitate a mastectomy. Therefore, lesions with enhancement need to be biopsied, even if the enhancement is non-specific.
Conclusions
It may be possible to omit SVAB for microcalcifications if there is no enhancement on MRI; however, any kind of enhancement indicates the need for biopsy in cases of BI-RADS 3 calcifications on MG.
Acknowledgements
None.
Footnote
Conflicts of Interest: The authors have no conflicts of interest to declare.
Ethical Statement: The study was approved by our hospital’s ethics committee (No. 26-59) and written informed consent was obtained from all patients.
References
- Berry DA, Cronin KA, Plevritis SK, et al. Effect of screening and adjuvant therapy on mortality from breast cancer. N Engl J Med 2005;353:1784-92. [Crossref] [PubMed]
- Kneeshaw PJ, Lowry M, Manton D, et al. Differentiation of benign from malignant breast disease associated with screening detected microcalcifications using dynamic contrast enhanced magnetic resonance imaging. Breast 2006;15:29-38. [Crossref] [PubMed]
- Barreau B, de Mascarel I, Feuga C, et al. Mammography of ductal carcinoma in situ of the breast: review of 909 cases with radiographic-pathologic correlations. Eur J Radiol 2005;54:55-61. [Crossref] [PubMed]
- Bennani-Baiti B, Bennani-Baiti N, Baltzer PA. Diagnostic Performance of Breast Magnetic Resonance Imaging in Non-Calcified Equivocal Breast Findings: Results from a Systematic Review and Meta-Analysis. PLoS One 2016;11:e0160346. [Crossref] [PubMed]
- Houserkova D, Prasad SN, Svach I, et al. The value of dynamic contrast enhanced breast MRI in mammographically detected BI-RADS 5 microcalcifications. Biomed Pap Med Fac Univ Palacky Olomouc Czech Repub 2008;152:107-15. [Crossref] [PubMed]
- Uematsu T, Yuen S, Kasami M, et al. Dynamic contrast-enhanced MR imaging in screening detected microcalcification lesions of the breast: is there any value? Breast Cancer Res Treat 2007;103:269-81. [Crossref] [PubMed]


