
Preoperative phosphorus levels may serve as a predictor of recurrent/persistent lesions after surgery for primary hyperparathyroidism: a cross-sectional study
Highlight box
Key findings
• In primary hyperparathyroidism (PHPT), satisfactory surgical success rates can be achieved without intraoperative parathyroid hormone monitoring. Blood phosphorus levels are important predictive indicators for postoperative recurrence or persistent lesions, and can therefore guide clinical decision-making.
What is known and what is new?
• Surgery is considered the definitive treatment for PHPT. However, the results of surgical procedures can be affected by a variety of factors, some of which remain contentious.
• Our study has revealed a close correlation between preoperative hypophosphatemia and an elevated incidence of postoperative recurrence/persistent lesions, which can serve as a guide for clinical decision-making.
What is the implication, and what should change now?
• In the diagnostic and therapeutic decision-making for PHPT, low blood phosphorus has been overlooked for a long time. When evaluating the indications for PHPT surgery, more consideration should be given to low blood phosphorus levels. Larger, prospective, multicenter studies with broader sample sizes will be required in the future.
Introduction
Primary hyperparathyroidism (PHPT) is a disease caused by excessive secretion of parathyroid hormone (PTH) due to primary parathyroid disease, which may lead to hypercalcemia, damage to the skeletal or urinary system. Surgery remains the preferred treatment and the only potential cure for PHPT (1,2). Historically, bilateral neck exploration (BNE) was the primary surgical approach for PHPT (3,4); however, in recent years, there has been a gradual shift toward minimally invasive parathyroidectomy (MIP) as the predominant method, as most cases of PHPT (80–90%) are caused by a single parathyroid adenoma (5-10).
The success rate of the surgery is affected by numerous factors. The advent of intraoperative parathyroid hormone (IOPTH) monitoring has further enhanced surgical success rates (11-15). However, not every center is equipped for IOPTH monitoring, just as the First Affiliated Hospital of Zhejiang University is not. The use of IOPTH monitoring can increase both the financial costs and surgical time (16,17). In cases in which preoperative ultrasound and 99mTc-methoxyisobutylisonitrile parathyroid scintigraphy with single-photon emission computed tomography/computed tomography (99mTc-MIBI SPECT/CT or MIBI) accurately localize the lesion, some centers that do not use IOPTH monitoring intraoperatively have reported similar success rates to those that routinely employ IOPTH monitoring (18-20).
In addition to IOPTH monitoring, numerous other factors affect the success rate of surgery. We collected data at the Department of Thyroid Surgery, First Affiliated Hospital, School of Medicine, Zhejiang University and conducted several relevant retrospective analysis studies. We present this article in accordance with the STROBE reporting checklist (available at https://gs.amegroups.com/article/view/10.21037/gs-2025-156/rc).
Methods
This study was approved by the Ethics Committee of the First Affiliated Hospital of Zhejiang University (No. 20230053) and was conducted in accordance with the Declaration of Helsinki and its subsequent amendments. As this was a retrospective study, informed consent was not required.
Patients who underwent surgery for PHPT at the First Affiliated Hospital of Zhejiang University, a tertiary hospital, between August 2017 and June 2022, were initially identified. To be included in the study, the patients had to have complete data, have benign parathyroid disease, and have only undergone parathyroid surgery; otherwise, they were excluded from the study. Due to the retrospective nature of this single-center study and limitations in the sample size, there might be some inherent biases in the results.
All patients in this study underwent routine blood vitamin D level testing, and none exhibited vitamin D deficiency. Since IOPTH monitoring was unavailable at the First Affiliated Hospital of Zhejiang University, all surgeries were performed without it. Rapid intraoperative frozen section pathology was used to confirm the removal of diseased parathyroid. Postoperative success was defined as a ≥50% reduction in PTH levels on the first postoperative day, followed by the normalization of PTH levels. Patients were considered cured if during a follow-up period exceeding 6 months, there was no abnormal increase in the calcium or PTH levels. Recurrence refers to the normalization of PTH and blood calcium levels within 6 months post-surgery, followed by an elevation thereafter. Persistence, on the other hand, denotes an increase in PTH and blood calcium levels within the first 6 months after surgery. And if creatinine levels exceed the normal range, it is considered renal dysfunction.
The following data were collected for all patients: age, gender, surgical method, number of pathological parathyroid glands and their maximum diameter, preoperative image examination data, osteoporosis or fracture (yes/no) (together, they are defined as a new variable: skeletal damage), and postoperative pathology type, as well as urolithiasis, alkaline phosphatase (ALP), creatinine, PTH, calcium, albumin, and phosphorus levels on the first postoperative day and 6 months postoperatively. Additionally, osteoporosis, fracture, and urolithiasis together were defined as a new variable: target organ damage.
Statistical analysis
We analyzed and compared the surgical success rates, cure rates, recurrence rates, and the incidence of persistent lesions. Univariate and multivariate analyses were conducted to identify the risk factors for surgical success, recurrence rates, and the incidence of persistent lesions. The statistical methods used included chi-square tests, t-tests, one-way analysis of variance, multivariate binary logistic regression analysis, and receiver operating characteristic (ROC) curve analysis. All the statistical analyses were performed using International Business Machines Corp (IBM) Statistical Package for the Social Sciences (SPSS) version 24.0. A P value <0.05 was considered statistically significant.
Results
Between August 2017 and June 2022, the data of 255 patients who underwent surgery for PHPT at the First Affiliated Hospital of Zhejiang University were reviewed. After excluding five patients with parathyroid carcinoma, 11 patients with concurrent papillary thyroid carcinoma, and six patients with incomplete data, 233 patients were included in the study. The cohort comprised 76 males (32.6%) and 157 females (67.4%), with a male-to-female ratio of approximately 1:2. The mean age of the patients was 55.08±12.46 years. The mean follow-up duration was 11.30±5.20 months (Table 1).
Table 1
| Index | Values |
|---|---|
| Sex | |
| Male | 76 (32.6) |
| Female | 157 (67.4) |
| Age (years) | 55.08±12.46 |
| Weight (kg) | 62.55±8.99 |
| BMI (kg/m2) | 23.45±2.88 |
| Tumor size (cm) | 2.38±1.14 |
| Tumor number | |
| Single | 208 (89.3) |
| Unilateral multiple | 9 (3.9) |
| Bilateral multiple | 16 (6.9) |
| Bone complications | |
| Osteoporosis | 75 (32.2) |
| Bone fracture | 9 (3.9) |
| Urolithiasis | 87 (37.3) |
| Renal dysfunction | 46 (19.7) |
| Operation type | |
| MIP | 208 (89.3) |
| UE | 9 (3.9) |
| BNE | 16 (6.9) |
| Ultrasound positive | 162 (69.5) |
| MIBI positive | 226 (97.0) |
| Pathological type | |
| Adenoma | 180 (77.3) |
| Hyperplasia | 53 (22.7) |
| Pre-creatinine (μmoI/L) | 84±33.4 |
| Pre-ALP (UL) | 110 |
| PTH (pg/mL) | |
| Pre | 179 |
| 1st day post | 7.3 |
| 6th month post | 56.19±51.51 |
| Albumin (g/L) | |
| Pre | 45.94±4.77 |
| 1st day post | 39.25±3.40 |
| 6th month post | 45.93±4.81 |
| Serum calcium (mmol/L) | |
| Pre | 2.80±0.36 |
| 1st day post | 2.37±0.30 |
| 6th month post | 2.34±0.15 |
| Phosphorus (mmol/L) | |
| Pre | 0.98±0.25 |
| 1st day post | 0.90±0.30 |
| 6th month post | 0.99±0.32 |
| Surgical success | 228 (97.8) |
| Cure | 209 (89.6) |
| Recurrence | 19 (8.2) |
| Persistent | 5 (2.2) |
| Follow-up time (months) | 11.30±5.20 |
Data are presented as n (%) or mean ± standard deviation or median. 1st day post: the first day after the operation; 6th month post: the sixth month after the operation. ALP, alkaline phosphatase; BMI, body mass index; BNE, bilateral neck exploration; MIBI, 99mTc-methoxyisobutylisonitrile parathyroid scintigraphy with single-photon emission computed tomography/computed tomography; MIP, minimally invasive parathyroidectomy; Pre, pre-operative; PTH, parathyroid hormone; UE, unilateral exploration.
In this study, IOPTH monitoring was not used in any case. Intraoperative frozen section pathology confirmed that all cases were either parathyroid adenomas or parathyroid hyperplasia. The overall surgical success rate was 97.8%, the cure rate was 89.6%, the recurrence rate was 8.2%, and the persistent lesion incidence was 2.2%. Of the total cases, 208 (89.3%) underwent MIP for a single adenoma, 9 (3.9%) underwent unilateral neck exploration (UNE), and 16 (6.9%) underwent BNE (Table 1). There were no significant differences in the surgical success rate, cure rate, recurrence rate, or persistent lesion incidence among the different surgical approaches (P>0.05).
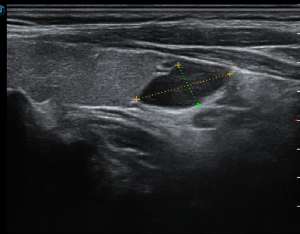
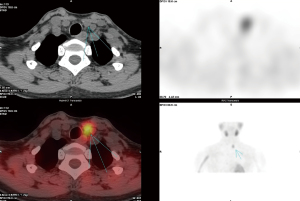
All patients underwent ultrasound (Figure 1) and 99mTc-MIBI SPECT/CT (Figure 2) examinations. Compared with postoperative pathology, the accuracy rates for MIBI and ultrasound were 97% and 69.5%, respectively, and the difference between the rates was significant (P<0.05). Postoperative pathology revealed 180 cases of parathyroid adenoma (77.3%) and 53 cases of parathyroid hyperplasia (22.7%); no significant differences were observed in the recurrence or persistent lesion rates (P>0.05).
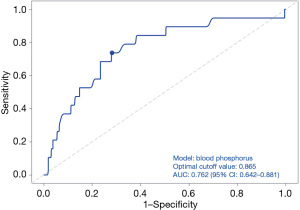
The univariate analysis of the risk factors for surgical success indicated that preoperative PTH and serum calcium were significantly negatively correlated with surgical success (P<0.05) (Table 2). However, multivariate analysis did not show any significance. The univariate analysis identified preoperative target organ damage, urolithiasis, and preoperative PTH, ALP, and phosphorus levels as risk factors for postoperative recurrence/persistent lesions (P<0.05) (Table 3). The multivariate analysis revealed that only the preoperative phosphorus level was a significant risk factor for recurrence/persistent lesions (P<0.05) (Table 4). In addition, ROC curve analysis showed that blood phosphorus levels below 0.865 mmol/L were associated with a higher incidence of recurrence/persistent lesions, with a sensitivity of 0.718, specificity of 0.67 and youden index of 0.388 (Figure 3).
Table 2
| Related factors | Chi-square test value/F value | P value |
|---|---|---|
| Operation type (MIP vs. UE vs. BNE) | 0.614 | 0.74 |
| Bone complications (osteoporosis and bone fracture) | 0.098 | 0.76 |
| Urolithiasis | 1.121 | 0.29 |
| Pre-PTH | 10.803 | 0.01 |
| Pre-serum calcium | 11.187 | 0.02 |
| Pre-creatinine | 0.879 | 0.92 |
| Pre-phosphorus | 0.056 | 0.85 |
| Adenoma vs. hyperplasia | 0.022 | 0.88 |
| Tumor size | 0.225 | 0.54 |
BNE, bilateral neck exploration; MIP, minimally invasive parathyroidectomy; Pre, pre-operative; PTH, parathyroid hormone; UE, unilateral exploration.
Table 3
| Related factors | Chi-square test value/F value | P value |
|---|---|---|
| Operation type (MIP vs. UE vs. BNE) | 0.308 | 0.86 |
| Bone complications (osteoporosis and bone fracture) | 0.195 | 0.66 |
| Urolithiasis | 5.04 | 0.03 |
| Pre-PTH | 85.486 | <0.001 |
| Pre-ALP | 113.185 | <0.001 |
| Pre-serum calcium | 1.185 | 0.94 |
| Pre-phosphorus | 0.160 | 0.02 |
| Pre-creatinine | 0.008 | 0.14 |
| Adenoma vs. hyperplasia | 0.077 | 0.78 |
| Tumor size | 0.048 | 0.99 |
| Target organ damages | 4.062 | 0.044 |
Target organ damage: osteoporosis plus bone fracture plus urolithiasis. ALP, alkaline phosphatase; BNE, bilateral neck exploration; MIP, minimally invasive parathyroidectomy; Pre, pre-operative; PTH, parathyroid hormone; UE, unilateral exploration.
Table 4
| Related factors | B | Wals | P value |
|---|---|---|---|
| Pre-phosphorus | −2.728 | 5.941 | 0.02 |
Pre, pre-operative.
Discussion
Surgery remains the primary treatment for PHPT. With the continuous advancement of imaging technology, the preoperative localization of diseased parathyroid glands has become highly accurate in the vast majority of cases (21-24). Consequently, MIP has emerged as the mainstream approach, replacing BNE (25-29). At the First Affiliated Hospital of Zhejiang University, all the patients underwent preoperative ultrasound and MIBI examinations, 89.3% of the patients suffered from a single parathyroid adenoma, and all of the patients underwent MIP, which is consistent with the literature (5). Our study found no significant differences in the surgical success rate, cure rate, recurrence rate, or incidence of persistent lesions between MIP, UNE, and BNE.
Many studies have shown that IOPTH monitoring can enhance surgical success rates for PHPT (11-15). However, satisfactory surgical outcomes can also be achieved without IOPTH monitoring (18-20). In fact, not all centers have access to IOPTH monitoring. As IOPTH monitoring was unavailable, we used rapid frozen section pathology as an alternative to ensure the accurate removal of the diseased parathyroid tissue. Additionally, we determined the number and location of diseased parathyroid glands based on preoperative examinations, and selected the appropriate surgical method accordingly. For solitary lesions, MIP was performed; for multiple unilateral lesions, UE was performed; and for multiple bilateral lesions or negative ultrasound and MIBI results, BNE was performed. The overall surgical success rate of our study was 97.8%, which is comparable to that reported by other centers (14-19). Some studies suggest that IOPTH monitoring offers little benefit for patients with both positive MIBI and ultrasound results (30,31). Thus, if the number and localization of the lesions are accurately determined preoperatively by high-resolution ultrasound or MIBI, rapid frozen section pathology can achieve satisfactory surgical outcomes even without IOPTH monitoring.
Previous report has indicated that MIBI is more accurate than ultrasound, and is the only method capable of locating ectopic parathyroid glands (32). In our study, MIBI achieved an accuracy rate of 97%, while that of ultrasound was only 69.5% (P<0.05). Ultrasound is a cost-effective examination method; however, it is often limited by thyroid nodules, blood vessels, cervical lymph nodes, and the skill level of the examiner. In our study, diseased parathyroid glands failed to be detected in many ultrasound examinations because they were mistaken for thyroid nodules. Given the close proximity of the parathyroid glands to the thyroid gland, the inexperienced sonographers often misidentified enlarged lesions as thyroid nodules. Thus, MIBI was found to be more reliable than ultrasound in the preoperative localization of diseased parathyroid glands.
In PHPT, elevated PTH and calcium levels, along with decreased phosphorus levels, are correlated with disease severity. Previous studies have shown that preoperative PTH and serum calcium levels, and tumor size are related to the surgical success rates and postoperative recurrence (33-36). In our study, the univariate analysis revealed that higher preoperative PTH and serum calcium were associated with lower surgical success rates (P<0.05). But multivariate analysis failed.
Additionally, the univariate analysis showed that preoperative target organ damage, urolithiasis, and preoperative PTH, ALP, and phosphorus levels were risk factors for postoperative recurrent/persistent lesions (P<0.05) (Table 3). The multivariate analysis revealed that only the preoperative phosphorus level was a significant risk factor for postoperative recurrence/persistent lesions (P<0.05) (Table 4). The ROC curve analysis indicated that a preoperative phosphorus level below 0.865 mmol/L was associated with a higher incidence of recurrent/persistent lesions, and had a sensitivity of 0.718, a specificity of 0.67 and youden index of 0.388 (Figure 3).
Recent study has suggested that the role of phosphates in PHPT may have been underestimated and warrants renewed attention (1). Low serum phosphate levels may indicate a poorer prognosis and a less favorable clinical phenotype for PHPT patients (37). It may be that preoperative phosphorus levels could be used to predict the incidence of postoperative recurrence or persistent lesions, and offer clinically valuable insights for decision making. For example, when evaluating the indications for PHPT surgery, in addition to considering hypercalcemia, more consideration should be given to low blood phosphorus.
This was a retrospective study with a limited sample size, and thus had numerous shortcomings. A multicenter study with a larger sample size may yield more accurate and convincing results.
Conclusions
At centers where IOPTH is unavailable, rapid frozen section pathology can achieve satisfactory surgical outcomes for PHPT. The phosphorus level is a predictor of postoperative recurrence/persistent lesions. These findings may guide clinical decisions. For instance, when assessing the indications for surgery in patients with PHPT, greater attention should be paid to low blood phosphorus levels. Prospective studies with larger sample sizes, conducted across multiple centers may yield more convincing results.
Acknowledgments
None.
Footnote
Reporting Checklist: The authors have completed the STROBE reporting checklist. Available at https://gs.amegroups.com/article/view/10.21037/gs-2025-156/rc
Data Sharing Statement: Available at https://gs.amegroups.com/article/view/10.21037/gs-2025-156/dss
Peer Review File: Available at https://gs.amegroups.com/article/view/10.21037/gs-2025-156/prf
Funding: This work was supported by
Conflicts of Interest: All authors have completed the ICMJE uniform disclosure form (available at https://gs.amegroups.com/article/view/10.21037/gs-2025-156/coif). The authors have no conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved. This study was conducted in accordance with the Declaration of Helsinki and its subsequent amendments. This study was approved by the Ethics Committee of the First Affiliated Hospital of Zhejiang University (No. 20230053). As this was a retrospective study, informed consent was not required.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Carpentier L, Bouillet B. Primary hyperparathyroidism: From diagnosis to treatment. Rev Med Interne 2025;46:204-10. [Crossref] [PubMed]
- Govind K, Paruk IM, Motala AA. Characteristics, management and outcomes of primary hyperparathyroidism from 2009 to 2021: a single centre report from South Africa. BMC Endocr Disord 2024;24:53. [Crossref] [PubMed]
- Khokar AM, Kuchta KM, Moo-Young TA, et al. Increasing trend of bilateral neck exploration in primary hyperparathyroidism. Am J Surg 2020;219:466-70. [Crossref] [PubMed]
- Laird AM, Libutti SK. Minimally Invasive Parathyroidectomy Versus Bilateral Neck Exploration for Primary Hyperparathyroidism. Surg Oncol Clin N Am 2016;25:103-18. [Crossref] [PubMed]
- Wells SA Jr, Leight GS, Ross AJ 3rd. Primary hyperparathyroidism. Curr Probl Surg 1980;17:398-463.
- Shapey IM, Jabbar S, Khan Z, et al. Scan-directed mini-incision focused parathyroidectomy: how accurate is accurate enough? Ann R Coll Surg Engl 2017;99:123-8. [Crossref] [PubMed]
- Kim WW, Lee YM, Sung TY, et al. Selection of parathyroidectomy methods for primary hyperparathyroidism according to concordance between ultrasonography and MIBI scan results. Gland Surg 2021;10:298-306. [Crossref] [PubMed]
- Korwar V, Yuen Chang F, Teasdale E, et al. Stepwise Approach for Parathyroid Localisation in Primary Hyperparathyroidism. World J Surg 2020;44:803-9. [Crossref] [PubMed]
- Schneider R, Hinrichs J, Meier B, et al. Minimally Invasive Parathyroidectomy without Intraoperative PTH Performed after Positive Ultrasonography as the only Diagnostic Method in Patients with Primary Hyperparathyroidism. World J Surg 2019;43:1525-31. [Crossref] [PubMed]
- Grant CS, Thompson G, Farley D, et al. Primary hyperparathyroidism surgical management since the introduction of minimally invasive parathyroidectomy: Mayo Clinic experience. Arch Surg 2005;140:472-8; discussion 478-9. [Crossref] [PubMed]
- Hargitai L, Boryshchuk D, Arikan M, et al. Is intraoperative parathyroid monitoring during minimally invasive parathyroidectomy still justified? Front Endocrinol (Lausanne) 2024;15:1442972. [Crossref] [PubMed]
- Bátora D, Iskandar R, Gertsch J, et al. Impact of perioperative diagnostic tools on clinical outcomes and cost-effectiveness in parathyroid surgery: a decision model-based analysis. BMJ Open 2024;14:e082901. [Crossref] [PubMed]
- Bachar G, Mizrachi A, Hadar T, et al. Role of parathyroid hormone monitoring during parathyroidectomy. Head Neck 2011;33:1754-7. [Crossref] [PubMed]
- Barczynski M. Minimally invasive parathyroidectomy without intraoperative parathyroid hormone monitoring: when and why? J Postgrad Med 2009;55:239-40. [Crossref] [PubMed]
- Irvin GL 3rd, Dembrow VD, Prudhomme DL. Clinical usefulness of an intraoperative "quick parathyroid hormone" assay. Surgery 1993;114:1019-22; discussion 1022-3.
- Morris LF, Zanocco K, Ituarte PH, et al. The value of intraoperative parathyroid hormone monitoring in localized primary hyperparathyroidism: a cost analysis. Ann Surg Oncol 2010;17:679-85. [Crossref] [PubMed]
- Zawawi F, Mlynarek AM, Cantor A, et al. Intraoperative parathyroid hormone level in parathyroidectomy: which patients benefit from it? J Otolaryngol Head Neck Surg 2013;42:56. [Crossref] [PubMed]
- Suliburk JW, Sywak MS, Sidhu SB, et al. 1000 minimally invasive parathyroidectomies without intra-operative parathyroid hormone measurement: lessons learned. ANZ J Surg 2011;81:362-5. [Crossref] [PubMed]
- Neychev VK, Ghanem M, Blackwood SL, et al. Parathyroid surgery can be safely performed in a community hospital by experienced parathyroid surgeons: A retrospective cohort study. Int J Surg 2016;27:72-6. [Crossref] [PubMed]
- Vaid S, Pandelidis S. Minimally invasive parathyroidectomy: a community hospital experience. Arch Surg 2011;146:876-8. [Crossref] [PubMed]
- Quak E, Lasne-Cardon A, Cavarec M, et al. F18-Choline PET/CT or MIBI SPECT/CT in the Surgical Management of Primary Hyperparathyroidism: A Diagnostic Randomized Clinical Trial. JAMA Otolaryngol Head Neck Surg 2024;150:658-65. [Crossref] [PubMed]
- Noltes ME, Kruijff S, Appelman APA, et al. Head-to-head comparison of [11C]methionine PET, [11C]choline PET, and 4-dimensional CT as second-line scans for detection of parathyroid adenomas in primary hyperparathyroidism. Eur J Nucl Med Mol Imaging 2024;51:1050-9.
- Wachtel H, Bartlett EK, Kelz RR, et al. Primary hyperparathyroidism with negative imaging: a significant clinical problem. Ann Surg 2014;260:474-80; discussion 480-2. [Crossref] [PubMed]
- Mortenson MM, Evans DB, Lee JE, et al. Parathyroid exploration in the reoperative neck: improved preoperative localization with 4D-computed tomography. J Am Coll Surg 2008;206:888-95; discussion 895-6. [Crossref] [PubMed]
- Garas G, Holsinger FC, Grant DG, et al. Is robotic parathyroidectomy a feasible and safe alternative to targeted open parathyroidectomy for the treatment of primary hyperparathyroidism? Int J Surg 2015;15:55-60. [Crossref] [PubMed]
- Reilly DJ, Chew GL, Eckhaus J, et al. Outcomes for minimally invasive parathyroidectomy: widening inclusion criteria based on preoperative imaging results. ANZ J Surg 2016;86:701-5. [Crossref] [PubMed]
- Brunaud L, Li Z, Van Den Heede K, et al. Endoscopic and robotic parathyroidectomy in patients with primary hyperparathyroidism. Gland Surg 2016;5:352-60. [Crossref] [PubMed]
- Wilhelm SM, Wang TS, Ruan DT, et al. The American Association of Endocrine Surgeons Guidelines for Definitive Management of Primary Hyperparathyroidism. JAMA Surg 2016;151:959-68. [Crossref] [PubMed]
- Tolley N, Arora A, Palazzo F, et al. Robotic-assisted parathyroidectomy: a feasibility study. Otolaryngol Head Neck Surg 2011;144:859-66. [Crossref] [PubMed]
- Bhangu JS, Riss P. The role of intraoperative parathyroid hormone (IOPTH) determination for identification and surgical strategy of sporadic multiglandular disease in primary hyperparathyroidism (pHPT). Best Pract Res Clin Endocrinol Metab 2019;33:101310. [Crossref] [PubMed]
- Dobrinja C, Santandrea G, Giacca M, et al. Effectiveness of Intraoperative Parathyroid Monitoring (ioPTH) in predicting a multiglandular or malignant parathyroid disease. Int J Surg 2017;41:S26-33. [Crossref] [PubMed]
- Pata G, Casella C, Magri GC, et al. Financial and clinical implications of low-energy CT combined with 99m Technetium-sestamibi SPECT for primary hyperparathyroidism. Ann Surg Oncol 2011;18:2555-63. [Crossref] [PubMed]
- Stewart ZA, Blackford A, Somervell H, et al. 25-hydroxyvitamin D deficiency is a risk factor for symptoms of postoperative hypocalcemia and secondary hyperparathyroidism after minimally invasive parathyroidectomy. Surgery 2005;138:1018-25; discussion 1025-6. [Crossref] [PubMed]
- Mazzaglia PJ, Milas M, Berber E, et al. Normalization of 2-week postoperative parathyroid hormone values in patients with primary hyperparathyroidism: four-gland exploration compared to focused-approach surgery. World J Surg 2010;34:1318-24. [Crossref] [PubMed]
- Beyer TD, Solorzano CC, Prinz RA, et al. Oral vitamin D supplementation reduces the incidence of eucalcemic PTH elevation after surgery for primary hyperparathyroidism. Surgery 2007;141:777-83. [Crossref] [PubMed]
- Yen TW, Wilson SD, Krzywda EA, et al. The role of parathyroid hormone measurements after surgery for primary hyperparathyroidism. Surgery 2006;140:665-72; discussion 672-4. [Crossref] [PubMed]
- Columbu C, Rendina D, Gennari L, et al. Phosphate metabolism in primary hyperparathyroidism: a real-life long-term study. Endocrine 2025;88:571-80. [Crossref] [PubMed]
(English Language Editor: L. Huleatt)


