
The triple-plane technique: a surgical technique for subpectoral implant-based breast reconstruction
Highlight box
Surgical highlights
• Triple-plane breast reconstruction is a technique in which the breast prosthesis is placed subpectorally without the use of mesh. The prosthesis is completely covered—medially by the pectoralis major muscle, and inferolaterally by the serratus anterior and latissimus dorsi fascia flap. This technique greatly reduces postoperative complications, lowers the possibility of prosthesis loss, and ensures satisfactory breast reconstruction results.
What is conventional and what is novel/modified?
• The conventional subpectoral implant-based breast reconstruction does not provide adequate autologous tissue coverage for the prosthesis, especially when the prosthesis is large. As a result, it often requires the use of mesh or acellular dermal matrix to achieve complete coverage.
• The novel approach of breast reconstruction surgery using a triple-plane technique creates three anatomical planes, allowing for the formation of a prosthetic pocket with a volume tailored to the size of the prosthesis as required. This new technique can achieve complete coverage of the prosthesis, thereby reducing the need for mesh or acellular dermal matrix.
What is the implication, and what should change now?
• The triple-plane technique can greatly reduce postoperative complications, decrease the likelihood of prosthesis loss, and ensure satisfactory breast reconstruction results. Clinical randomized controlled trials of the surgical triple-plane technique need to be conducted to confirm its clinical value.
Introduction
Implant-based breast reconstruction is an essential component of breast reconstruction surgery (1). Subpectoral implant-based breast reconstruction is still the commonest technique in China. However, in the absence of effective coverage, direct contact between the prosthesis and subcutaneous tissue may indirectly increase the risk of erythema through localized friction or microcirculatory disturbances (2). Additionally, partial stripping of the pectoralis major muscle may also leave a residual risk of mild animation deformity (3). In case of inadequate autologous tissue coverage for the prosthesis in traditional subpectoral implant-based breast reconstruction, mesh or the latissimus dorsi (or both combined) can be used for better support and coverage; however, the disadvantages of this approach cannot be ignored. For example, the combination of a prosthesis and a latissimus dorsi muscle flap can damage the entire latissimus dorsi muscle and cause donor site morbidity (4), while prosthesis combined with mesh may lead to adverse events, such as infection, prosthesis exposure, and prosthesis loss (5,6). Moreover, the high cost of mesh and the resultant significant economic burden on patients have led to a low rate of uptake in Chinese hospitals (7). Finding a breast reconstruction method that is safe and cost-effective in our region is necessary.
In this study, we propose a new approach for breast reconstruction: prosthetic breast reconstruction using the triple-plane technique. During the surgery, the serratus anterior and latissimus dorsi fascia are dissociated, and the prosthetic is implanted subpectorally, and the fascia is then sutured (Figure 1). The blood supply to the fasciocutaneous flap is derived from the perforating vessels at the lateral border of the pectoralis major muscle. Intraoperatively, the perforating branch is preserved by blunt dissection to ensure the axial blood supply of the fasciocutaneous flap. Theoretically, the triple-plane technique ensures the blood supply of the fascial flap by expanding the anterior serratus muscle fascia and partial latissimus dorsi muscle fascia, pedicled on the perforator vessels at the lateral margin of pectoralis major muscle. The technique thus provides vascularized coverage of the prosthesis, thus minimizing the risk of prosthesis exposure and infection, and obviates the need for mesh. In addition, the triple-plane technique can potentially reduce the complications of conventional subpectoral implantation by preserving the medial attachment point of the pectoralis major muscle and the combined fascial coverage. To date, no research appears to have been conducted abroad or at home to confirm the specific clinical application effect of this procedure.

Recently, we popularized the use of the triple-plane technique in breast reconstruction. Unlike the anatomical prosthesis coverage technique proposed by Gardani et al. (1), the technique proposed herein achieves vascularized coverage of the prosthesis by preserving the perforating vessels of the anterior serratus fascia without the need for additional patch support, thereby reducing the risk of infection and prosthesis exposure. We have now accumulated enough cases, as reported herein, to provide a new reference and guidance as a new technique in prosthetic breast reconstruction. We present this article in accordance with the SUPER reporting checklist (available at https://gs.amegroups.com/article/view/10.21037/gs-2025-116/rc).
Preoperative preparations and requirements
We conducted a retrospective cohort study. Ninety-six patients with breast cancer admitted to Hunan Cancer Hospital from January 2023 to October 2023 were selected as the research participants. The patients were aged between 30 and 60 years. All patients underwent unilateral nipple-sparing mastectomy, followed by immediate breast reconstruction at Hunan Cancer Hospital. To be eligible for inclusion in this study, the patients had to meet the following inclusion criteria: (I) with a pathological diagnosis of breast cancer and a pathological stage of 0–II; (II) having no involvement of the pectoral fascia, the nipple-areolar complex, the skin, or subcutaneous tissue; (III) the breasts with minimal ptosis; and (IV) with no preoperative radiotherapy. Patients were excluded from the study if they met any of the following exclusion criteria: (I) having received neoadjuvant and postoperative chemotherapy; (II) with non-epithelial breast malignancy such as metastatic breast malignancy of hematological, renal, neuroendocrine or skin origin, or malignant phyllodes tumor; (III) having undergone delayed reconstruction; (IV) with diseases of the vital organs (e.g., heart or lung), or coagulation disorders; and/or (V) having incomplete clinical data, or being lost to follow-up. The mean age of these patients was 53.32±8.47 years, and their average body mass index (BMI) was 20.51±2.91 kg/m2. Among them, 34 patients had comorbid diabetes and/or hypertension. The average intraoperative mastectomy volume was 292.84±88.45 mL and the average prosthesis volume was 273.04±88.83 mL. Most of them (n=38) were diagnosed with stage 1A, while 12 had stage 0, 29 had stage 1B, and 17 had stage 2A. A total of 74 patients were diagnosed as invasive carcinoma and 22 as intraductal carcinoma. Of these, 86 received postoperative chemotherapy and 10 patients received postoperative radiotherapy. Informed consent was obtained from all study subjects for the publication of this article and accompanying images. A copy of the written consent is available for review by the editorial office of this journal. The study was conducted in strict accordance with the Declaration of Helsinki and its subsequent amendments. The study was approved by the Ethics Committee of Hunan Cancer Hospital (No. 2025-53).
All the patients received the immediate implant-based reconstruction using the triple-plane technique after mastectomy prior to receiving adjuvant chemotherapy or radiotherapy. All surgeries of these patients performed in this study were led by the same surgeon. Before the mastectomy, the surgeon marked the patients’ midline of the sternum, bilateral breast contours, and inframammary folds using a marking pen. In addition, the patients’ breast base width, skin and subcutaneous tissue thickness, breast protrusion, and the distance from the suprasternal notch to the bilateral nipples were measured.
Step-by-step description
After general anesthesia, the patient was placed in a supine position with the affected upper limb abducted at 90 degrees. An incision was made along the inframammary fold, extending laterally for approximately 10 cm in the 6 o’clock direction. The skin was incised to the deep layer of the superficial fascia, and the retromammary space was separated along this layer, extending beyond the lateral margin of the pectoralis major muscle by approximately 2 cm. The deep layer of the superficial fascia on the surface of the pectoralis major muscle was removed. Posterior dissection of the breast was carried out without exceeding the circular mammary ligament (Figure 2A).
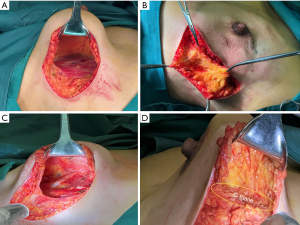
Upon completion of the dissection, the sentinel lymph node biopsy was performed through the same incision. The breast was then resected from inferior to superior along the subcutaneous superficial fascia plane. Following breast resection, the breast specimen was weighed, and its volume was measured to estimate the size of the prosthesis needed. The width and height of the pectoralis major muscle were measured to estimate the required extent and area of tissue coverage laterally and inferiorly.
Dissection was proceeded laterally along the deep layer of the superficial fascia. The length of separation was calculated based on the breast base width minus the bilateral skin tissue thickness, which was then subtracted from the semicircular perimeter that could be covered by the pectoralis major muscle (Figure 2B). Once sufficient fascia had been dissected, the fascia and underlying tissues were transected down to the surface of the serratus anterior or latissimus dorsi muscle (B-plane). The fascia was then separated from the outer to the inner along the muscle surface to the deep aspect of the pectoralis major muscle, and the rib attachments of the pectoralis major muscle were divided to the parasternal region. The specific range was determined based on the location of the medial breast contour (cleavage line).
Dissection continued deep to the pectoralis major muscle, dividing all muscle bundles below it and extending towards the abdominal direction. The length and area of dissection were determined based on the height required for prosthesis placement, calculated as the semicircular perimeter minus the portion of the prosthesis covered by the pectoralis major (at this point, the muscle bundles inferior to the pectoralis major were completely detached. However, as they remained connected to the deep layer of superficial fascia and the skin beneath the breast, the pocket encapsulating the lower pole of the prosthesis remained intact) (Figure 2C).
The prosthesis was inserted through the incision, and its lateral aspect was covered by the lateral tissue flap posterior to the pectoralis major and secured to the surface of the serratus anterior. Whether using round or anatomical prosthesis was determined based on the patient’s breast protrusion, volume, and base width. Following prosthesis insertion, the original inframammary fold was displaced superiorly. To reconstruct the lower pole coverage (C-plane), electrocautery was used to dissect the deep layer of the superficial fascia along the surface of the pectoralis major to the inferior margin of the prosthesis (Figure 2D).
Postoperative considerations and tasks
Postoperatively, antibiotics were administered intravenously for 24–48 hours (each time 8–12 hours apart). Surgical time, incision length, intraoperative blood loss, and hospitalization time of all patients were all recorded.
In addition, the patients maintained regular monthly follow-up in clinic for 6–10 months after the surgery. The reconstruction and physical rehabilitation were assessed at each follow-up visit. The interval between each follow-up visit was no more than 1 month, and each patient completed at least five follow-up visits.
The effect of breast reconstruction was categorized as follows: excellent: the breast position was symmetrical, and the shape and size were similar, and the reconstruction did not significantly affect the patient’s daily life and social interactions. Good: the position and size of both breasts were basically symmetrical, and the appearance was basically normal or slightly smaller. Fair: the bilateral breasts were obviously asymmetrical, and the difference was clearly observable under clothes. Poor: the breasts were obviously deformed. Any type of postoperative complications, such as hematoma, infection, and skin necrosis, were recorded. The satisfaction with the breast reconstruction was evaluated 6 months after surgery. At the last follow-up visit, a satisfaction survey was conducted with the BREAST-Q patient-reported outcome measure (8) to assess patient satisfaction with their breasts, surgeons, nursing staff, and other members of the team. The total score of each subscale was 100 points after conversion, and the scoring result was directly proportional to patient satisfaction.
Tips and pearls
When making an incision through the skin and subcutaneous tissue at the inframammary fold, it is crucial to first identify the deep layer of the superficial fascia and ensure its integrity. The retromammary space should be separated along the deep layer of the superficial fascia until it extends beyond the lateral margin of the pectoralis major muscle. Another technique involves placing the prosthesis into the pocket before dissecting C-plane (Figure 2D), which can simplify the procedure and facilitate a more accurate assessment of the extent of fascial separation required.
Data analysis
This study employed SPSS 24.0 software for the data processing and analysis. Patient age, operation time, and other continuous variables were calculated and statistically described as the mean ± standard deviation (SD). Categorical variables, such as pathological stage and complication rate, were expressed as numbers (n) and percentages (%).
Surgery
The average operation time of the patients was 88.40±12.13 minutes. The average incision was 12.24±4.01 cm. The average intraoperative blood loss was 46.40±10.73 mL, and the average hospitalization time was 7.23±1.00 days.
Surgical effect
Breast appearance was rated as excellent by 92 (95.83%) patients, good by 4 (4.17%) patients. None of the subjects rated their results as fair nor poor. During the study period, the shape and size of all the reconstructed breasts were similar and symmetrical, with no obvious deformity (Figure 3). Mild chest wall fibrosis was seen in 2 of 10 postoperative radiotherapy patients (20%), but it did not significantly affect the symmetry (Figure 4).
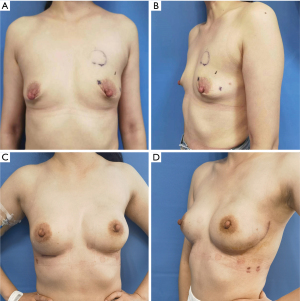
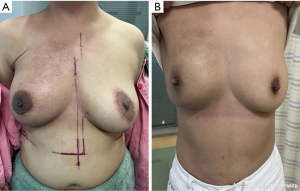
Surgical safety
No major complications, such as infection and hematoma, were observed in this cohort of patients. One patient (1.04%) developed skin necrosis at the incision margin. It healed without complications after debridement and closure.
Objective indicators
According to the BREAST-Q scoring results, the scores of patient satisfaction with their breasts, surgeons, nursing staff, and other doctors were 86.05±3.80, 90.40±5.85, 91.59±4.39, and 89.73±5.02 points, respectively.
Discussion
This study examined a new breast reconstruction technique after nipple-sparing mastectomy—the triple-plane technique. This procedure not only has a high safety profile, but also has the potential to achieve significant patient and clinical satisfaction. Our findings can serve as a new reference and provide guidance for future breast reconstruction.
In China, subpectoral implant-based breast reconstruction is the most common type of breast reconstruction (9). As such, the presence or absence of good soft tissue coverage on the prosthesis surface is critical in determining surgical outcomes (10). Small prostheses can be placed under the pectoralis major muscle; however, for larger prostheses, the pectoralis major can only cover the upper half of the prosthesis, thus the lower part has to be covered with adjacent tissues or meshes (11). A study has shown that if the prosthesis is covered with the pectoralis major alone, or a sling formed by the serratus anterior and pectoralis major, the muscle flap contraction can easily lead to prosthesis displacement and animation deformity, resulting in the reconstructed breast being poorly positioned or shaped (12). Conversely, meshes can provide a more natural inframammary fold preserving the lower pole contour, minimizing the displacement while enhancing the aesthetic appearance, with its advantages being more obvious in large or ptotic breasts (13). Available evidence suggests that patches may indirectly improve periosteal contracture by reducing prosthesis displacement (14), but their efficacy in patients undergoing radiotherapy still needs to be validated with long-term follow-up. However, the cost of meshes can be high, which in turn increases the cost of surgery, making it unaffordable for most families (15). Additionally, in our experience, mastectomy skin flap ischemia, wound infection, prosthesis loss, and other short-term postoperative complications are common, which is also the reason why primary hospitals with insufficient resources do not recommend the use of mesh combined with prosthetic reconstruction; rather, they prefer to preserve subcutaneous tissue as much as possible, or use the latissimus dorsi combined with prosthesis to reduce postoperative complications related to prosthesis or meshes (16). However, the preservation of the pectoralis major fascia remains controversial in terms of cancer safety. In addition, while the latissimus dorsi can perfectly cover the prosthesis, it sacrifices the complete function of the latissimus dorsi muscle, which can cause significant donor site morbidity and potentially decrease patient’s quality of life.
The triple-plane breast reconstruction surgery involves freeing the serratus anterior and latissimus dorsi fascia, and implanting a prosthetic subpectorally for suture and fixation. This technique follows the “no-contact principle” which reduces the possibility of prosthesis exposure, and lowers surgical costs, and thus has high clinical applicability. In this study, the operation time, incision length, and intraoperative blood loss of the triple-plane breast reconstruction surgery patients were basically consistent with those previously reported for conventional breast reconstruction (17), confirming the feasibility of this procedure.
In China, the length of hospital stay after breast reconstruction surgery is usually between 10 and 15 days, while the length of hospital stay after surgery of the visible patients in this study was 7.23±1.00 days, confirming that the triple-plane breast reconstruction is less traumatic for patients and can accelerate their rehabilitation. However, in Western countries, the length of hospital stay for similar surgical procedures is typically 24–48 hours. The longer hospital stay observed in this study may be influenced by differences in healthcare systems, cultural factors, or postoperative care practices. Future research and healthcare policy improvements may focus on adopting enhanced recovery protocols, improving perioperative care to further shorten hospitalization time while maintaining patient safety and outcomes.
In addition, this study achieved satisfactory breast reconstruction results. Specifically, 96% of the patients rated their postoperative breast appearance as excellent, 4% as good, and no patients rated as fair nor poor, and the BREAST-Q scores ranged between 80 and 100 points for all of the patients, demonstrating the satisfactory results of the triple-plane breast reconstruction technique. We believe that the high level of satisfaction may be related to patient selection criteria (mild breast ptosis, moderate prosthesis volume) and strict postoperative management. Mild fibrosis was seen in two of the radiotherapy patients, but it did not significantly affect the symmetry of the reconstructed breasts.
Most importantly, only one patient developed mastectomy skin flap necrosis in this study, which went on to heal uneventfully, confirming the safety of the technique. This result may be related to the incisional tension rather than directly attributable to the fascial flap technique. In a follow-up survey of 6,855 patients undergoing autologous breast reconstruction by Massenburg et al., the incidence of complications in patients undergoing latissimus dorsi flap, rectus abdominis transverse muscle flap, and free flap reconstruction was 10.8%, 20.6%, and 26.1%, respectively (18). In a statistical analysis conducted by Pérez-Rubio et al., the complication rate of 57 cases after breast reconstruction with mesh combined with prosthesis was 28.1%, among whom eight patients had prosthesis dislocation (19). Conversely, the risk of short-term complications after the triple-plane breast reconstruction was significantly reduced, and to date, no patient has experienced prosthesis exposure nor prosthesis loss. This may be because the complete coverage of the prosthesis with the fascial flap in the triple-plane breast reconstruction reduces the possibility of postoperative prosthesis exposure. Further, with its perforating branches, the fascial flap has a better blood supply and a higher protective effect on the prosthesis, which greatly reduces the likelihood of postoperative complications. Of course, it is also possible that this is related to the extremely low incidence of flap necrosis (1/96) in this study. Therefore, subsequent follow-up investigations of these study subjects are still needed to verify the validity of this observation. In addition, Chan et al. reported that the anterior serratus fascia flap is suitable for small-volume prostheses (up to B cup) (20), whereas the prostheses in the present study were larger (mean 273 mL), suggesting that a modified fascia flap design could extend its indications. In addition, no cases of capsular contracture (Baker classification ≥ grade III) were observed during this study. The possible reasons are as follows: the anterior serratus and latissimus dorsi fascial flaps are supplied with blood through perforating vessels, which enhances tissue viability and reduces foreign body reaction and chronic inflammation, thus decreasing the risk of capsular contracture. In addition, 10 patients received postoperative radiotherapy (dose 50 Gy), of which two developed mild chest wall fibrosis that did not result in prosthesis displacement nor morphologic abnormalities. This is consistent with the study by Rocco et al. (21) indicating that completion of prosthesis implantation prior to radiotherapy reduces the impact of fibrosis on reconstructive outcome.
Based on the findings discussed above, the main indications for the triple-plane breast reconstruction surgery can be summarized as follows. First, it is suitable for patients with early-stage breast cancer (pathological stage 0–II), provided that the tumor has not invaded the pectoral muscle fascia or skin, thereby ensuring surgical safety. Second, patients with mild breast ptosis (grade I–II) are ideal candidates, as the fascial flap can effectively cover the lower pole of the prosthesis and preserve a natural breast contour. Third, the procedure is best suited for individuals who do not require postoperative radiotherapy; if radiotherapy is anticipated, pre-radiotherapy reconstruction is preferred. In cases where radiotherapy is necessary, a low-segmentation treatment plan is recommended to reduce tissue damage. Lastly, this approach is appropriate for patients who decline the use of a patch or are unable to afford one, making it an appealing option for those seeking a lower-cost procedure with reduced risk of complications. However, certain contraindications must be considered, including a history of axillary lymph node dissection—which may compromise fascial penetrating vessels—and any prior chest wall radiotherapy or the presence of an active infection.
The need to free the fascia of the serratus anterior and latissimus dorsi disrupts the lateral contour of the breast, which may lead to changes in the position of the lateral inframammary fold. Also, the fascial flap blood supply is dependent on the perforating vessels, and if the patient has undergone axillary lymph node dissection, the integrity of the blood supply should be carefully evaluated. Currently, as the use of this technique has not yet been fully popularized, there is a lack of sufficient cases for clinical randomized controlled trials and long-term follow-up data to support our claim. Future multicenter randomized controlled trials are needed to compare the efficacy of this technique with patch co-implantation and to assess its long-term safety in patients after radiotherapy. In the meantime, we plan to conduct a 5-year follow-up study to further evaluate the long-term incidence of capsular contracture.
Conclusions
Triple-plane breast reconstruction using the pectoralis major muscle, the serratus anterior fascia, and the deep layer of the superficial fascia can create a well-defined prosthetic pocket with volume tailored to the prosthesis size, which can achieve complete coverage of the prosthesis. This technique has the potential to reduce postoperative complications, lower the possibility of prosthesis loss, and ensure satisfactory breast reconstruction outcomes. In the future, we intend to conduct clinical research on the application effect of triple-plane breast reconstruction surgery to validate the findings of this study, and thus lay a foundation for the clinical application and use of this procedure.
Acknowledgments
None.
Footnote
Reporting Checklist: The authors have completed the SUPER reporting checklist. Available at https://gs.amegroups.com/article/view/10.21037/gs-2025-116/rc
Peer Review File: Available at https://gs.amegroups.com/article/view/10.21037/gs-2025-116/prf
Funding: None.
Conflicts of Interest: All authors have completed the ICMJE uniform disclosure form (available at https://gs.amegroups.com/article/view/10.21037/gs-2025-116/coif). The authors have no conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved. Informed consent was obtained from all study subjects for the publication of this article and accompanying images. A copy of the written consent is available for review by the editorial office of this journal. The study was conducted in strict accordance with the Declaration of Helsinki and its subsequent amendments. The study was approved by the Ethics Committee of Hunan Cancer Hospital (No. 2025-53).
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Gardani M, Bertozzi N, Grieco MP, et al. Breast reconstruction with anatomical implants: A review of indications and techniques based on current literature. Ann Med Surg (Lond) 2017;21:96-104. [Crossref] [PubMed]
- Colwell AS, Taylor EM. Recent Advances in Implant-Based Breast Reconstruction. Plast Reconstr Surg 2020;145:421e-32e. [Crossref] [PubMed]
- Zehra S, Doyle F, Barry M, et al. Health-related quality of life following breast reconstruction compared to total mastectomy and breast-conserving surgery among breast cancer survivors: a systematic review and meta-analysis. Breast Cancer 2020;27:534-66. [Crossref] [PubMed]
- Santosa KB, Qi J, Kim HM, et al. Long-term Patient-Reported Outcomes in Postmastectomy Breast Reconstruction. JAMA Surg 2018;153:891-9. [Crossref] [PubMed]
- Xing J, Jia Z, Xu Y, et al. A Bayesian Network Meta-Analysis of Complications Related to Breast Reconstruction Using Different Skin Flaps After Breast Cancer Surgery. Aesthetic Plast Surg 2022;46:1525-41. [Crossref] [PubMed]
- Karoobi M, Yazd SMM, Nafissi N, et al. Comparative clinical outcomes of using three-dimensional and TIGR mesh in immediate breast reconstruction surgery for breast cancer patients. J Plast Reconstr Aesthet Surg 2023;86:321-8. [Crossref] [PubMed]
- Zhang T, Ye J, Tian T. Implant Based Breast Reconstruction Using a Titanium-Coated Polypropylene Mesh (TiLOOP® Bra): A Systematic Review and Meta-analysis. Aesthetic Plast Surg 2024;48:925-35. [Crossref] [PubMed]
- Seth I, Seth N, Bulloch G, et al. Systematic Review of Breast-Q: A Tool to Evaluate Post-Mastectomy Breast Reconstruction. Breast Cancer (Dove Med Press) 2021;13:711-24. [Crossref] [PubMed]
- Xu X, Gao X, Pan C, et al. Postoperative outcomes of minimally invasive versus conventional nipple-sparing mastectomy with prosthesis breast reconstruction in breast cancer: a meta-analysis. J Robot Surg 2024;18:274. [Crossref] [PubMed]
- Seitz AJ, Attaluri PK, Edalatpour A, et al. The Relationship Between Neuropsychiatric Diagnoses and Revision Surgery After Breast Reconstruction. Ann Plast Surg 2022;89:615-21. [Crossref] [PubMed]
- Michalopoulos NV, Frountzas M, Karathanasis P, et al. Implant infections after breast reconstruction surgery following mastectomy: Experience from a Greek breast unit. Breast Dis 2022;41:37-44. [Crossref] [PubMed]
- Whisker L, Barber M, Egbeare D, et al. Biological and synthetic mesh assisted breast reconstruction procedures: Joint guidelines from the Association of Breast Surgery and the British Association of Plastic, Reconstructive and Aesthetic Surgeons. Eur J Surg Oncol 2021;47:2807-13. [Crossref] [PubMed]
- Guliyeva G, Torres RA, Avila FR, et al. The Impact of implant-based reconstruction on persistent pain after breast cancer surgery: A systematic review. J Plast Reconstr Aesthet Surg 2022;75:519-27. [Crossref] [PubMed]
- Logan Ellis H, Asaolu O, Nebo V, et al. Biological and synthetic mesh use in breast reconstructive surgery: a literature review. World J Surg Oncol 2016;14:121. [Crossref] [PubMed]
- Kaviani A, Ashraf-Ganjouei A, Vasigh M, et al. Immediate Breast Reconstruction Using the Autologous Dermal Flap. J Surg Res 2023;283:713-8. [Crossref] [PubMed]
- Escandón JM, Aristizábal A, Langstein HN, et al. Single versus Double Drainage for Immediate Two-Stage Implant-Based Breast Reconstruction: A Propensity Score-Matched Analysis. Aesthetic Plast Surg 2024;48:3304-16. [Crossref] [PubMed]
- Dieterich M, Dragu A, Stachs A, et al. Clinical Approaches to Breast Reconstruction: What Is the Appropriate Reconstructive Procedure for My Patient? Breast Care (Basel) 2017;12:368-73. [Crossref] [PubMed]
- Massenburg BB, Sanati-Mehrizy P, Ingargiola MJ, et al. Flap Failure and Wound Complications in Autologous Breast Reconstruction: A National Perspective. Aesthetic Plast Surg 2015;39:902-9. [Crossref] [PubMed]
- Pérez-Rubio Á, Estellés Vidagany N, Martínez López E, et al. Immediate Breast Reconstruction with Prosthesis and Titanized Mesh Using a Dual-Plane Approach: Complications and Risk Factors Analysis. Aesthetic Plast Surg 2022;46:115-22. [Crossref] [PubMed]
- Chan YH, Yue IK, Ho CM, et al. The Use of Serratus Anterior Fascial Flap in Integrated Mastectomy and Implant Reconstruction. World J Surg 2020;44:825-30. [Crossref] [PubMed]
- Rocco N, Catanuto G, Nava MB. Radiotherapy and breast reconstruction. Minerva Chir 2018;73:322-8. [Crossref] [PubMed]
(English Language Editor: L. Huleatt)


