
Evidence-based integration of clinicopathological factors with the risk of papillary thyroid carcinoma lateral cervical lymph node metastasis: systematic review and meta-analysis and subgroup study
Highlight box
Key findings
• We found that the following clinicopathological features were significantly correlated with lateral neck lymph node metastasis, such as male, extrathyroidal extension (ETE), tumor size >2 cm, multifocality, central lymph node metastasis (CLNM), capsular invasion, high tumor location, calcification, and hyperechoic.
What is known, and what is new?
• Males, ETE, tumor size >2 cm, neoplastic patients, bilobal involvement, and CLNM could predict lateral neck lymph node metastasis. Still, the included studies were small, so we further verified.
• Combining the latest findings to complement previous studies, there was no significant association between age, bilaterality, Hashimoto’s thyroiditis, BRAF V600E mutation, thyroid-stimulating hormone, thyroglobulin antibody, and papillary thyroid carcinoma (PTC).
What is the implication, and what should change now?
• We found that the following clinicopathological and ultrasonic features were significantly correlated with lateral neck lymph node metastasis: male, ETE, tumor size >2 cm, multifocality, CLNM, capsular invasion, high tumor location, calcification, and hyperechoic. It is suggested that for suspected metastatic lymph nodes, lateral neck lymph node dissection can be considered in patients with the above risk factors to reduce the recurrence and distant metastasis of PTC.
Introduction
Thyroid cancer is the most common endocrine malignancy, accounting for about 3.4% of annual malignancy diagnoses (1). Papillary thyroid carcinoma (PTC) is the most common type of thyroid cancer, accounting for about 80% of all thyroid cancers (2). Over the past few decades, thyroid cancer has become a significant public health problem in many countries around the world (3). After standard surgery and adjuvant radioiodine therapy, the long-term prognosis for PTC is good (4). The 2015 American Thyroid Association (ATA) differentiated thyroid cancer (DTC) guidelines state that “Prophylactic central-compartment neck dissection (ipsilateral or bilateral) should be considered in patients with papillary thyroid carcinoma with clinically union evolved central neck lymph nodes (cN0) who have advanced primary tumors (T3 or T4) or clinically involved lateral neck nodes (cN1b), or if the information will be used to plan further steps in therapy”. However, prophylactic lateral neck dissection is still controversial, and how to minimize the residual or recurrence of tumors while reducing unnecessary trauma caused by surgery is the key to the following research (5). Preoperative ultrasonography (USG) has limited diagnostic sensitivity for lateral cervical lymph node metastasis (LLNM), reportedly as low as 27.3% (6). Therefore, it is essential to understand the risk factors of LLNM in patients with thyroid papillary carcinoma and predict LLNM.
Although many studies on LLNM and central lymph node metastasis (CLNM), including some meta-analyses, have been conducted, the number of studies focusing on LLNM is limited and remains partially controversial. In this meta-analysis, we aimed to investigate the risk factors for LLNM in PTC. According to preoperative or intraoperative detection, the contralateral neck lymph node dissection has guiding significance for patients with high-risk factors of lateral neck lymph node metastasis. We present this article in accordance with the PRISMA reporting checklist (available at https://gs.amegroups.com/article/view/10.21037/gs-2025-60/rc).
Methods
Literature retrieval and screening
We conducted a search on the literature related to the risk factors of LLNM in PTC published between 2019 and 2024 in PubMed, Embase, Web of Science, the Chinese biomedical literature database, CNKI, and WANFANG DATA. The English database search is “(Thyroid Cancer Papillary OR papillary thyroid cancer OR PTC OR papillary thyroid carcinoma) AND ((lateral cervical OR lateral neck) AND (lymph nodes)) AND (Risk Factors) Filters: in the last 5 years”. The Chinese database search type was “(subject: (thyroid papillary carcinoma) and subject: (lateral cervical lymph node OR lateral cervical region lymph node) and subject: (risk factors)) and publication time: 2019-*”. Two authors completed the search independently, and differences of opinion were resolved through negotiation.
Inclusion and exclusion criteria
Inclusion criteria
Inclusion criteria: (I) pathologically confirmed PTC; (II) the surgical procedures were total thyroidectomy or unilateral thyroidectomy plus regional cervical lymph node dissection; (III) the risk factors of cervical lymph node metastasis could be obtained; and (IV) assess clinicopathological and/or ultrasound risk factors for LLNM.
Exclusion criteria
Exclusion criteria: (I) duplicate literature; (II) reviews, case reports, conference minutes, and other documents; (III) the subjects were patients with non-PTC, such as follicular thyroid cancer, medullary thyroid cancer, or anaplastic cancer; (IV) the risk factors of cervical lymph node metastasis could not be obtained; and (V) study center or time limit replicated with other studies.
Literature screening and data extraction
Two researchers independently extracted data from studies that met the inclusion criteria and resolved their differences through discussion. The two researchers independently extracted data from the included studies, and differences were resolved through joint discussion. The following data were extracted from each study: first author name, year of publication, country, study duration, study design, diagnosis, number of cases, mode of surgery, investigated risk factors [i.e., gender, age, extrathyroidal extension (ETE), tumor size, multifocality, bilaterality, thyroiditis, Hashimoto thyroiditis, CLNM, capsular invasion, tumor location, BRAF V600E mutation].
Literature quality evaluation
The quality of the included literature was evaluated by two reviewers using the Newcastle-Ottawa scale (NOS). In the evaluation process, if there was any disagreement, it was discussed in focus or ruled by a third party. The maximum score of this scale is 9 points; 7–9 points are considered high-quality literature, and 4–6 points are regarded as medium-quality literature. A score of 4 is considered as low-quality literature.
Statistical analysis
Meta-analysis was performed by RevMan 5.2 software. Odds ratio (OR) and 95% confidence interval (CI) were used to represent the categorical variables, and P<0.05 was considered statistically significant. Heterogeneity was quantified using I2 statistics. The fixed effect model was used for meta-analysis when I2≤50%. If I2>50%, the cause of heterogeneity was further analyzed, and after excluding obvious clinical influencing factors, the random effects model was used for meta-analysis. The results of each analysis will be presented in the form of a forest map. When the number of eligible original studies was more than 10, Begg’s funnel plot was used to determine publication bias.
In the face of missing data, the original data were first obtained. Then, a reasonable imputation strategy was selected to calculate the missing value based on the existing statistics (such as mean and sample size), and the standard deviation was imputed through the regression model. Multiple imputation method was used to generate various data sets for comprehensive analysis of complex missing patterns, and the robustness of the results was verified by sensitivity analysis.
Results
Research description
A total of 915 studies were retrieved, of which 252 were replicated and immediately excluded. Another 663 studies were excluded after careful reading of the title and abstract. After thoroughly evaluating the remaining 54 articles, another 30 studies were excluded for various reasons (did not meet the inclusion criteria, research center or time limit repetitive with other studies, no data available, irrelevant studies). Finally, 24 retrospective studies (a total of 40,190 patients; among them, 4,991 had LLNM) were included in this meta-analysis. The NOS scores of 24 literatures were all ≥7 points, and the quality was good. Figure 1 shows the research selection process, and Table 1 summarizes the characteristics of the included research. All risk factors for LLNM are shown in Table 2.
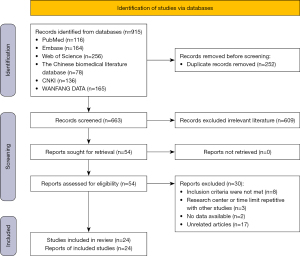
Table 1
| Authors | Year | Area | Study period | Study design | Diagnosis | Recorded/total | Surgery | Quality score |
|---|---|---|---|---|---|---|---|---|
| Cai et al. | 2024 | China | February 2016 to January 2020 | Retrospective | PTC | 396/499 | TT/lobectomy + CND ± LND | 7 |
| Caliskan et al. | 2023 | Türkiye | May 2012 to September 2020 | Retrospective | PTC | 36/346 | TT/lobectomy ± CND ± LND | 7 |
| Fan et al. | 2024 | China | June 2017 to February 2023 | Retrospective | PTC | 206/3,336 | Thyroidectomy | 7 |
| Feng et al. | 2022 | China | March 2019 to May 2020 | Retrospective | PTC | 115/528 | TT/lobectomy + CND±LND | 8 |
| Heng et al. | 2020 | China | 2017 to 2019 | Retrospective | PTC | 142/434 | TT/lobectomy + CND ± LND | 7 |
| Huang et al. | 2023 | China | January 2013 to June 2018 | Retrospective | PTMC | 157/4,872 | TT/lobectomy + CND ± LND | 7 |
| Kim et al. | 2020 | Korea | January 2015 to December 2017 | Retrospective | PTMC | 157/3,578 | TT + CND + LND | 9 |
| Kwon et al. | 2023 | Korea | 2007 to 2021 | Retrospective | PTMC | 246/5,329 | TT/lobectomy + CND ± LND | 8 |
| Lai et al. | 2022 | China | January 2015 to December 2020 | Retrospective | PTC | 1,135/1,815 | TT + CND + LND | 7 |
| Liu et al. | 2019 | China | January 2013 to December 2015 | Retrospective | PTC | 211/420 | TT/lobectomy + CND ± LND | 8 |
| Liu et al. | 2023 | China | February 2011 to April 2022 | Retrospective | PTC | 509/4,247 | TT/lobectomy+ CND ± LND | 7 |
| Lou et al. | 2023 | China | December 2019 to April 2022 | Retrospective | PTC | 51/69 | Thyroidectomy + CND + LND | 7 |
| Luo et al. | 2019 | China | October 2010 to January 2017 | Retrospective | PTMC | 58/1,031 | TT/lobectomy + CND ± LND | 7 |
| Masui et al. | 2023 | Japan | January 2009 to December 2018 | Retrospective | PTC | 78/274 | TT/lobectomy + LND | 8 |
| Ruan et al. | 2024 | China | January 1997 to December 2016 | Retrospective | PTMC | 128/5,241 | TT/lobectomy | 9 |
| Song et al. | 2020 | China | January 2010 to December 2019 | Retrospective | PTMC | 256/3,686 | TT/lobectomy + CND ± LND | 8 |
| Wang et al. | 2023 | China | 2015 to 2018 | Retrospective | PTC | 147/355 | Thyroidectomy + LND | 7 |
| Wu et al. | 2019 | China | January 2017 to December 2017 | Retrospective | PTMC | 126/936 | TT + CND ± LND | 7 |
| Yoon et al. | 2024 | Korea | 2004 to 2013 | Retrospective | PTMC | 90/358 | TT + CND ± LND | 7 |
| Zhang et al. | 2021 | China | January 2017 to May 2017 | Retrospective | PTC | 35/345 | TT/lobectomy + CND ± LND | 7 |
| Zhao et al. | 2019 | China | January 2014 to July 2015 | Retrospective | PTMC | 163/215 | TT + CND ± LND | 8 |
| Zhu et al. | 2023 | China | January 2013 to June 2018 | Retrospective | PTC | 374/1,732 | TT/lobectomy + LND | 7 |
| Zhuo et al. | 2022 | China | January 2013 to June 2019 | Retrospective | PTC | 47/138 | Thyroidectomy | 7 |
| Zou et al. | 2021 | China | July 2015 to June 2019 | Retrospective | PTC | 128/406 | TT + LND | 9 |
CND, central lymph node dissection; LND, lateral neck lymph node dissection; PTC, papillary thyroid carcinoma; PTMC, papillary thyroid microcarcinoma; TT, total thyroidectomy.
Table 2
| Risk factors | OR | 95% CI | P | I2 (%) |
|---|---|---|---|---|
| Gender (male) | 1.51 | 1.34 to 1.70 | <0.001 | 52 |
| Age (<55 years) | 1.17 | 0.95 to 1.44 | 0.15 | 63 |
| ETE | 4.16 | 2.82 to 6.14 | <0.001 | 87 |
| Tumor size (2 cm) | 0.35 | 0.20 to 0.59 | <0.001 | 89 |
| Multifocality | 1.94 | 1.50 to 2.52 | <0.001 | 87 |
| Bilaterality | 1.18 | 0.77 to 1.80 | 0.45 | 88 |
| Hashimoto thyroiditis | 1.06 | 0.91 to 1.22 | 0.45 | 0 |
| CLNM | 5.38 | 2.62 to 11.07 | <0.001 | 97 |
| Capsular invasion | 0.07 | 0.05 to 0.08 | <0.001 | 77 |
| Tumor location (high) | 1.84 | 1.63 to 2.09 | <0.001 | 0 |
| BRAF V600E mutation | 1.17 | 0.98 to 1.41 | 0.09 | 22 |
| Calcification | 1.97 | 1.34 to 2.91 | <0.001 | 74 |
| Echogenicity (hyperechoic) | 1.55 | 1.16 to 2.08 | 0.003 | 39 |
| TSH | Mean difference =0.01 | −0.15 to 0.18 | 0.86 | 0 |
| TGAB | Mean difference =8.98 | −12.21 to 30.17 | 0.41 | 65 |
CI, confidence interval; CLNM, central lymph node metastasis; ETE, extrathyroidal extension; LLNM, lateral cervical lymph node metastasis; OR, odds ratio; PTC, papillary thyroid carcinoma; TGAB, thyroglobulin antibody; TSH, thyroid-stimulating hormone.
Gender and LLNM
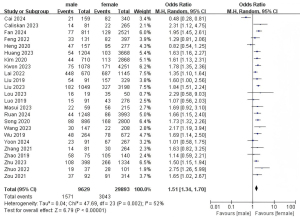
A total of 24 studies with 39,522 patients were included to investigate the effect of gender on the risk of LLNM (Figure 2) (7-30). Due to the low heterogeneity, a random-effects model was used (P=0.002; I2=52%). Pooled analysis showed a significantly higher risk of LLNM in male patients (OR =1.51; 95% CI: 1.34–1.70; P<0.001).
Age and LLNM
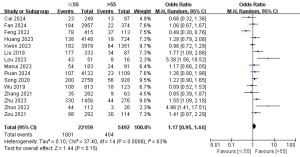
A total of 15 studies with 27,651 patients were included to investigate the effect of age on the risk of LLNM (Figure 3) (7,9,10,12,14,16,18,20-22,24,26,28-30). Due to the high heterogeneity, a random-effects model was used (P=0.006; I2=63%). Pooled analysis showed that the risk of LLNM was not significantly associated with age (OR =1.17; 95% CI: 0.95–1.44; P=0.15).
ETE and LLNM
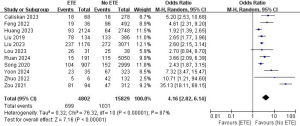
A total of 11 studies with 20,631 patients were included to investigate the effect of ETE on the risk of LLNM (Figure 4) (8,10,12,16-18,21,22,25,29,30). Due to the high heterogeneity, a random-effects model was used (P<0.001; I2=87%). Pooled analysis showed a significantly increased risk of lateral neck lymph node metastasis in ETE patients (OR =4.16; 95% CI: 2.82–6.14; P<0.001).
Tumor size and LLNM
A total of seven studies with 7,850 patients were included to investigate the effect of tumor size on the risk of lateral neck lymph node metastasis (Figure 5) (7,10,16-18,23,28). Due to the high heterogeneity, a random-effects model was used (P<0.001; I2=89%). Pooled analysis showed that the risk of LLNM increased significantly when the tumor was larger than 2 cm (OR =0.35; 95% CI: 0.20–0.59; P<0.001).
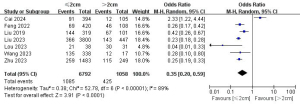
Multifocality and LLNM
A total of 15 studies with 26,906 patients were included to investigate the effect of multifocality on the risk of LLNM (Figure 6) (7-10,14,16,17,19,21-23,25-28). Due to the high heterogeneity, a random-effects model was used (P<0.001; I2=87%). Pooled analysis showed a significantly increased risk of lateral neck lymph node metastasis in multifocality patients (OR =1.94; 95% CI: 1.50–2.52; P<0.001).
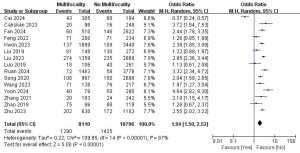
Bilaterality and LLNM
A total of 10 studies with 14,934 patients were included to investigate the effect of bilaterality on the risk of LLNM (Figure 7) (7,9-11,16,18,19,21,22,25). Due to the high heterogeneity, a random-effects model was used (P<0.001; I2=88%). Pooled analysis showed that bilaterality was not significantly associated with LLNM (OR =1.18; 95% CI: 0.77–1.80; P=0.45).
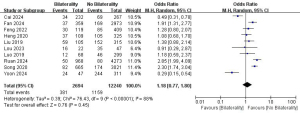
Hashimoto thyroiditis and LLNM
A total of 10 studies with 14,402 patients were included to investigate the effect of Hashimoto thyroiditis on the risk of LLNM (Figure 8) (10,12,16,18,21,24,26-29). Due to the low heterogeneity, a fixed-effects model was used (P=0.53; I2=0%). Pooled analysis showed that Hashimoto thyroiditis was not significantly associated with LLNM (OR =1.06; 95% CI: 0.91–1.22; P=0.45).
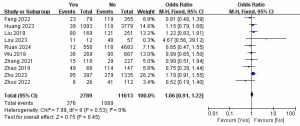
CLNM and LLNM
A total of 11 studies with 19,252 patients were included to investigate the effect of CLNM on the risk of LLNM (Figure 9) (7,8,10,12,13,16,17,22,24,26,27). Due to the high heterogeneity, the random-effects model was used (P<0.001; I2=97%). Pooled analysis showed a significantly increased risk of LLNM when patients developed CLNM (OR =5.38; 95% CI: 2.62–11.07; P<0.001).
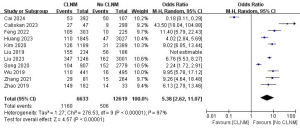
Capsular invasion LLNM
A total of five studies with 8,083 patients were included to investigate the effect of capsular invasion on the risk of LLNM (Figure 10) (11,14,15,19,29). Due to the high heterogeneity, the random-effects model was used (P=0.002; I2=77%). Pooled analysis showed a significantly increased LLNM risk when the tumor developed capsular invasion (OR =0.07; 95% CI: 0.05–0.08; P<0.001).
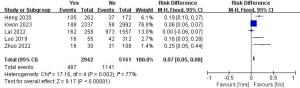
Tumor location and LLNM
A total of nine studies with 13,747 patients were included to investigate the effect of tumor location on the risk of LLNM (Figure 11) (7,9,10,12,15,26-28,30). Due to the high heterogeneity, the random-effects model was used (P<0.001; I2=0%). Pooled analysis showed a significantly increased risk of LLNM when patients had higher tumor locations (OR =1.84; 95% CI: 1.63–2.09; P<0.001).
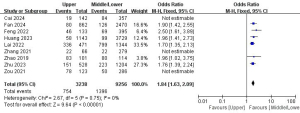
BRAF V600E mutation and LLNM
A total of four studies with 7,992 patients were included to investigate the effect of BRAF V600E mutation on the risk of LLNM (Figure 12) (10,13,26,28). Due to the low heterogeneity, a fixed-effects model was used (P=0.28; I2=22%). Pooled analysis showed that BRAF V600E mutation was not significantly associated with LLNM (OR =1.17; 95% CI: 0.98–1.41; P=0.09).
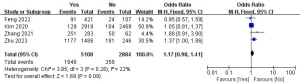
Calcification and LLNM
A total of six studies with 4,830 patients were included to investigate the effect of calcification on the risk of LLNM (Figure 13) (15,16,19,25,28,29). Due to the high heterogeneity, a random-effects model was used (P=0.002; I2=74%). Pooled analysis showed a significantly increased risk of LLNM when patients had calcification on ultrasound examination (OR =1.97; 95% CI: 1.34–2.91; P<0.001).
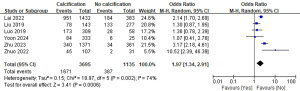
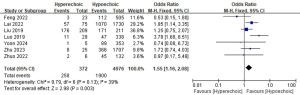
Echogenicity and LLNM
A total of seven studies with a total of 5,348 patients were included to investigate the effect of echogenicity on the risk of LLNM (Figure 14) (10,15,16,19,25,28,29). Due to the low heterogeneity, a fixed-effect model was used (P=0.13; I2=39%). Pooled analysis showed a significantly increased risk of LLNM when patients had hyperechoic on ultrasound examination (OR =1.55; 95% CI: 1.16–2.08; P=0.003).
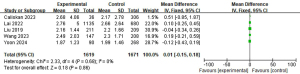
Thyroid-stimulating hormone (TSH) and LLNM
A total of five studies with 3,290 patients were included to investigate the effect of TSH on the risk of LLNM (Figure 15) (8,15,16,23,25). Due to the low heterogeneity, a fixed-effects model was used (P=0.68; I2=0%). Pooled analysis showed that TSH was not significantly associated with LLNM (mean difference =0.01; 95% CI: −0.15 to 0.18; P=0.86).
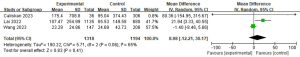
Thyroglobulin antibody (TGAB) and LLNM
A total of three studies with 2,512 patients were included to investigate the effect of anti-TGABs on the risk of LLNM (Figure 16) (8,15,23). Due to the high heterogeneity, a random-effects model was used (P=0.06; I2=65%). Pooled analysis showed that TGAB was not significantly associated with LLNM (mean difference =8.98; 95% CI: −12.21 to 30.17; P=0.41).
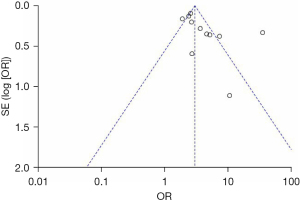
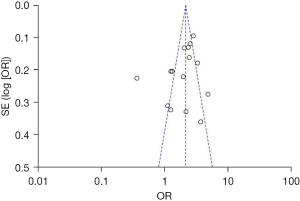
Publication bias
Begg’s funnel plot showed no significant evidence of asymmetry in meta-analyses of gender, age, and multifocality ETE (Figures 17-20).
Discussion
PTC is a low-grade malignant tumor with a good prognosis and a 10-year survival rate of more than 90% (31), but 30% to 80% of cervical lymph node metastases occur (32,33). The increasing incidence of PTC compared to other major histological types has largely accelerated the global trend of thyroid cancer incidence (34). Due to the limited sensitivity of preoperative USG in the diagnosis of LLNM, the analysis of the predictors of cervical lymph node metastasis provides more powerful evidence for clinical decision-making (6). The results of this study showed that the clinical risk factors for LLNM of PTC are male, ETE, tumor size >2 cm, multifocality, CLNM, capsular invasion, high tumor location, calcification, and hyperechoic. In contrast, age, bilaterality, Hashimoto’s thyroiditis, BRAF V600E mutation, TSH, and TAGB were not considered as risk factors for LLNM in these patients.
Age was not associated with the risk of LLNM. The 8th edition of the American Joint Committee on Cancer (AJCC) guidelines raised the age threshold from 45 to 55 years, which had been used as the threshold in most previous studies. Zhao et al. suggested that PTC patients than 45 years old were at an increased risk of LLNM (35). On the contrary, Qu et al. concluded that LLNM was significantly higher in younger people (age <45 years) (36). It is more common in patients age <45 years, and a study has shown no statistical significance between age and lymph node metastasis (37). The latest study showed no correlation between age and Delphian lymph node metastasis (38). As the relationship between age and lateral neck lymph node metastasis is not clear, most studies use 55 years old as the critical value. Therefore, this study was also conducted at 55 years old. Fifteen studies were included, with a total of 27,651 patients, and no significant statistical significance was found, which is still controversial, and the critical value should be changed and a larger sample should be investigated.
The incidence of thyroid cancer in women is significantly higher than that in men; the ratio of male to female is about 1:3, but the incidence of cervical lymph node metastasis in men is higher than that in women. The incidence of PTC in women is significantly correlated with estrogen level (39). Fan et al. found that estrogen receptor α can promote the growth of PTC by enhancing autophagy, an essential pro-survival catabolic process in PTC cells (40). Men are more likely to have LLNM; the mechanism is not completely clear, mainly related to hormone levels. Jiang et al. pointed out that testosterone can promote the malignant behavior of PTC, such as growth, migration, invasion, and epithelial-to-mesenchymal transition (EMT) process by up-regulating Tnnt1 expression, and the function of testosterone may be achieved by activating the p38/JNK signaling pathway (41). In addition, female patients often have Hashimoto’s thyroiditis, whose immunomodulatory effect may reduce the risk of metastasis, while the proportion of male patients with HT is low. Male PTC is more aggressive, characterized by larger tumor size (>1 cm), higher proportion of multifocality, and more invasion of the capsule and surrounding tissues, which increase the probability of lymphatic metastasis. Male patients have a higher rate of BRAF V600E mutation, which may accelerate cancer cell invasion and metastasis. Men are more likely to develop LLNM than women in this study, which is consistent with previous studies (39,42).
A study has shown that ETE is an independent risk factor for LLNM in PTC patients, and the prognosis with ETE includes worse lymph node metastasis compared with patients without ETE (21). ETE is a risk factor for LLNM, which may be due to the tendency of tumor cells to metastasize along the abundant lymphoid tissue surrounding the thyroid gland to peripheral lymph nodes once they invade the thyroid capsule and extrathyroidal tissues. Our findings are consistent with previous studies showing that ETE increases the risk of LLNM in PTC patients (37,42).
In our study, biological indicators, such as TSH, TGAB, and BRAF V600E mutation, were innovatively included, which were rarely included in a previous similar study (42). Studies have found that TSH, thyroglobulin (Tg), anti-TGAB, thyroid peroxidase antibody (TPOAb), TSH receptor antibody (TRAb), and other thyroid-related serological indicators can be used as reliable indicators to predict the occurrence, metastasis, and recurrence of PTC. It is a simple, efficient, and cost-effective clinical method to identify PTC disease progression (43). TSH is the main stimulator of thyroid cell function, differentiation, and proliferation. TSH induces thyrocyte growth directly by binding to its autoreceptor and indirectly by stimulating other growth factors such as vascular endothelial growth factor and amyloid precursor. The relationship between serum TSH and lymph node metastasis in PTC patients remains controversial. The mechanism of its occurrence may be that TSH stimulates the massive release of growth factors, which may induce cancer cell growth and neovascularization and may assist malignant cell immune escape to avoid apoptosis, thereby increasing the risk of metastasis (44). Vasileiadis et al. pointed out that patients with DTC with positive TGAB were more likely to have lymph node metastasis, and the results of this study showed that preoperative TGAB had certain predictive value for DTC lymph node metastasis (45). However, there are few studies on preoperative TGAB and DTC, so the value of preoperative TGAB in predicting distant metastasis needs further study. BRAF is the strongest activator of the MAPK signaling pathway, which can regulate cell proliferation, differentiation, and apoptosis. It is composed of 18 exons. BRAF mutation is the missense mutation of exon 15 leading to sequence change at codon 600, which accounts for most mutations, so BRAF V600E mutation is also known as BRAF mutation (46). BRAF mutation can cause the continuous proliferation and differentiation of cells, leading to the occurrence and development of tumors, which is closely related to the invasive biological characteristics of thyroid cancer (thyroid capsule invasion, lymph node metastasis, etc.) (47). However, the predictive value of BRAF V600E mutation remains inconclusive (48). The BRAF V600E mutation will remain important in the preoperative diagnosis of PTC (49). Therefore, whether BRAF V600E mutation can be used as an independent risk factor for lateral neck lymph node metastasis in PTC is still controversial, and further study is needed. To our surprise, BRAF V600E mutation, TSH, and TGAB had no significant difference in our research. Considering the small samples we included and the discrepancies between different studies, a comprehensive evaluation should be performed with other clinicopathological features.
The 2015 ATA showed that cervical lymph node dissection in area VI is not recommended for patients with PTC (clinical stage cN0), while cervical lymph node dissection in area VI is required for patients with clinical stage cN1a, but preventive cervical lymph node dissection is not necessary, and cervical lymph node dissection is required for patients with cN1b. Lymph nodes and adipose tissue in regions II to V were the scope of dissection (6). At present, there is still controversy about whether preventive lymph node dissection should be performed in the central region. Preventive lymph node dissection may reduce the risk of tumor recurrence and the risk of second operation. Chinese experts unanimously recommend preventive dissection of central lymph nodes on the premise of protecting nerve and parathyroid function (50). However, the current domestic and foreign guidelines do not recommend preventive dissection of lateral cervical lymph nodes (6,50). In this study, it was found that when patients developed CLNM, the risk of LLNM was significantly increased. Therefore, patients with CLNM can be regarded as a risk factor for lateral neck lymph node metastasis, and preventive lateral neck lymph node dissection can be performed with sufficient evidence, which requires more studies to provide sufficient data. The process of glucose oxidation can provide energy for cell growth, development, invasion, and metastasis (51). Fasting serum glucose (FSG) can predict lymph node metastasis in patients with PTC, and higher FSG is a risk factor for CLNM and CLNM combined with LLNM (52). Diabetes may be associated with the development of thyroid cancer, and patients with diabetes mellitus (DM) have a higher risk of thyroid cancer, with a 20% increased risk of thyroid papillary cancer relative to non-diabetic patients, and patients with diabetes and elevated fasting blood sugar levels are more likely to develop thyroid papillary cancer (53). Hyperglycemia can promote the production of advanced glycation end products (AGEs), which then proliferate tumor cells and directly or indirectly promote IGF-1R phosphorylation (54,55). Therefore, glucose concentration can also be used as a predictor of lateral neck lymph node metastasis. Due to the lack of research data, glucose concentration was not included in this study, which is the defect of this meta-analysis. The deepening of research data can be further analyzed in the following studies to better predict lateral neck lymph node metastasis and provide better strategies for sweeping surgery.
However, this study has some limitations, such as no new biomarkers were included, high heterogeneity, and geographic bias. For some risk factors, only a small number of studies have been analyzed, such as the meta-analysis between capsular invasion, tumor location, and BRAF V600E mutation. Different studies used different size intervals and data representation methods, and many studies were excluded from the meta-analysis. The included studies were mainly from Asian countries, and there may be differences along ethnic lines.
Conclusions
In conclusion, this study identified several risk factors for lateral neck lymph node metastasis in patients with PTC, which play an important role in its prediction. We found that the following clinicopathological features were significantly correlated with lateral neck lymph node metastasis, such as male, ETE, tumor size >2 cm, multifocality, CLNM, capsular invasion, high tumor location, calcification, and hyperechoic. It is suggested that for suspected metastatic lymph nodes, patients with the above risk factors can consider lateral cervical lymph node puncture or further treatment to reduce the recurrence and distant metastasis of PTC.
In future studies, more samples can be included to reduce the risk of bias. The inclusion of new biomarkers, such as lipid profile studies, protein molecular signatures, molecular biomarkers, and glucose-related factors, can better predict the risk of lymph node metastasis and disease progression.
Acknowledgments
None.
Footnote
Reporting Checklist: The authors have completed the PRISMA reporting checklist. Available at https://gs.amegroups.com/article/view/10.21037/gs-2025-60/rc
Peer Review File: Available at https://gs.amegroups.com/article/view/10.21037/gs-2025-60/prf
Funding: This work was supported by
Conflicts of Interest: All authors have completed the ICMJE uniform disclosure form (available at https://gs.amegroups.com/article/view/10.21037/gs-2025-60/coif). The authors have no conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Prete A, Borges de Souza P, Censi S, et al. Update on Fundamental Mechanisms of Thyroid Cancer. Front Endocrinol (Lausanne) 2020;11:102. [Crossref] [PubMed]
- Shi J, Wu P, Sheng L, et al. Ferroptosis-related gene signature predicts the prognosis of papillary thyroid carcinoma. Cancer Cell Int 2021;21:669. [Crossref] [PubMed]
- Kitahara CM, Sosa JA. The changing incidence of thyroid cancer. Nat Rev Endocrinol 2016;12:646-53. [Crossref] [PubMed]
- Pacini F, Fuhrer D, Elisei R, et al. 2022 ETA Consensus Statement: What are the indications for post-surgical radioiodine therapy in differentiated thyroid cancer? Eur Thyroid J 2022;11:e210046. [Crossref] [PubMed]
- Cai HZ, Huang ZH, Huang YC, et al. Update on diagnosis and treatment of lateral cervical lymph node metastasis in papillary thyroid carcinoma. Zhonghua Er Bi Yan Hou Tou Jing Wai Ke Za Zhi 2023;58:398-402. [PubMed]
- Haugen BR, Alexander EK, Bible KC, et al. 2015 American Thyroid Association Management Guidelines for Adult Patients with Thyroid Nodules and Differentiated Thyroid Cancer: The American Thyroid Association Guidelines Task Force on Thyroid Nodules and Differentiated Thyroid Cancer. Thyroid 2016;26:1-133. [Crossref] [PubMed]
- Cai H, Zhuge L, Huang Z, et al. Distinct risk factors of lateral lymph node metastasis in patients with papillary thyroid cancer based on age stratification. BMC Surg 2024;24:24. [Crossref] [PubMed]
- Caliskan O, Unlu MT, Yanar C, et al. Predictive Factors Affecting the Development of Lateral Lymph Node Metastasis in Papillary Thyroid Cancer. Sisli Etfal Hastan Tip Bul 2023;57:312-9. [Crossref] [PubMed]
- Fan Y, Zheng X, Ran Y, et al. Analysis of risk factors for lateral lymph node metastasis in T1 stage papillary thyroid carcinoma: a retrospective cohort study. Gland Surg 2024;13:314-24. [Crossref] [PubMed]
- Feng JW, Wu WX, Qi GF, et al. Nomograms based on sonographic and clinicopathological characteristics to predict lateral lymph node metastasis in classic papillary thyroid carcinoma. J Endocrinol Invest 2022;45:2043-57. [Crossref] [PubMed]
- Heng Y, Yang Z, Zhou L, et al. Risk stratification for lateral involvement in papillary thyroid carcinoma patients with central lymph node metastasis. Endocrine 2020;68:320-8. [Crossref] [PubMed]
- Huang H, Xu S, Ni S, et al. A nomogram for predicting lateral lymph node metastasis in cN0 unifocal papillary thyroid microcarcinoma. BMC Cancer 2023;23:718. [Crossref] [PubMed]
- Kim K, Zheng X, Kim JK, et al. The contributing factors for lateral neck lymph node metastasis in papillary thyroid microcarcinoma (PTMC). Endocrine 2020;69:149-56. [Crossref] [PubMed]
- Kwon O, Lee S, Bae JS. Risk factors associated with high-risk nodal disease in patients considered for active surveillance of papillary thyroid microcarcinoma without extrathyroidal extension. Gland Surg 2023;12:1179-90. [Crossref] [PubMed]
- Lai SW, Fan YL, Zhu YH, et al. Machine learning-based dynamic prediction of lateral lymph node metastasis in patients with papillary thyroid cancer. Front Endocrinol (Lausanne) 2022;13:1019037. [Crossref] [PubMed]
- Liu C, Xiao C, Chen J, et al. Risk factor analysis for predicting cervical lymph node metastasis in papillary thyroid carcinoma: a study of 966 patients. BMC Cancer 2019;19:622. [Crossref] [PubMed]
- Liu W, Zhang D, Jiang H, et al. Prediction model of cervical lymph node metastasis based on clinicopathological characteristics of papillary thyroid carcinoma: a dual-center retrospective study. Front Endocrinol (Lausanne) 2023;14:1233929. [Crossref] [PubMed]
- Lou J, Yang J, Luo Y, et al. Analysis of the influence factors of cervical lymph node metastasis in Papillary thyroid carcinoma: A retrospective observational study. Medicine (Baltimore) 2023;102:e35045. [Crossref] [PubMed]
- Luo Y, Zhao Y, Chen K, et al. Clinical analysis of cervical lymph node metastasis risk factors in patients with papillary thyroid microcarcinoma. J Endocrinol Invest 2019;42:227-36. [Crossref] [PubMed]
- Masui T, Adachi S, Uemura H, et al. Risk factors for the lateral cervical lymph node metastasis of papillary thyroid carcinoma: A clinical study. Mol Clin Oncol 2023;18:25. [Crossref] [PubMed]
- Ruan J, Chen Z, Chen S, et al. Lateral lymph node metastasis in papillary thyroid microcarcinoma: a study of 5241 follow-up patients. Endocrine 2024;83:414-21. [Crossref] [PubMed]
- Song J, Yan T, Qiu W, et al. Clinical Analysis of Risk Factors for Cervical Lymph Node Metastasis in Papillary Thyroid Microcarcinoma: A Retrospective Study of 3686 Patients. Cancer Manag Res 2020;12:2523-30. [Crossref] [PubMed]
- Wang J, Gao Y, Zong Y, et al. Nomogram Model Based on Iodine Nutrition and Clinical Characteristics of Papillary Thyroid Carcinoma to Predict Lateral Lymph Node Metastasis. Cancer Control 2023;30:10732748231193248. [Crossref] [PubMed]
- Wu X, Li B, Zheng C, et al. Predicting factors of lateral neck lymph node metastases in patients with papillary thyroid microcarcinoma. Medicine (Baltimore) 2019;98:e16386. [Crossref] [PubMed]
- Yoon JH, Park JY, Hong AR, et al. Predictors of lateral lymph node metastasis and skip metastasis in patients with papillary thyroid microcarcinoma. Front Endocrinol (Lausanne) 2024;15:1392247. [Crossref] [PubMed]
- Zhang X, Chen W, Fang Q, et al. Lateral Lymph Node Metastases in T1a Papillary Thyroid Carcinoma: Stratification by Tumor Location and Size. Front Endocrinol (Lausanne) 2021;12:716082. [Crossref] [PubMed]
- Zhao W, Chen S, Hou X, et al. Predictive Factors of Lateral Lymph Node Metastasis in Papillary Thyroid Microcarcinoma. Pathol Oncol Res 2019;25:1245-51. [Crossref] [PubMed]
- Zhu J, Chang L, Li D, et al. Nomogram for preoperative estimation risk of lateral cervical lymph node metastasis in papillary thyroid carcinoma: a multicenter study. Cancer Imaging 2023;23:55. [Crossref] [PubMed]
- Zhuo X, Yu J, Chen Z, et al. Dynamic Nomogram for Predicting Lateral Cervical Lymph Node Metastasis in Papillary Thyroid Carcinoma. Otolaryngol Head Neck Surg 2022;166:444-53. [Crossref] [PubMed]
- Zou Y, Sun S, Liu Q, et al. A new prediction model for lateral cervical lymph node metastasis in patients with papillary thyroid carcinoma: Based on dual-energy CT. Eur J Radiol 2021;145:110060. [Crossref] [PubMed]
- Mazzaferri EL, Kloos RT. Clinical review 128: Current approaches to primary therapy for papillary and follicular thyroid cancer. J Clin Endocrinol Metab 2001;86:1447-63. [Crossref] [PubMed]
- Wagner JM, Alleman AM. Ultrasonography of Cervical Lymph Nodes. Radiol Clin North Am 2019;57:485-500. [Crossref] [PubMed]
- Lee SH, Roh JL, Gong G, et al. Risk Factors for Recurrence After Treatment of N1b Papillary Thyroid Carcinoma. Ann Surg 2019;269:966-71. [Crossref] [PubMed]
- Kilfoy BA, Zheng T, Holford TR, et al. International patterns and trends in thyroid cancer incidence, 1973-2002. Cancer Causes Control 2009;20:525-31. [Crossref] [PubMed]
- Zhao L, Wu F, Zhou T, et al. Risk factors of skip lateral cervical lymph node metastasis in papillary thyroid carcinoma: a systematic review and meta-analysis. Endocrine 2022;75:351-9. [Crossref] [PubMed]
- Qu N, Zhang L, Ji QH, et al. Risk Factors for Central Compartment Lymph Node Metastasis in Papillary Thyroid Microcarcinoma: A Meta-Analysis. World J Surg 2015;39:2459-70. [Crossref] [PubMed]
- So YK, Kim MJ, Kim S, et al. Lateral lymph node metastasis in papillary thyroid carcinoma: A systematic review and meta-analysis for prevalence, risk factors, and location. Int J Surg 2018;50:94-103. [Crossref] [PubMed]
- Chen Y, Wang Y, Li C, et al. Meta-analysis of the effect and clinical significance of Delphian lymph node metastasis in papillary thyroid cancer. Front Endocrinol (Lausanne) 2023;14:1295548. [Crossref] [PubMed]
- Zheng X, Peng C, Gao M, et al. Risk factors for cervical lymph node metastasis in papillary thyroid microcarcinoma: a study of 1,587 patients. Cancer Biol Med 2019;16:121-30. [Crossref] [PubMed]
- Fan D, Liu SY, van Hasselt CA, et al. Estrogen receptor α induces prosurvival autophagy in papillary thyroid cancer via stimulating reactive oxygen species and extracellular signal regulated kinases. J Clin Endocrinol Metab 2015;100:E561-71. [Crossref] [PubMed]
- Jiang C, Xu F, Yi D, et al. Testosterone promotes the migration, invasion and EMT process of papillary thyroid carcinoma by up-regulating Tnnt1. J Endocrinol Invest 2024;47:149-66. [Crossref] [PubMed]
- Mao J, Zhang Q, Zhang H, et al. Risk Factors for Lymph Node Metastasis in Papillary Thyroid Carcinoma: A Systematic Review and Meta-Analysis. Front Endocrinol (Lausanne) 2020;11:265. [Crossref] [PubMed]
- Giovanella L, Milan L, Roll W, et al. Thyroglobulin measurement is the most powerful outcome predictor in differentiated thyroid cancer: a decision tree analysis in a European multicenter series. Clin Chem Lab Med 2024;62:2307-15. [Crossref] [PubMed]
- Malaguarnera R, Frasca F, Garozzo A, et al. Insulin receptor isoforms and insulin-like growth factor receptor in human follicular cell precursors from papillary thyroid cancer and normal thyroid. J Clin Endocrinol Metab 2011;96:766-74. [Crossref] [PubMed]
- Vasileiadis I, Boutzios G, Charitoudis G, et al. Thyroglobulin antibodies could be a potential predictive marker for papillary thyroid carcinoma. Ann Surg Oncol 2014;21:2725-32. [Crossref] [PubMed]
- Śmiech M, Leszczyński P, Kono H, et al. Emerging BRAF Mutations in Cancer Progression and Their Possible Effects on Transcriptional Networks. Genes (Basel) 2020;11:1342. [Crossref] [PubMed]
- Liang WZ, Liu YX, Xu DD, et al. Decoding the Molecular Mechanisms of BRAF (V600E)-Induced Nevi Formation. Biomed Environ Sci 2024;37:774-84. [PubMed]
- Chen B, Shi Y, Xu Y, et al. The predictive value of coexisting BRAFV600E and TERT promoter mutations on poor outcomes and high tumour aggressiveness in papillary thyroid carcinoma: A systematic review and meta-analysis. Clin Endocrinol (Oxf) 2021;94:731-42. [Crossref] [PubMed]
- Hlozek J, Rotnagl J, Holy R, et al. BRAF V600E positive papillary thyroid carcinoma (TERT and TP53 mutation coexistence excluded): Correlation of clinicopathological features and the extent of surgical treatment and its complications. J Appl Biomed 2024;22:214-20. [Crossref] [PubMed]
- National Cancer Center. Quality control index for standardized diagnosis and treatment of thyroid cancer in China (2022 edition). Zhonghua Zhong Liu Za Zhi 2022;44:902-7. [PubMed]
- Hantzidiamantis PJ, Awosika AO, Lappin SL. Physiology, Glucose. In: StatPearls. Treasure Island: StatPearls Publishing; 2024.
- Liu Y, Gong J, Huang Y, et al. Fasting serum glucose and lymph node metastasis in non-diabetic PTC patients: a 10-Year multicenter retrospective study. J Cancer 2022;13:2673-82. [Crossref] [PubMed]
- Jin L, Zheng D, Mo D, et al. Glucose-to-Lymphocyte Ratio (GLR) as a Predictor of Preoperative Central Lymph Node Metastasis in Papillary Thyroid Cancer Patients With Type 2 Diabetes Mellitus and Construction of the Nomogram. Front Endocrinol (Lausanne) 2022;13:829009. [Crossref] [PubMed]
- Yang SJ, Chen CY, Chang GD, et al. Activation of Akt by advanced glycation end products (AGEs): involvement of IGF-1 receptor and caveolin-1. PLoS One 2013;8:e58100. [Crossref] [PubMed]
- Lopez R, Arumugam A, Joseph R, et al. Hyperglycemia enhances the proliferation of non-tumorigenic and malignant mammary epithelial cells through increased leptin/IGF1R signaling and activation of AKT/mTOR. PLoS One 2013;8:e79708. [Crossref] [PubMed]




