
The impact of serum interleukin-17a as a predictive biomarker for neoadjuvant therapy response in breast cancer: a retrospective study
Highlight box
Key findings
• High plasma interlukin-17a (IL-17a) expression before and after neoadjuvant therapy is positively correlated with poor responses [i.e., stable disease (SD) and progressive disease (PD)] in breast cancer (BC) patients.
• IL-17a levels are significantly decreased in patients with good responses [i.e., a complete response (CR) and pathologic partial response] and are more pronounced in early stage patients.
• Elevated IL-17a is associated with advanced tumor stage (T3–T4), nodal involvement (N3), and high Ki67 levels, indicating a link to tumor aggressiveness.
• The results suggests that higher post-treatment IL-17a levels may be correlated with worse progression-free survival (PFS).
What is known, and what is new?
• IL-17a has been implicated in tumor progression, angiogenesis, and immune escape in various cancers, including BC. Neoadjuvant therapy is a standard approach for downstaging tumors, but predictive biomarkers are lacking.
• This study identified IL-17a as a potential biomarker for predicting the efficacy of neoadjuvant therapy in BC. It showed that IL-17a levels are modulated by chemotherapy, and may reflect treatment sensitivity and prognosis.
What is the implication, and what should change now?
• IL-17a could serve as a non-invasive biomarker for stratifying patients for neoadjuvant therapy, optimizing treatment strategies, and improving outcomes.
• Further large-scale studies need to be conducted to validate the predictive role of IL-17a and explore its mechanistic involvement in therapy resistance. Clinically, integrating IL-17a assessment into diagnostic protocols may enhance personalized treatment planning.
Introduction
Breast cancer (BC) is the most common malignancy in women worldwide (1). Chemotherapy plays an irreplaceable and vital role in the systemic treatment of BC. Neoadjuvant therapy for BC refers to the systemic treatment of recently diagnosed BC patients without distant metastasis before their planned surgical treatment, or local treatment involving surgery and radiotherapy (2). Generally, neoadjuvant therapy refers to systemic treatment before surgery to reduce the size or stage of the tumor (3,4). Accordingly, Patients who were initially unable to undergo breast-conserving surgery may become candidates for the procedure after receiving neoadjuvant chemotherapy (NACT). Similarly, patients who were previously deemed ineligible for surgery may become suitable candidates for surgical treatment following NACT (5,6). Additionally, NACT can be used to assess the sensitivity of tumors to drugs and guide decisions about follow-up medication. Sensitive patients to NACT have a better prognosis, while insensitive patients need intensive postoperative treatment to improve their prognosis (7,8).
For human epidermal growth factor receptor 2 (HER2)-positive BC, neoadjuvant therapy has changed from a single chemotherapy regimen to a multi-drug combination regimen of chemotherapy and targeted therapy. The double-targeted trastuzumab regimen combined with pertuzumab has become the first-line regimen of neoadjuvant therapy for HER2-positive BC (9,10). Moreover, a study has shown that the 5-years overall response rate of HER2-positive BC is at least 80% (11). A taxane-based chemotherapy regimen is the cornerstone of neoadjuvant therapy for triple-negative BC patients. However, immune checkpoint inhibitors have made certain breakthroughs in neoadjuvant therapy for triple-negative BC. The KEYnote-522 study explored the use of pembrolizumab in neoadjuvant therapy for triple-negative BC based on the 4 cycles of paclitaxel combined with carboplatin followed by 4 cycles of doxorubicin or epirubicin combined with cyclophosphamide chemotherapy regimen, and reported that the patients who received this regimen had significantly better event-free survival (EFS) benefits than those who received chemotherapy alone (EFS rate at 36 months: 84.5% vs. 76.8%; P<0.001) and a significantly increased pathological complete response (pCR) rate (64.8% vs. 51.2%; P<0.001) (12). Moreover, estrogen receptor (ER)-positive BC is the least effective subtype of neoadjuvant therapy, and its complete pathological remission remains uncommon. To assess the efficacy of NACT, clinical parameters such as disease response, particularly the residual cancer burden (RCB), are commonly used. The RCB index is a continuous measure that considers tumor size, cellularity, and lymph node involvement. It classifies therapy response into four categories: pCR and three RCB levels—minimal (RCBI), moderate (RCBII), and extensive (RCBIII). Histopathological techniques, including cancer biomarker evaluation in surgical specimens, along with Cox regression analyses, are also applied to assess NACT outcomes. To enhance predictions of NACT response and cancer prognosis, various experimental methods are employed. These include immunofluorescence, tissue microarrays, DNA/RNA analysis, sequencing, protein quantification, and epigenetic assessments such as methylation and cell cycle analysis. However, challenges remain, particularly in personalizing chemotherapy. Sub-optimal treatments not tailored to individual patients may lead to resistance to NACT and long-term toxicity (e.g., from anthracyclines), diminishing treatment success and increasing mortality rates. Therefore, potential prognostic biomarkers for predicting the efficacy of neoadjuvant therapy urgently need to be investigated.
Inflammatory status is a prognostic factor for metastatic BC and contributes to cancer development and progression. Notably, immune cells, including T cells, tumor-associated macrophages, neutrophils, natural killer cells, and B cells, play a role in regulating tumor immunity and tumor angiogenesis in microenvironment, and affecting tumor cell proliferation, invasion, and migration (13,14). Th-17 cells are a type of inflammatory cluster of differentiation 4+ (CD4+) T cell that are crucial in tumor pathogenesis and anti-tumor immunity (15,16). In BC, Th-17 expression is negatively correlated with interleukin (IL)-6, IL-1b, and IL-17a expression levels, and is positively correlated with increased metastatic lymph nodes and tumor cells (17). It has been reported that IL-17a induced inflammatory mediators, including granulocyte colony-stimulating factor (G-CSF), interleukin-6 (IL-6) and chemokine (C-X-C motif) ligand 1 (CXCL1) secretion, stimulate the proliferation and recruitment of macrophages (18). Further, IL-17a promotes angiogenesis and immune escape (19). However, the correlation between IL-17a expression and the efficacy of neoadjuvant therapy in BC patients remains unclear. Therefore, this study evaluated the predictive and interactive effects of IL-17a plasma levels on the efficacy of neoadjuvant therapy in BC patients. We present this article in accordance with the REMARK reporting checklist (available at https://gs.amegroups.com/article/view/10.21037/gs-2025-197/rc).
Methods
Blood sample handing
After collecting 2 mL of venous blood sample from each patient, the samples were centrifuged (15,000 ×g for 20 min at 4 ℃) to remove cellular debris. Consequently, the sera were stored at −80 ℃ awaiting use for the enzyme-linked immunoassay (ELISA) assessment of Il-17a protein levels.
ELISA
A human IL-17 ELISA kit (ab100556) was purchased from Abcam (Cambridge, CB2 0AX, UK). The IL-17 serum levels were interpolated from reference curves generated using the provided reference standards of known concentrations, and all experiments were performed in accordance with the manufacturer’s instructions.
Patient enrolment
The plasma samples of 54 BC patients at The Affiliated Hospital of Guangdong Medical University (Zhanjiang, China) who received neoadjuvant therapy before and after treatment between 2019 and 2021 were obtained. The inclusion criteria for the study were as follows: women aged 18 years or older who provided informed consent, non-metastatic BC patients with TNM stage II or higher, and those with no prior history of chemotherapy, endocrine therapy, surgery, or radiotherapy. Participants were also required to have normal cardiac function, an ECOG score of 2 or lower, and adequate organ function. The exclusion criteria included patients who had been diagnosed with other malignancies within the last five years, or those who were deemed unsuitable for chemotherapy. The NACT regimen involved adriamycin or epirubicin with cyclophosphamide every three weeks for four cycles, followed by either weekly paclitaxel, three-weekly docetaxel, docetaxel with cyclophosphamide for four to six cycles, or docetaxel with carboplatin for six cycles. HER2-positive patients received trastuzumab, either alone or with pertuzumab. Therapeutic efficacy was evaluated using RECIST criteria. pCR was defined as no residual invasive carcinoma or ductal carcinoma in situ (DCIS) in the breast or lymph nodes, confirmed by microscopic examination. This retrospective observational study focused on evaluating pCR after NACT, with secondary outcomes including progression-free survival (PFS) and overall survival (OS). All the patients provided informed consent. The study was conducted in accordance with the Declaration of Helsinki and its subsequent amendments. The study was approved by the ethics board of The Affiliated Hospital of Guangdong Medical University (approval No. PJKT2024-003).
Statistical analysis
The two-tailed student’s t-test, paired-sample t-test, and the Mann-Whitney U-test were used to detect any significant differences in various molecular, cellular, and physiological parameters between the means or medians of the treatment and control groups. A P value <0.05 was considered statistically significant. The error bars in the experiments indicate the standard error of the mean (SEM) for a minimum of three independent experiments.
Results
Study patients
A retrospective study of therapeutic regimens for newly diagnosed BC or advanced BC patients who had never received neoadjuvant therapy was conducted to examine whether IL-17a expression is related to neoadjuvant therapy efficacy in BC. In total, 54 patients were recruited from the Affiliated Hospital of Guangdong Medical University, Zhanjiang, China from 2019 to 2022. Table S1 shows the detailed clinicopathological characteristics of the patients. The age range of the patients was 28–71 years. Among the 54 patients, 25 (46.3%) were premenopausal and 29 (53.7%) were postmenopausal. Further, 34 (63%) patients were ER-positive and 20 (37%) were ER-negative, while 25 (46.3%) patients were progesterone receptor-positive and 29 (53.7%) were progesterone receptor-negative. Moreover, 26 (48.1%) patients had positive HER2 expression, and 28 (51.9%) had negative HER2 expression. Tumor size measurements were obtained before and after treatment using magnetic resonance imaging (MRI) or ultrasonography. The study cut-off date was September 1, 2023, and the median follow-up duration ranged from 17 to 52 months.
High IL-17a expression is related to a poor response to neoadjuvant therapy
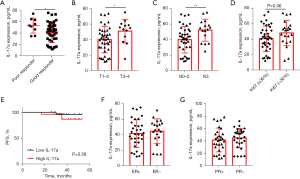
ELISA was used to measure the secretion of IL-17a in the blood; the mean concentration of serum IL-17a before treatment was 42.52±17.09 pg/mL. The patients who showed a pathologic CR and pathologic partial response were defined as having a better response to NACT, while those who showed stable disease (SD) and progressive disease (PD) were defined as having a poor response to NAC. The results showed that the patients with a poor response had higher IL-17a levels than those with a good response (P=0.04) (Figure 1A). Moreover, the baseline IL-17a levels of the patients varied by stage (T1–T2 vs. T3–T4). Nodal status (N0–2 vs. N3) and higher IL-17a expression were positively related to T3–4 and N3 (Figure 1B,1C). Additionally, high IL-17a expression was correlated with high Ki67 levels, indicating that IL-17a promotes tumor cell proliferation (Figure 1D). However, when the patients were divided into the IL-17a-high and IL-17a-low subgroups based on the median expression of IL-17a, the Kaplan-Meier analysis failed to show that the patients in the IL-17a-high subgroup had a worse response to neoadjuvant therapy than those in the IL-17a-low subgroup (P=0.58) (Figure 1E). Further, the baseline plasma IL-17a levels were not found to be correlated with hormone receptor status (Figure 1F,1G) or HER2 status (Table S1).
Further, ELISA was used to measure the serum IL-17a levels of the patients after treatment. The mean serum IL-17a after treatment was 36.00±19.31 pg/mL, and a similar result was found in the serum IL-17a after-treatment analysis. First, the results showed that the high expression of serum IL-17a after treatment was significantly correlated with a poor response to NAC (Figure 2A). Similarly, in relation to the clinicopathologic data, higher serum IL-17a levels after treatment were positively correlated with later stage and more nodal involvement (Figure 2B,2C), and higher Ki67 levels (Figure 2D). However, the Kaplan-Meier analysis showed that high IL-17a levels after treatment were positively associated with poor PFS (P=0.09), although this finding was limited by the small sample size (Figure 2E). Finally, the plasma IL-17a levels after treatment were not found to be correlated with hormone receptor status (Figure 2F,2G) or HER2 status (Table S1).
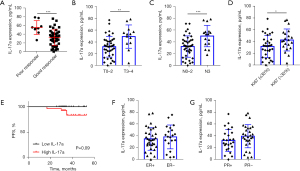
These findings indicate that pre- and after-NAC IL-17a is an important index in predicting the curative effect of NAC. IL-17a may be regulated by NAC, and its expression after treatment may predict the clinical prognosis of patients.
Decreased IL-17a correlated with a good response to neoadjuvant therapy
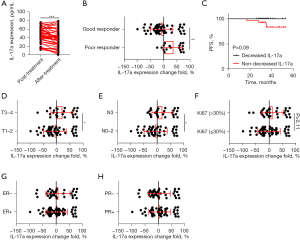
The baseline and after-treatment serum levels of patients were compared to explore whether IL-17a is regulated by NAC. The paired t-test showed that IL-17a was significantly downregulated by NAC, and patients with good responses showed decreased IL-17a levels compared to those with poor responses (Figure 3A,3B). The patients with a decrease > 20% in IL-17a were allocated to the decreased group while those with a decrease <20% or an increase were allocated to the non-decreased group. A Kaplan-Meier analysis between the decreased group and non-decreased group indicated that the patients with a significant decrease in IL-17a had better PFS (P=0.09) (Figure 3C). Further, patients with early stage (T1–T2) and less lymphatic metastasis (N0–2) showed more decrease of IL-17a. The T3–T4 or N3 patients showed no IL-17a decrease after chemotherapy (Figure 3D,3E). Moreover, NAC significantly downregulated IL-17a expression in patients whose Ki67 level was ≤30% (Figure 3F). Overall, no significant decrease in IL-17a was detected among patients with different levels of ER or progesterone receptor expression (Figure 3G,3H). These results suggest that in low-risk patients, IL-17a was more sensitively downregulated by NAC.
Discussion
BC has surpassed lung cancer to be the most commonly diagnosed cancer worldwide (1). Chemotherapy has played a pivotal role in BC treatment. Neoadjuvant chemotherapy (NAC) is particularly effective in addressing distant metastases earlier and more efficiently. It may also improve the long-term survival rate of BC patients, especially those who achieve complete pathological remission after chemotherapy. Moreover, it can provide valuable information about the chemotherapeutic sensitivity of tumors in vivo, and thus provide a basis for selecting postoperative adjuvant chemotherapy regimens. NAC also enables cytotoxic drugs to reach the tumor’s interior via intact tumor blood vessels, and eliminates the need to reduce chemotherapy drug concentrations in tumor tissues due to changes in the tumor vascular bed after surgery. Consequently, this approach enhances the efficacy of chemotherapy. Consistent with the findings of a recent study (8), the effect of NAC predicts the clinical prognosis of patients. Given that 20–30% of patients do not benefit from NAC (11), we sought to identify a biomarker to predict the effectiveness of this treatment.
Th-17 cells are a type of CD4+ T cells that produce IL-17a and act as a heterodimeric form with IL-17F (20,21). In BC, IL-17a has been reported to promote tumor proliferation through the MAPK signaling pathway, including key components such as c-Jun N-terminal kinases (JNK) and extracellular signal-regulated kinases 1 and 2 (ERK1/2), as well as through the NF-κB pathway (22-25). Furthermore, IL-17a enhances the expression of pro-inflammatory cytokines, such as transforming growth factor beta (TGF-β) and tumor necrosis factor alpha (TNF-α), which play critical roles in tumor progression, immune evasion, and the modulation of tumor microenvironment, ultimately contributing to BC pathogenesis and metastasis (26). Moreover, IL-17a is reported to be involved in tumor cell invasion and metastasis via the MMP pathway. MMP-9 inhibitors were shown to decrease BC cell invasion in mouse models (27). Further, the IL-17A pathway activates NF-κB, and the subsequent NF-κB-mediated expression of matrix metalloproteinases 2 (MMP-2) and matrix metalloproteinases 9 (MMP-9) plays a crucial role in driving the invasiveness and metastasis of various human cancers, including colorectal cancer, hepatocellular carcinoma, nasopharyngeal carcinoma, and non-small cell lung cancer (28-31). These reports provide evidence that IL-17a may act as a prognostic indicator of BC. Our results did not prove that IL-17a is correlated with prognosis, however, a higher IL-17a level was found to be correlated with a poor response to NACT. Elevated IL-17a expression is strongly linked to adverse outcomes in BC patients, as already confirmed by immunohistochemistry (32).
Interestingly, our results showed that IL-17a was a predictor of chemotherapy and was downregulated by chemotherapy. A previous study showed that IL-17a promotes docetaxel chemoresistance via the ERK1/2 pathway and epidermal growth factor receptor phosphorylation (33). However, the specific mechanism by which chemotherapy leads to IL-17a downregulation remains unclear. IL-17a has been shown to promote angiogenesis by promoting vascular endothelial growth factor secretion by tumor cells (33). This suggests that promoting angiogenesis by IL-17a can improve the accessibility of chemotherapy drugs to tumors. Overall, IL-17a directly promotes tumor growth, invasion, and metastasis. Additionally, research has shown IL-17a negatively regulates the immune response in cancer patients by recruiting myeloid-derived suppressor cells (34). Moreover, an increase in IL-17a has been shown to be associated with the neutrophil-to-lymphocyte ratio and the stimulation of programmed death ligand 1, which are positively related to immune escape (35). Our study provides important evidence that IL-17a expression levels significantly predicts NAC sensitivity. All the above-mentioned studies showed that higher IL-17a expression is related to poor responses both pre- and post-treatment.
Additionally, our study is limited by several factors, including the relatively small sample size and the single-center design, which restricts the generalizability of our findings to broader populations. Furthermore, the relatively short follow-up period and the issue of heterogeneity across different BC subtypes also pose limitations. Nonetheless, these factors provide valuable insights for further research in this area.
Conclusions
Briefly, we showed the important role of IL-17a in the context of neoadjuvant therapy. Specifically, we found that neoadjuvant therapy downregulated IL-17a expression, and significantly predicted NAC sensitivity. Moreover, we showed that reduced IL-17a expression at the baseline or after treatment was significantly correlated with early stage disease, less lymphatic metastasis, and a lower expression of ki67. IL-17a did not predict PFS; however, after treatment, plasma IL-17a and its decreased expression were associated with the prognosis of patients undergoing NAC.
Acknowledgments
None.
Footnote
Reporting Checklist: The authors have completed the REMARK reporting checklist. Available at https://gs.amegroups.com/article/view/10.21037/gs-2025-197/rc
Data Sharing Statement: Available at https://gs.amegroups.com/article/view/10.21037/gs-2025-197/dss
Peer Review File: Available at https://gs.amegroups.com/article/view/10.21037/gs-2025-197/prf
Funding: None.
Conflicts of Interest: All authors have completed the ICMJE uniform disclosure form (available at https://gs.amegroups.com/article/view/10.21037/gs-2025-197/coif). The authors have no conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved. All the patients provided informed consent. The study was conducted in accordance with The Declaration of Helsinki and its subsequent amendments. The study was approved by the ethics board of the Affiliated Hospital of Guangdong Medical University (approval No. PJKT2024-003).
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Arnold M, Morgan E, Rumgay H, et al. Current and future burden of breast cancer: Global statistics for 2020 and 2040. Breast 2022;66:15-23. [Crossref] [PubMed]
- Vaidya JS, Massarut S, Vaidya HJ, et al. Rethinking neoadjuvant chemotherapy for breast cancer. BMJ 2018;360:j5913. [Crossref] [PubMed]
- Charfare H, Limongelli S, Purushotham AD. Neoadjuvant chemotherapy in breast cancer. Br J Surg 2005;92:14-23. [Crossref] [PubMed]
- Wang H, Mao X. Evaluation of the Efficacy of Neoadjuvant Chemotherapy for Breast Cancer. Drug Des Devel Ther 2020;14:2423-33. [Crossref] [PubMed]
- Morrow M, Jagsi R, Alderman AK, et al. Surgeon recommendations and receipt of mastectomy for treatment of breast cancer. JAMA 2009;302:1551-6. [Crossref] [PubMed]
- Schott AF, Hayes DF. Defining the benefits of neoadjuvant chemotherapy for breast cancer. J Clin Oncol 2012;30:1747-9. [Crossref] [PubMed]
- von Minckwitz G, Blohmer JU, Costa SD, et al. Response-guided neoadjuvant chemotherapy for breast cancer. J Clin Oncol 2013;31:3623-30. [Crossref] [PubMed]
- Krishnan Y, Alawadhi SA. Pathological responses and long-term outcome analysis after neoadjuvant chemotheraphy in breast cancer patients from Kuwait over a period of 15 years. Ann Saudi Med 2013;33:443-50. [Crossref] [PubMed]
- Gianni L, Pienkowski T, Im YH, et al. Efficacy and safety of neoadjuvant pertuzumab and trastuzumab in women with locally advanced, inflammatory, or early HER2-positive breast cancer (NeoSphere): a randomised multicentre, open-label, phase 2 trial. Lancet Oncol 2012;13:25-32. [Crossref] [PubMed]
- Shao Z, Pang D, Yang H, et al. Efficacy, Safety, and Tolerability of Pertuzumab, Trastuzumab, and Docetaxel for Patients With Early or Locally Advanced ERBB2-Positive Breast Cancer in Asia: The PEONY Phase 3 Randomized Clinical Trial. JAMA Oncol 2020;6:e193692. [Crossref] [PubMed]
- Wu J, Jiang Z, Liu Z, et al. Neoadjuvant pyrotinib, trastuzumab, and docetaxel for HER2-positive breast cancer (PHEDRA): a double-blind, randomized phase 3 trial. BMC Med 2022;20:498. [Crossref] [PubMed]
- Schmid P, Cortes J, Dent R, et al. Event-free Survival with Pembrolizumab in Early Triple-Negative Breast Cancer. N Engl J Med 2022;386:556-67. [Crossref] [PubMed]
- Fasoulakis Z, Kolios G, Papamanolis V, et al. Interleukins Associated with Breast Cancer. Cureus 2018;10:e3549. [Crossref] [PubMed]
- Sun J, Cui H, Gao Y, et al. TGF-α Overexpression in Breast Cancer Bone Metastasis and Primary Lesions and TGF-α Enhancement of Expression of Procancer Metastasis Cytokines in Bone Marrow Mesenchymal Stem Cells. Biomed Res Int 2018;2018:6565393. [Crossref] [PubMed]
- Dong C. Diversification of T-helper-cell lineages: finding the family root of IL-17-producing cells. Nat Rev Immunol 2006;6:329-33. [Crossref] [PubMed]
- Amatya N, Garg AV, Gaffen SL. IL-17 Signaling: The Yin and the Yang. Trends Immunol 2017;38:310-22. [Crossref] [PubMed]
- Rodriguez C, Araujo Furlan CL, Tosello Boari J, et al. Interleukin-17 signaling influences CD8(+) T cell immunity and tumor progression according to the IL-17 receptor subunit expression pattern in cancer cells. Oncoimmunology 2023;12:2261326. [Crossref] [PubMed]
- Yang B, Kang H, Fung A, et al. The role of interleukin 17 in tumour proliferation, angiogenesis, and metastasis. Mediators Inflamm 2014;2014:623759. [Crossref] [PubMed]
- Fares J, Fares MY, Khachfe HH, et al. Molecular principles of metastasis: a hallmark of cancer revisited. Signal Transduct Target Ther 2020;5:28. [Crossref] [PubMed]
- Pires BRB, Binato R, Ferreira GM, et al. Twist1 Influences the Expression of Leading Members of the IL-17 Signaling Pathway in HER2-Positive Breast Cancer Cells. Int J Mol Sci 2021;22:12144. [Crossref] [PubMed]
- Brembilla NC, Senra L, Boehncke WH. The IL-17 Family of Cytokines in Psoriasis: IL-17A and Beyond. Front Immunol 2018;9:1682. [Crossref] [PubMed]
- Song X, Qian Y. The activation and regulation of IL-17 receptor mediated signaling. Cytokine 2013;62:175-82. [Crossref] [PubMed]
- Kawai T, Akira S. Signaling to NF-kappaB by Toll-like receptors. Trends Mol Med 2007;13:460-9. [Crossref] [PubMed]
- Bie Q, Jin C, Zhang B, et al. IL-17B: A new area of study in the IL-17 family. Mol Immunol 2017;90:50-6. [Crossref] [PubMed]
- Monin L, Gaffen SL. Interleukin 17 Family Cytokines: Signaling Mechanisms, Biological Activities, and Therapeutic Implications. Cold Spring Harb Perspect Biol 2018;10:a028522. [Crossref] [PubMed]
- Patnaik A, Haluska P, Tolcher AW, et al. A First-in-Human Phase I Study of the Oral p38 MAPK Inhibitor, Ralimetinib (LY2228820 Dimesylate), in Patients with Advanced Cancer. Clin Cancer Res 2016;22:1095-102. [Crossref] [PubMed]
- Feng M, Wang Y, Chen K, et al. IL-17A promotes the migration and invasiveness of cervical cancer cells by coordinately activating MMPs expression via the p38/NF-κB signal pathway. PLoS One 2014;9:e108502. [Crossref] [PubMed]
- Ren H, Wang Z, Zhang S, et al. IL-17A Promotes the Migration and Invasiveness of Colorectal Cancer Cells Through NF-κB-Mediated MMP Expression. Oncol Res 2016;23:249-56. [Crossref] [PubMed]
- Li J, Lau GK, Chen L, et al. Interleukin 17A promotes hepatocellular carcinoma metastasis via NF-kB induced matrix metalloproteinases 2 and 9 expression. PLoS One 2011;6:e21816. [Crossref] [PubMed]
- Wang L, Ma R, Kang Z, et al. Effect of IL-17A on the migration and invasion of NPC cells and related mechanisms. PLoS One 2014;9:e108060. [Crossref] [PubMed]
- Wu Z, He D, Zhao S, et al. IL-17A/IL-17RA promotes invasion and activates MMP-2 and MMP-9 expression via p38 MAPK signaling pathway in non-small cell lung cancer. Mol Cell Biochem 2019;455:195-206. [Crossref] [PubMed]
- Chen WC, Lai YH, Chen HY, et al. Interleukin-17-producing cell infiltration in the breast cancer tumour microenvironment is a poor prognostic factor. Histopathology 2013;63:225-33. [Crossref] [PubMed]
- Abotaleb M, Kubatka P, Caprnda M, et al. Chemotherapeutic agents for the treatment of metastatic breast cancer: An update. Biomed Pharmacother 2018;101:458-77. [Crossref] [PubMed]
- Noll C, Hiltensperger M, Aly L, et al. Association of the retinal vasculature, intrathecal immunity, and disability in multiple sclerosis. Front Immunol 2022;13:997043. [Crossref] [PubMed]
- Motomura T, Shirabe K, Mano Y, et al. Neutrophil-lymphocyte ratio reflects hepatocellular carcinoma recurrence after liver transplantation via inflammatory microenvironment. J Hepatol 2013;58:58-64. [Crossref] [PubMed]


