
Pathological metastatic lymph node density (ND) predicts early recurrence in papillary thyroid cancer patients after curative resection
Highlight box
Key findings
• A high pathological metastatic lymph node density (ND) (≥17.4%) strongly predicts early recurrence in both early and locally advanced papillary thyroid cancer (PTC), particularly in young patients.
What is known and what is new?
• Research indicate that the ratio of metastatic lymph nodes-to-harvested lymph nodes (LNR), ND, is an independent prognostic factor in both central neck nodes and lateral neck nodes at optimal offs.
• This study found that ND ≥17.4% independently predicts disease recurrence regardless of the tumor progression and the extent of lymph node dissection in PTC patients after curative resection. The prognostic impact was stronger in young patients. The two peaks of best cutoffs (around 17% and 35%) were suggested as the optimal cutoffs. Notably, disease recurrence in patients with high ND occurs earlier than in patients with low ND status. ND ≥17.4% was significantly associated with lymph node/local recurrence as the initial recurrence site.
What is the implication, and what should change now?
• The study found that pathological ND is reliable prognostic indicator that identifies high-risk patients with high specificity. Therefore, patients with ND ≥17.4% should be treated with postoperative therapy (thyroid-stimulating hormone suppression and ablative/adjuvant radioactive iodine therapy), even in young patients.
Introduction
Papillary thyroid cancer (PTC) is the most common histological type of differentiated thyroid malignancies, comprising approximately 95% of all thyroid cancer cases (1,2). Although the 10-year disease-specific survival rate exceeds 90%, around 10% of patients eventually require additional therapy for recurrence or metastasis (3). Fifteen percent to 60% of PTC patients develop lymph node metastasis because of the feature prone to spread through the lymphatic system (4). Lymph node metastasis is classified as central neck (N1a) or lateral neck (N1b) based on location and is included in the Union for International Cancer Control (UICC) TNM Staging System as a reliable prognostic indicator. Furthermore, large metastatic lymph node and extranodal tumor extension are well-known indicators of a high-risk for disease recurrence (5-14).
Recently, several indicators on harvested lymph nodes such as the number of harvested lymph nodes (15-17) or metastatic lymph nodes (11,18), as well as the ratio of metastatic lymph nodes-to-harvested lymph nodes, have been reported as prognostic indicators in multiple solid cancers, including thyroid cancers (19-22). Among them, growing evidence has proposed a high ratio of metastatic lymph nodes-to-harvested lymph nodes, known as lymph node ratio (LNR), as a robust prognostic factor (6,8,16,23-35), that is associated with poor recurrence-free survival (RFS) or worse disease-specific survival. Here, we used the term “node density (ND)” to describe LNR to avoid confusion with well-known inflammatory markers, lymphocyte-to-neutrophil ratio and lymphocyte-to-monocyte ratio (LMR) (36). Because the clinical impact of ND is still unknown, we assessed the clinical significance of ND to identify patients with high-risk for recurrence. We present this article in accordance with the STROBE reporting checklist (available at https://gs.amegroups.com/article/view/10.21037/gs-2025-36/rc).
Methods
A total of 988 patients who underwent curative surgery for PTC without evidence of de novo distant metastasis, as evaluated by preoperative computed tomography (CT), were retrospectively analyzed at Kitasato University Hospital between January 2001 and March 2023. All patients routinely underwent preoperative neck ultrasonography and neck-chest CT scan. Fifty-two of these patients were excluded because they dropped out or had incomplete follow-ups with insufficient data. The remaining 936 patients were examined in this study. The disease was staged using the medical records and the 8th edition of the UICC TNM Staging System. The institutional pathologists conducted routine pathological diagnoses.
As mentioned earlier, we used the term “node density (ND)” to describe metastatic lymph node-to-harvested lymph node ratio which is also known as LNR. We pathologically determined the ND as the percentage of metastatic lymph nodes among the total number of harvested lymph nodes, calculated by dividing the number of metastatic nodes by the total number of harvested lymph nodes and multiplied by 100. ND was further classified as ND1a or ND1b, based on the numbers of metastatic nodes in the central neck or lateral neck areas divided by the total harvested nodes. The clinicopathological parameters studied were age, gender, extrathyroidal extension, pathological lymph node metastasis (pN), the maximum size of lymph node(s), ND, and UICC staging using the 8th edition of the UICC TNM Staging System. Patients were categorized into three groups based on the extent of neck dissection: central, lateral, and others. The “others” group included patients who underwent less-than-standard central neck dissection (CND).
A preoperative laboratory blood test, including complete blood counts with leukocyte fractions, and biochemical profiles [e.g., thyroglobulin (Tg)], was performed as part of the preoperative routine workup one month before surgery. The neutrophil-to-lymphocyte ratio (NLR) and a LMR were also determined from leukocyte (neutrophil, monocyte, or lymphocyte) counts and platelet counts, as described previously (36,37).
Ethics
The Institutional Review Board (IRB) of the Kitasato University School of Medicine approved this study (No. B23-057), which was conducted in accordance with the IRB’s clinical research guidelines and the Declaration of Helsinki and its subsequent amendments. All individuals gave written informed consent for their samples to be pathologically assessed, routine laboratory tests performed, and clinical data analyzed.
Statistical analysis
Categorical variables were evaluated with chi-square or Fisher’s exact tests where appropriate, while continuous variables were examined using the Wilcoxon rank-sum test. Clinicopathological characteristics and follow-up outcomes were analyzed in relation to 10-year RFS. The follow-up period was calculated from the date of surgery. To identify optimal cutoff values for recurrence, receiver operating characteristic (ROC) curves were generated for continuous variables, and Youden’s index was applied to determine thresholds at the latest follow-up. These cutoff values were then used to stratify patients into two groups for prognostic analysis. ND was not calculated in 17 patients in whom lymph node dissection was performed, and these cases were excluded from ROC analysis of ND, correlation analysis on ND, and Kaplan-Meier analysis on ND. The optimal cutoff value of ND for disease recurrence was also determined by X-tile software (Yale University, version 3.6.1) based on previous literature (27,38). The X-tile software tests every possible cutoff value and automatically calculates the RFS differences between subgroups using the Kaplan-Meier method and the log-rank test, determining the optimal cutoff value when the most significant difference in RFS is achieved. RFS was calculated using Kaplan-Meier curves and compared with a log-rank test. Moreover, variables that showed prognostic relevance in the univariate analysis (P<0.05) were subsequently included in the multivariate Cox proportional hazards model. To determine factors independently associated with ND, a multivariate logistic regression analysis was performed. Statistical significance was defined when P<0.05. All analyses were conducted using JMP Pro17 software (SAS Institute, Cary, NC, USA).
Results
Patient and disease characteristics
Table 1 summarizes the demographic and clinicopathological characteristics of 936 PTC patients who underwent curative resection. According to the medical records, the lymph node(s) were clinically palpable in 145 patients. The median number of harvested lymph nodes was 11 (IQR: 6–17) in the central compartment, and 32 (IQR: 22–49) in the lateral compartment. The median observation period was 48.5 (IQR: 22.3–91.8) months, and the median age was 57 [15–90] years. Females outnumbered males by 75% to 25%. Patients in stages I, II, III, and IVA were 493 (52.7%), 324 (34.6%), 118 (12.6%), and 1 (0.1%), respectively, with 82 (8.8%) experiencing recurrence(s). One hundred and seventy (18.2%) patients had gross extrathyroidal extension into adjacent structures. pN0, pN1a, and pN1b were responsible for 257 (27.5%), 412 (44.2%), and 250 (26.7%). In 17 patients (1.6%), the area of dissected metastatic lymph node(s) were unable to be determined (pNx). Thirty-four (3.6%) patients had metastatic lymph node(s) greater than 3cm. The median ND value was 12.5% (range: 0.0–100.0%).
Table 1
| Variables | No. of patients (%) | 10-year RFS (%)* | Univariate | |
|---|---|---|---|---|
| HR (95% CI) | P** | |||
| Age (years) | 0.11 | |||
| <55 | 411 (43.9) | 89.3 | 1.00 | |
| ≥55 | 525 (56.1) | 82.6 | 1.45 (0.92–2.29) | |
| Gender | <0.001 | |||
| Female | 699 (74.7) | 88.5 | 1.00 | |
| Male | 237 (25.3) | 76.0 | 2.35 (1.52–3.63) | |
| Tumor size (cm) | <0.001 | |||
| ≤2 | 554 (60.0) | 93.9 | 1.00 | |
| >2, ≤4 | 281 (30.4) | 75.6 | 3.71 (2.17–6.34) | |
| >4 | 89 (9.6) | 69.2 | 6.45 (3.46–12.02) | |
| Gross extrathyroidal extension | <0.001 | |||
| No | 766 (81.8) | 89.4 | 1.00 | |
| Yes | 170 (18.2) | 65.8 | 3.87 (2.50–5.99) | |
| pN | <0.001 | |||
| N0 | 257 (27.5) | 93.4 | 1.00 | |
| N1a | 412 (44.2) | 90.7 | 2.91 (1.10–7.70) | |
| N1b | 250 (26.7) | 71.6 | 10.89 (4.34–27.31) | |
| Nx | 17 (1.6) | – | – | |
| Lymph node size (cm) | <0.001 | |||
| ≤3 | 902 (96.4) | 87.2 | 1.00 | |
| >3 | 34 (3.6) | 18.5 | 12.92 (7.36–22.69) | |
| ND (%) | <0.001 | |||
| <17.4 | 437 (47.6) | 91.5 | 1.00 | |
| ≥17.4 | 482 (52.4) | 79.5 | 3.96 (2.31–6.77) | |
| Stage (UICC 8th) | <0.001 | |||
| I | 493 (52.7) | 89.9 | 1.00 | |
| II | 324 (34.6) | 87.7 | 1.05 (0.65–1.92) | |
| III | 118 (12.6) | 60.5 | 4.69 (2.82–7.79) | |
| IVA | 1 (0.1) | – | – | |
| Preoperative Tg (ng/mL) | <0.001 | |||
| <57.9 | 558 (60.3) | 89.2 | 1.00 | |
| ≥57.9 | 367 (39.7) | 79.9 | 3.05 (1.91–4.88) | |
| Preoperative NLR | 0.62 | |||
| <1.9 | 598 (64.6) | 85.9 | 1.00 | |
| ≥1.9 | 328 (35.4) | 86.0 | 1.13 (0.71–1.80) | |
| Preoperative LMR | 0.008 | |||
| <5.3 | 368 (39.7) | 80.5 | 1.86 (1.18–2.94) | |
| ≥5.3 | 558 (60.3) | 90.2 | 1.00 | |
| Initial surgery | <0.001 | |||
| Lobectomy | 478 (51.1) | 92.7 | 1.00 | |
| Subtotal thyroidectomy | 39 (4.2) | 83.5 | 3.59 (1.55–8.31) | |
| Total thyroidectomy | 415 (44.3) | 78.7 | 3.75 (2.12–6.62) | |
| Others† | 4 (0.3) | – | – | |
| Extent of lymph node dissection | <0.001 | |||
| Central | 621 (66.3) | 93.3 | 1.00 | |
| Lateral | 287 (30.7) | 74.2 | 4.80 (2.94–7.85) | |
| Others‡ | 28 (3.0) | – | – | |
| Ablative/adjuvant RAI | <0.001 | |||
| No | 824 (88.0) | 89.5 | 1.00 | |
| Yes | 112 (12.0) | 51.7 | 7.35 (4.73–11.41) | |
Total number of cases is not 936 in several variables because of missing data. *, a Kaplan-Meier curve compared using a log-rank test. **, a Cox proportional hazards model. †, others include isthmusectomy, partial thyroidectomy, and tumorectomy. ‡, others, either no lymph node dissection or sampling of lymph node(s). CI, confidence interval; HR, hazard ratio; LMR, lymphocyte-to-monocyte ratio; ND, node density; NLR, neutrophil-to-lymphocyte ratio; PTC, papillary thyroid cancer; RAI, radioactive iodine; RFS, recurrence-free survival; Tg, thyroglobulin; UICC, Union for International Cancer Control.
High ND (≥17.4%) predicts disease recurrence in PTC after curative resection
Clinicopathological variables including ND were evaluated for their prognostic value. Patients with recurrence(s) had significantly higher ND (median 35.2%; mean 34.2%±21.8%) compared to those without recurrence during the most recent visit (median 17.2%; mean 24.7%±26.1%) (Figure 1A). Based on the ROC curve for disease recurrence, the optimal cutoff value for ND was 17.4% with an AUC of 0.644, and the sensitivity and specificity were 50.2% and 79.3%, respectively (Figure 1B). Four hundred eighty-two (52.4%) patients had high ND (≥17.4%). In the Kaplan-Meier analysis, patients with ND equal to or more than 17.4% had a 10-year RFS of 79.4%, while the counterpart showed 91.4% (P<0.001, Figure 1C). Univariate analyses showed that male gender, larger tumor size, gross extrathyroidal extension, pathological (p) N1b, lymph node larger than 3 cm, ND ≥17.4%, advanced stage, high preoperative serum Tg level, and low preoperative LMR were all associated with poor RFS among tumor factors (Table 1). As treatment factors, subtotal or total thyroidectomy, lateral lymph node dissection, and ablative/adjuvant radioactive iodine (RAI) were all associated with a lower RFS, reflecting the advanced disease. Additionally, ND status showed more powerful prognostic impact in patients without RAI (Figure S1). Maybe partly because 88% patients (n=824) did not undergo ablative/adjuvant RAI, and only 12% patients (n=112) received RAI after initial surgery in this cohort.
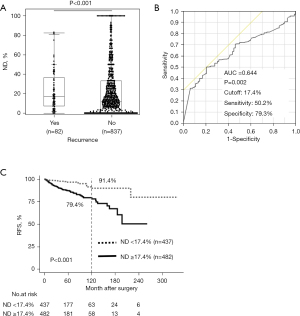
The multivariate prognostic analysis included factors with prognostic potential (P<0.05) and age, a known prognostic factor, in univariate analyses except for UICC stages which include tumor size, extrathyroidal extension, and pN. Older age [P=0.02; hazard ratio (HR) =1.88, 95% confidence interval (CI): 1.09–3.23], larger tumor size [P=0.02; HR =2.11 (95% CI: 1.16–3.81) (2< and ≤4 cm); HR =2.43 (95% CI: 1.69–5.04) (>4 cm)], lymph node greater than 3 cm (P<0.001; HR =5.25, 95% CI: 2.69–10.27), ND ≥17.4% (P=0.03; HR =2.20, 95% CI: 1.06–4.58), high preoperative Tg level (P=0.003; HR =2.28, 95% CI: 1.33–3.91), and low preoperative LMR (P=0.03; HR =1.83, 95% CI: 1.05–3.19) were the independent predictors of recurrence, marginally followed by gross extrathyroidal extension (P=0.051; HR =1.73, 95% CI: 1.00–3.01) (Table 2).
Table 2
| Variables | Multivariate | |
|---|---|---|
| HR (95% CI) | P* | |
| Age (years) | 0.02 | |
| <55 | 1.00 | |
| ≥55 | 1.88 (1.09–3.23) | |
| Gender | 0.047 | |
| Female | 1.00 | |
| Male | 1.65 (1.01–2.71) | |
| Tumor size (cm) | 0.02 | |
| ≤2 | 1.00 | |
| >2, ≤4 | 2.11 (1.16–3.81) | |
| >4 | 2.43 (1.69–5.04) | |
| Gross extrathyroidal extension | 0.051 | |
| No | 1.00 | |
| Yes | 1.73 (1.00–3.01) | |
| pN | 0.40 | |
| N0 | 1.00 | |
| N1a | 1.35 (0.43–4.25) | |
| N1b | 1.41 (0.75–6.10) | |
| Lymph node size (cm) | <0.001 | |
| ≤3 | 1.00 | |
| >3 | 5.25 (2.69–10.27) | |
| ND (%) | 0.03 | |
| <17.4 | 1.00 | |
| ≥17.4 | 2.20 (1.06–4.58) | |
| Preoperative Tg (ng/mL) | 0.003 | |
| <57.9 | 1.00 | |
| ≥57.9 | 2.28 (1.33–3.91) | |
| Preoperative LMR | 0.03 | |
| <5.3 | 1.83 (1.05–3.19) | |
| ≥5.3 | 1.00 | |
*, a Cox proportional hazards model. CI, confidence interval; HR, hazard ratio; LMR, lymphocyte-to-monocyte ratio; ND, node density; PTC, papillary thyroid cancer; RFS, recurrence-free survival; Tg, thyroglobulin.
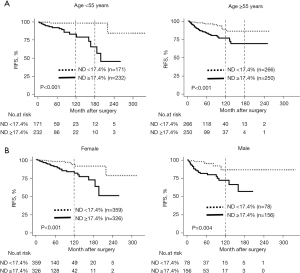
In the correlation analysis (Table 3), the multivariate logistic regression analysis showed that ND ≥17.4% was independently correlated with younger age (<55 years) [P<0.001; odds ratio (OR) =1.65, 95% CI: 1.17–2.45], male gender (P<0.001; OR =2.02, 95% CI: 1.46–2.79), gross extrathyroidal extension (P<0.001; OR =1.98, 95% CI: 1.35–2.93), and lymph node larger than 3 cm (P=0.002; OR =4.32, 95% CI: 1.46–12.76). Even in subgroup analyses considering either age or gender that independently correlated with ND status, patients with ND ≥17.4% had a significantly poorer prognosis than their counterparts (Figure 2). Of note, in younger patients (<55 years), the ND <17.4% group had an excellent prognosis with both the 10-year and the 15-year RFS at 98.4%, while the ND ≥17.4% group had a significantly poor prognosis with the 10-year RFS at 82.7% and the 15-year RFS at 65.5%.
Table 3
| Variables | ND (%) | Multivariate | ||||
|---|---|---|---|---|---|---|
| ≥17.4 (n=482), n (%) | <17.4 (n=437), n (%) | P* | OR (95% CI) | P** | ||
| Age (years) | 0.006 | <0.001 | ||||
| <55 | 232 (57.6) | 171 (42.4) | 1.65 (1.17–2.45) | |||
| ≥55 | 250 (48.5) | 266 (51.5) | 1.00 | |||
| Gender | <0.001 | <0.001 | ||||
| Female | 326 (47.6) | 359 (52.4) | 1.00 | |||
| Male | 156 (66.7) | 78 (33.3) | 2.02 (1.46–2.79) | |||
| Tumor size (cm) | 0.007 | 0.55 | ||||
| ≤2 | 264 (48.4) | 281 (51.6) | 1.00 | |||
| >2, ≤4 | 153 (55.6) | 122 (44.4) | 1.12 (0.82–1.52) | |||
| >4 | 57 (64.8) | 31 (35.2) | 1.28 (0.78–2.11) | |||
| Gross extrathyroidal extension | <0.001 | <0.001 | ||||
| No | 366 (48.8) | 384 (51.2) | 1.00 | |||
| Yes | 116 (68.6) | 53 (31.4) | 1.98 (1.35–2.93) | |||
| pN | NA | |||||
| N0 | 0 (0.0) | 257 (100) | <0.001 | − | ||
| N1a | 298 (72.3) | 114 (27.7) | 0.16 | − | ||
| N1b | 184 (73.6) | 66 (26.4) | − | |||
| Lymph node size (cm) | <0.001 | 0.002 | ||||
| ≤3 | 452 (51.1) | 433 (48.9) | 1.00 | |||
| >3 | 30 (88.2) | 4 (11.8) | 4.32 (1.46–12.76) | |||
| Preoperative Tg (ng/mL) | 0.22 | NA | ||||
| <57.9 | 280 (50.6) | 273 (49.4) | − | |||
| ≥57.9 | 195 (54.8) | 161 (45.2) | − | |||
| Preoperative NLR | 0.76 | NA | ||||
| <1.9 | 303 (51.9) | 281 (48.1) | − | |||
| ≥1.9 | 172 (52.9) | 153 (47.1) | − | |||
| Preoperative LMR | 0.47 | NA | ||||
| <5.3 | 195 (53.7) | 168 (46.3) | − | |||
| ≥5.3 | 280 (51.3) | 266 (48.7) | − | |||
*, Chi-square test or Fisher’s exact test, where appropriate. **, multivariate logistic regression analysis. CI, confidence interval; NA, not assessed; ND, node density; LMR, lymphocyte-to-monocyte ratio; NLR, neutrophil-to-lymphocyte ratio; OR, odds ratio; PTC, papillary thyroid cancer; Tg, thyroglobulin.
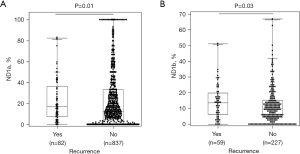
Both ND of central lymph nodes (ND1a) and ND of lateral lymph nodes (ND1b) were significantly higher in patients with disease recurrence
We also looked at the prognostic impact of ND1a (ND of central lymph nodes to total dissected nodes) and ND1b (ND of lateral lymph nodes to total dissected nodes) separately. Cases of N1b included patients with both CND and lateral neck dissections (LNDs) (n=287). Patients with recurrence(s) had significantly higher ND1a (P=0.01) and ND1b (P=0.03) than their counterparts (Figure 3A,3B). The median and mean ND1a of patients with recurrence(s) were 17.8% and 23.9%, while those without recurrence were 12.5% and 21.4%, respectively. The median and mean ND1b of patients with recurrence(s) were 13.5% and 14.3%, while those without recurrence were 10.1% and 12.0%, respectively.
High ND was significantly associated with disease recurrence regardless of the extent of lymph node dissection in PTC after curative resection
We then assessed the prognostic impact of ND based on the extent of lymph node dissection. Patients with only CND (CND only) (n=617) or both central and LND (n=287) were separately analyzed for ND status in terms of disease recurrence. Twenty-two patients (3.6%) had a recurrence in the CND-only group, compared to 59 patients (20.6%) in the LND group. Because patients in the LND group have a significantly poor prognosis due to advanced disease and more lymph node metastases, cutoffs of ND on recurrence were also separately determined (38.9% in the CND-only group, 34.2% in the LND group) (Figure S2A,S2B). In both groups of CND-only and LND, patients with recurrence(s) had significantly higher ND than those without (Figure 4A,4B). Furthermore, patients with higher ND than each cutoff had a significantly lower RFS regardless of the extent of lymph node dissection (Figure 4C,4D). In the CND-only group, the 10-year RFS was 85.1% and 95.5% in patients with ND ≥38.9% and ND <38.9%, respectively. In the LND group, patients with ND ≥34.2% had a 10-year RFS of 61.0%, which was significantly lower than that of their counterparts (79.8%). Similar results were observed in Kaplan-Meier curves using the previously determined cutoff (17.4%) in the entire patient population (Figure S2C,S2D). Collectively, ND status would significantly enrich patients at high-risk of recurrence.
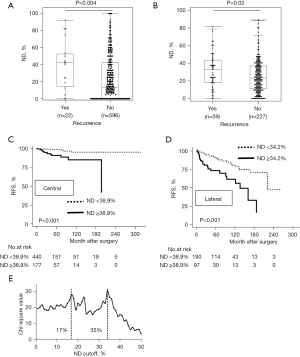
The optimal cutoff for ND on recurrence was 17.4% in all cases; however, in separate analyses based on the extent of lymph node dissection, the optimal cutoffs were 38.9% in the CND-only group and 34.2% in the LND group (both are around 35%). The X-tile software displayed the two best peaks of chi-square values, which corresponded to the optimal cutoffs of 17% and 35% (Figure 4E). A cutoff of 17.4% improved specificity, while cutoffs of 38.9% and 34.2% improved sensitivity (Figure 1B, Figures S1B,S2A). These findings may explain why the cutoffs differ. Considering the higher specificity in the entire cohort, the cutoff 17.4% may be better to predict disease recurrence more accurately across the patients. However, particularly among the patients with lateral lymph node metastasis, high cutoff [around 35% (34.2%)] would be appropriate to improve the sensitivity to select high-risk for recurrence considering the advanced disease.
PTC with lymph node/local recurrence showed significantly higher ND than that of hematogenous recurrence, and high ND (than each cutoff) showed early recurrence among patients with recurrence(s)
The relationship between ND and the first recurrence site(s) was investigated in 82 patients who had recurrence(s). Two patients experienced cervical lymph node and hematogenous (lung ± bone) recurrences simultaneously. Figure 5A shows significantly higher ND in patients with lymph nodes (including cervical and mediastinal nodes) or local recurrence (35.6%±21.8%) compared to those with hematogenous recurrence(s) (13.4%±10.9%) (P=0.003).
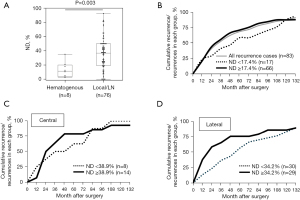
The time to recurrence among patients with recurrence(s) was also investigated. The cumulative rates of cases with recurrence-to-total cases with recurrence in each group were shown in Figure 5B (whole recurrence cases), Figure 5C (recurrence cases in the CND-only group), and Figure 5D (recurrence cases in the LND group). Interestingly, cumulative recurrence curves were convex upward in high ND patients with recurrence(s), whereas they were linear in low ND patients with recurrence(s) in all Figure 5B-5D. Particularly, the differences were noticeable within 72 months of surgery in both the CND only and LND groups.
These findings indicate that recurrence occurs earlier in patients with high ND than in patients with low ND, notwithstanding the extent of lymph node dissection.
Discussion
This study investigated the clinical significance of pathological ND in curatively resected PTC and showed that high ND independently predicted disease recurrence. Particularly among the younger population, ND selected patients with a significantly worse prognosis. The prognostic impact was significant in both the ND of the central neck (N1a) and the ND of the lateral neck (N1b). Furthermore, high ND predicted recurrence regardless of the extent of lymph node dissection. Notably, recurrence occurs earlier in patients with high ND than in patients with low ND.
Several indicators for harvested lymph nodes have been proposed, and in gastrointestinal cancers, the number of metastatic lymph nodes has already been included in the UICC TNM staging system (39). Actually, the number of metastatic nodes has been proposed as a prognostic factor for PTC (11,18). However, the number of metastatic nodes decreases when a metastatic node is large or forms a lump with multiple nodes. In this regard, ND has an advantage in clinical use as long as appropriate node dissection is conducted. Importantly, high ND (≥17.4%) independently predicted recurrence and had prognostic significance in both central and lateral nodes, implying that ND could be a genuine prognostic and independent predictor comparable to known factors already included in the TNM staging system.
In the current cohort of patients, a cutoff of 17.4% predicted recurrence with 79.3% specificity and 50.2% sensitivity (Figure 1B). The results show that ND ≥17.4% accurately selects high-risk patients for recurrence, but that other parameters may be required to increase sensitivity and cover high-risk patients. Particularly, young patients with ND ≥17.4% had worse RFS even than older patients with ND ≥17.4% (Figure 2A), implying that young patients with ND ≥17.4% should receive ablative/adjuvant RAI therapy as well as thyroid-stimulating hormone suppression. Previous studies have reported various cutoffs of ND (16,20,25,27-29,31,34), and Lindfors et al. proposed two cutoffs for nodal recurrence (40). Indeed, in the current study, ND represented bimodal optimal cutoffs of 17% and of 35% (Figure 4E), resulting in optimal cutoffs of 38.9% in the CND-only group and 34.2% in the LND group (Figure 4, Figure S2A,S2B), which show higher sensitivity but lower specificity than 17.4%. The bimodal distribution of optimal cutoffs could be attributed to lymph node yield caused by the laterality of thyroidectomy and lymph node dissection.
Interestingly, the first recurrence(s) in patients with higher ND occurred earlier than in patients with lower ND (Figure 5B-5D), and the difference was observed within 72 months after surgery notwithstanding the extent of lymph node dissection. These results are novel regarding the clinical implications of ND in thyroid cancer. Given that local or lymph node recurrence was significantly linked to high ND, patients with high ND should be followed up at short intervals for the first 72 months after surgery, preferably with neck ultrasonography, to detect lymph node or local recurrence.
Several limitations of this study should be addressed. First, this study was retrospectively performed at a single institution, which could have introduced selection bias, particularly given the relatively limited sample size. Actually, older age, a well-established high-risk factor, was not an independent prognostic indicator in our cohort, despite being strongly correlated with ND status. Second, we used a time-dependent ROC curve to determine the optimal cutoff value of ND on recurrence; thus, it may not accurately account for RFS in spite of the patient’s long-term follow-up. As a result, further validation by multiple institutions is necessary.
Conclusions
In conclusion, a high ND strongly predicts recurrence in both early and locally advanced PTC, especially in young patients. Recurrence in patients with high ND occurred earlier than in their counterparts. As a result, ND would be considered a strong prognostic factor when selecting high-risk patients in PTC after curative resection.
Acknowledgments
We are grateful to Enago (Crimson Interactive Japan Co., Ltd.) for proofreading this manuscript.
Footnote
Reporting Checklist: The authors have completed the STROBE reporting checklist. Available at https://gs.amegroups.com/article/view/10.21037/gs-2025-36/rc
Data Sharing Statement: Available at https://gs.amegroups.com/article/view/10.21037/gs-2025-36/dss
Peer Review File: Available at https://gs.amegroups.com/article/view/10.21037/gs-2025-36/prf
Funding: None.
Conflicts of Interest: All authors have completed the ICMJE uniform disclosure form (available at https://gs.amegroups.com/article/view/10.21037/gs-2025-36/coif). The authors have no conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved. The Institutional Review Board (IRB) of the Kitasato University School of Medicine approved this study (No. B23-057), which was conducted in accordance with the IRB’s clinical research guidelines and the Declaration of Helsinki and its subsequent amendments. All individuals gave written informed consent for their samples to be pathologically assessed, routine laboratory tests performed, and clinical data analyzed.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Sherman SI. Thyroid carcinoma. Lancet 2003;361:501-11. [Crossref] [PubMed]
- Siegel RL, Miller KD, Jemal A. Cancer Statistics, 2017. CA Cancer J Clin 2017;67:7-30. [Crossref] [PubMed]
- Haugen BR, Alexander EK, Bible KC, et al. 2015 American Thyroid Association Management Guidelines for Adult Patients with Thyroid Nodules and Differentiated Thyroid Cancer: The American Thyroid Association Guidelines Task Force on Thyroid Nodules and Differentiated Thyroid Cancer. Thyroid 2016;26:1-133. [Crossref] [PubMed]
- Mao J, Zhang Q, Zhang H, et al. Risk Factors for Lymph Node Metastasis in Papillary Thyroid Carcinoma: A Systematic Review and Meta-Analysis. Front Endocrinol (Lausanne) 2020;11:265. [Crossref] [PubMed]
- Genpeng L, Pan Z, Tao W, et al. Prognostic implications of extranodal extension in papillary thyroid carcinomas: A propensity score matching analysis and proposal for incorporation into current tumor, lymph node, metastasis staging. Surgery 2022;171:368-76. [Crossref] [PubMed]
- Hong YR, Lee SH, Lim DJ, et al. The stratification of patient risk depending on the size and ratio of metastatic lymph nodes in papillary thyroid carcinoma. World J Surg Oncol 2017;15:74. [Crossref] [PubMed]
- Ito Y, Miyauchi A, Masuoka H, et al. Prognostic Value of Extranodal Tumor Extension in Papillary Thyroid Carcinoma: Proposal for Upstaging of Cases with Extranodal Tumor Extension. World J Surg 2020;44:638-43. [Crossref] [PubMed]
- Kim HI, Kim TH, Choe JH, et al. Restratification of survival prognosis of N1b papillary thyroid cancer by lateral lymph node ratio and largest lymph node size. Cancer Med 2017;6:2244-51. [Crossref] [PubMed]
- Kim JW, Roh JL, Gong G, et al. Extent of Extrathyroidal Extension as a Significant Predictor of Nodal Metastasis and Extranodal Extension in Patients with Papillary Thyroid Carcinoma. Ann Surg Oncol 2017;24:460-8. [Crossref] [PubMed]
- Moritani S. Impact of invasive extranodal extension on the prognosis of patients with papillary thyroid carcinoma. Thyroid 2014;24:1779-83. [Crossref] [PubMed]
- Randolph GW, Duh QY, Heller KS, et al. The prognostic significance of nodal metastases from papillary thyroid carcinoma can be stratified based on the size and number of metastatic lymph nodes, as well as the presence of extranodal extension. Thyroid 2012;22:1144-52. [Crossref] [PubMed]
- Roh JL, Park JW, Jeong J, et al. Extranodal extension of lymph node metastasis as a prognostic indicator of recurrence and survival in papillary thyroid carcinoma. J Surg Oncol 2017;116:450-8. [Crossref] [PubMed]
- Sugitani I, Kasai N, Fujimoto Y, et al. A novel classification system for patients with PTC: addition of the new variables of large (3 cm or greater) nodal metastases and reclassification during the follow-up period. Surgery 2004;135:139-48. [Crossref] [PubMed]
- Wu MH, Shen WT, Gosnell J, et al. Prognostic significance of extranodal extension of regional lymph node metastasis in papillary thyroid cancer. Head Neck 2015;37:1336-43. [Crossref] [PubMed]
- Heaton CM, Chang JL, Orloff LA. Prognostic Implications of Lymph Node Yield in Central and Lateral Neck Dissections for Well-Differentiated Papillary Thyroid Carcinoma. Thyroid 2016;26:434-40. [Crossref] [PubMed]
- Vas Nunes JH, Clark JR, Gao K, et al. Prognostic implications of lymph node yield and lymph node ratio in papillary thyroid carcinoma. Thyroid 2013;23:811-6. [Crossref] [PubMed]
- Weitzman RE, Justicz NS, Kamani D, et al. How Many Nodes to Take? Lymph Node Ratio Below 1/3 Reduces Papillary Thyroid Cancer Nodal Recurrence. Laryngoscope 2022;132:1883-7. [Crossref] [PubMed]
- Zhan L, Feng HF, Yu XZ, et al. Clinical and prognosis value of the number of metastatic lymph nodes in patients with papillary thyroid carcinoma. BMC Surg 2022;22:235. [Crossref] [PubMed]
- Liu D, Chen Y, Deng M, et al. Lymph node ratio and breast cancer prognosis: a meta-analysis. Breast Cancer 2014;21:1-9. [Crossref] [PubMed]
- Xian K, Xu S, Huang H, et al. Synergy of Nodal Factors in Predicting Recurrence After Treatment of N1b Papillary Thyroid Carcinoma. J Clin Endocrinol Metab 2024;109:3137-45. [Crossref] [PubMed]
- Zhang MR, Xie TH, Chi JL, et al. Prognostic role of the lymph node ratio in node positive colorectal cancer: a meta-analysis. Oncotarget 2016;7:72898-907. [Crossref] [PubMed]
- Zhu J, Xue Z, Zhang S, et al. Integrated analysis of the prognostic role of the lymph node ratio in node-positive gastric cancer: A meta-analysis. Int J Surg 2018;57:76-83. [Crossref] [PubMed]
- Amit M, Tam S, Boonsripitayanon M, et al. Association of Lymph Node Density With Survival of Patients With Papillary Thyroid Cancer. JAMA Otolaryngol Head Neck Surg 2018;144:108-14. [Crossref] [PubMed]
- Chang YW, Kim HS, Jung SP, et al. Significance of micrometastases in the calculation of the lymph node ratio for papillary thyroid cancer. Ann Surg Treat Res 2017;92:117-22. [Crossref] [PubMed]
- Lee SG, Ho J, Choi JB, et al. Optimal Cut-Off Values of Lymph Node Ratio Predicting Recurrence in Papillary Thyroid Cancer. Medicine (Baltimore) 2016;95:e2692. [Crossref] [PubMed]
- Luo Z, Hei H, Qin J, et al. Lymph node ratio in lateral neck is an independent risk factor for recurrence-free survival in papillary thyroid cancer patients with positive lymph nodes. Endocrine 2022;78:484-90. [Crossref] [PubMed]
- Luo Z, Hei H, Qin J, et al. Lymph node ratio as a tool to stratify patients with N1b papillary thyroid cancer. Langenbecks Arch Surg 2023;408:315. [Crossref] [PubMed]
- Nam SH, Roh JL, Gong G, et al. Nodal Factors Predictive of Recurrence After Thyroidectomy and Neck Dissection for Papillary Thyroid Carcinoma. Thyroid 2018;28:88-95. [Crossref] [PubMed]
- Parvathareddy SK, Siraj AK, Qadri Z, et al. Lymph node ratio is superior to AJCC N stage for predicting recurrence in papillary thyroid carcinoma. Endocr Connect 2022;11:e210518. [Crossref] [PubMed]
- Ryu IS, Song CI, Choi SH, et al. Lymph node ratio of the central compartment is a significant predictor for locoregional recurrence after prophylactic central neck dissection in patients with thyroid papillary carcinoma. Ann Surg Oncol 2014;21:277-83. [Crossref] [PubMed]
- Schneider DF, Mazeh H, Chen H, et al. Lymph node ratio predicts recurrence in papillary thyroid cancer. Oncologist 2013;18:157-62. [Crossref] [PubMed]
- Yip J, Orlov S, Orlov D, et al. Predictive value of metastatic cervical lymph node ratio in papillary thyroid carcinoma recurrence. Head Neck 2013;35:592-8. [Crossref] [PubMed]
- Zheng CM, Ji YB, Song CM, et al. Number of Metastatic Lymph Nodes and Ratio of Metastatic Lymph Nodes to Total Number of Retrieved Lymph Nodes Are Risk Factors for Recurrence in Patients With Clinically Node Negative Papillary Thyroid Carcinoma. Clin Exp Otorhinolaryngol 2018;11:58-64. [Crossref] [PubMed]
- Schneider DF, Chen H, Sippel RS. Impact of lymph node ratio on survival in papillary thyroid cancer. Ann Surg Oncol 2013;20:1906-11. [Crossref] [PubMed]
- Hu Y, Wang Z, Dong L, et al. The prognostic value of lymph node ratio for thyroid cancer: a meta-analysis. Front Oncol 2024;14:1333094. [Crossref] [PubMed]
- Yokota M, Katoh H, Nishimiya H, et al. Lymphocyte-Monocyte Ratio Significantly Predicts Recurrence in Papillary Thyroid Cancer. J Surg Res 2020;246:535-43. [Crossref] [PubMed]
- Katoh H, Okamoto R, Yokota M, et al. CD163(+) Tumor-Associated Macrophage Recruitment Predicts Papillary Thyroid Cancer Recurrence. J Surg Res 2024;303:532-44. [Crossref] [PubMed]
- Wang Z, Tang C, Wang Y, et al. Inclusion of the Number of Metastatic Lymph Nodes in the Staging System for Medullary Thyroid Cancer: Validating a Modified American Joint Committee on Cancer Tumor-Node-Metastasis Staging System. Thyroid 2022;32:536-43. [Crossref] [PubMed]
- International Union Against Cancer TNM Classification of Malignant Tumors Eighth edition. Wilet Blackwell. Available online: https://www.uicc.org/resources/tnm-classification-malignant-tumours-8th-edition
- Lindfors H, Ihre Lundgren C, Zedenius J, et al. The Clinical Significance of Lymph Node Ratio and Ki-67 Expression in Papillary Thyroid Cancer. World J Surg 2021;45:2155-64. [Crossref] [PubMed]


