
Malignant risk prediction of cystic-solid thyroid nodules using a comprehensive model integrating clinical and ultrasound features, ultrasound radiomics, and deep transfer learning
Highlight box
Key findings
• In both the training set and the testing set, the deep transfer learning radiomics clinical nomogram (DTLRN) demonstrated good predictive performance, with a sensitivity of 87.50% and a specificity of 82.90%.
What is known and what is new?
• Cystic-solid thyroid nodules (CSTN) consist of solid and cystic components, with an incidence rate ranging from 15.0% to 53.8% and a malignancy risk ranging from 5% to 45.8%, indicating a wide risk span.
• In response to that the risk of malignancy in CSTN varies greatly and may be underestimated, we developed the DTLRN integrated model to predict the malignancy risk of CSTN.
What is the implication, and what should change now?
• The application of the integrated model can reduce the rate of missed diagnoses of malignant CSTN and unnecessary biopsy procedures, while also improving clinicians’ acceptance of deep learning-assisted ultrasound diagnostics.
• Our future research will include contrast-enhanced ultrasound for further analysis.
Introduction
Thyroid nodules are a common clinical condition and are palpable in approximately 5% of the population. Additionally, with advancements in imaging technology, these nodules are often incidentally found through ultrasound examinations, leading to a significant increase in detection rates, which can be as high as 70% (1). Cystic-solid thyroid nodules (CSTN) consist of solid and cystic components, with an incidence rate ranging from 15.0% to 53.8% (2) and a malignancy risk ranging from 5% to 45.8% (3), indicating a wide risk span. A recent study has reported an increased incidence of thyroid cancer (TC) among pathologically confirmed CSTN, accounting for 20% to 25% (4). Currently, various Thyroid Imaging Reporting and Data Systems (TI-RADS) (5-11) classifications primarily focus on solid thyroid nodules and emphasize several ultrasound characteristics that are significantly associated with malignancy. These features include vertical orientation, indistinct margins, microcalcifications, and hypoechogenicity. However, limited research has been conducted on the diagnostic significance of ultrasound characteristics in evaluating CSTN; moreover, these ultrasound features are subjective and variable, relying on the operator’s experience. Due to the mixed cystic and solid components of CSTN, they often present atypically on imaging, which increases the risk of missed diagnoses and can lead to unnecessary fine-needle aspirations. Therefore, differentiating between benign and malignant conditions is particularly challenging.
With the continuous development of artificial intelligence (AI) technology, the clinical applications of machine learning (ML) and deep learning (DL) methods in medical image analysis have gradually expanded. Nonetheless, solely relying on traditional ML methods still has certain limitations, such as the need for a large amount of labeled data and dependence on data quality. Deep transfer learning (DTL), as an emerging technology, can achieve better results in predicting thyroid nodules by transferring knowledge from pre-trained models in other domains to the prediction task of thyroid nodules, even with a smaller amount of labeled data. Research has shown that ML-assisted models have a diagnostic accuracy for thyroid nodules comparable to that of experienced ultrasound physicians (12-14). Despite progress in predicting the malignancy risk of thyroid nodules, there is limited research specifically focused on CSTN. Due to their complex imaging presentations and heterogeneity, CSTN pose greater challenges to traditional predictive models. Therefore, ensuring high diagnostic accuracy before surgery while reducing unnecessary fine-needle aspirations has emerged as a research hotspot and clinical challenge.
This study aims to combine the advantages of ML and DTL by constructing and validating a comprehensive model to enhance the effectiveness of predicting the malignancy risk of CSTN, providing an effective tool for clinical diagnosis and treatment. We present this article in accordance with the TRIPOD reporting checklist (available at https://gs.amegroups.com/article/view/10.21037/gs-2024-551/rc).
Methods
Study subjects
This retrospective study analyzed the ultrasound image data of CSTN from the First Affiliated Hospital of Guangxi Medical University from January 2023 to December 2023. Based on the inclusion and exclusion criteria, a total of 278 patients were included, comprising 278 CSTN. The entire dataset was randomly divided into training and testing sets in a 7:3 ratio. The training set was used to build the predictive model, while the testing set was used to validate the model. Inclusion criteria: (I) ultrasound examination showed that the thyroid nodules have both solid and cystic components; (II) patients underwent thyroidectomy or fine needle aspiration (FNA) cytopathology examination with clear pathological results; (III) complete ultrasound images; (IV) complete clinical and pathological data were available. Exclusion criteria: (I) patients with multiple lesions; (II) patients who have previously undergone surgical resection or radiofrequency ablation treatment; (III) patients with incomplete clinical, pathological, or ultrasound image data. This study was conducted in accordance with the Declaration of Helsinki and its subsequent amendments. This retrospective study was approved by the Ethics Committee of the First Affiliated Hospital of Guangxi Medical University (No. 2024-E568-01). Informed consent was not required for this study due to the retrospective nature. The patient inclusion process is illustrated in Figure 1.
Thyroid ultrasound examination
In this study, an ultrasound diagnostic device equipped with real-time high-frequency linear array transducers (5–12 or 8–15 MHz) was utilized for the ultrasound examinations. Image data were collected by two ultrasound physicians with over 5 years of experience in thyroid ultrasound. The thyroid ultrasound scanning protocol included transverse and longitudinal scans for thyroid nodules; the images were stored in JPEG format. Based on the ultrasound characteristics determined by the Chinese-TIRADS (C-TIRADS) and American College of Radiology TIRADS (ACR-TIRADS) guidelines (8,11), two experienced ultrasound specialists (with 10 years of experience in thyroid ultrasound) reached a consensus on the definitions of the ultrasound features to be analyzed. In cases of diagnostic disagreement, a consensus was reached by discussion between the two ultrasound physicians. Additionally, all ultrasound physicians were unaware of the patient’s clinical history, preoperative ultrasound reports, surgical records, or pathological results.
Image preprocessing
Due to the use of different ultrasound devices during image collection, the images in this study varied in size. Therefore, all ultrasound images were standardized to 1,024×1,024 pixels by either cropping or adding black bars. To ensure the quality of the ultrasound images, a physician with 5 years of experience in thyroid ultrasound screened all the images to exclude those with significant artifacts or low resolution.
Radiomics feature extraction and selection
Initially, a physician with 5 years of experience in thyroid ultrasound manually delineated the region of interest (ROI) of CSTN using ITK-SNAP software (version 3.8.0). This ROI was then reviewed and agreed upon by another thyroid ultrasound physician with seven years of experience in thyroid ultrasound. The manually created features were extracted using Pyradiomics (http://pyradiomics.readthedocs.io). The extracted ultrasound radiomics features included 18 first-order statistical features, 14 shape features, 24 gray-level co-occurrence matrix (GLCM) features, 14 gray-level dependence matrix (GLDM) features, 16 gray-level run length matrix (GLRLM) features, 16 gray-level size zone matrix (GLSZM) features, and 5 neighboring gray-tone difference matrix (NGTDM) features. Least absolute shrinkage and selection operator (LASSO) logistic regression analysis was employed on the training set to select ultrasound radiomics features.
ML model development
Feature selection was performed with LASSO, and supervised learning was conducted using eight different ML classifiers: random forest (RF), k-nearest neighbors (KNN), logistic regression (LR), multilayer perceptron (MLP), support vector machine (SVM), extreme gradient boosting (XGBoost), light gradient boosting machine (LightGBM), and Extra Trees. Five-fold cross-validation was employed to derive the final ultrasound radiomics features and to determine the optimal ML model.
DTL model development
A convolutional neural network (CNN) was established to predict the malignancy risk of CSTN using DTL features from ultrasound images. The ultrasound images from the training cohort and the testing cohort were cropped according to the defined ROI and resized uniformly to 224×224. During the preprocessing stage, the grayscale values were normalized to [0, 1]. To improve the model’s generalization performance, transfer learning techniques were employed to develop the model, initializing it with pre-trained parameters from ImageNet, and the best-performing DenseNet169 was selected to develop the predictive model. During the training phase, random cropping and horizontal flipping were utilized to augment the images and mitigate overfitting.
Development and interpretation of the comprehensive predictive model
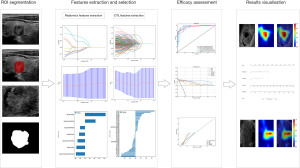
The comprehensive predictive model primarily consists of DTL, handcrafted radiomics features, as well as clinical and ultrasound characteristics. The workflow diagram of the comprehensive predictive model is shown in Figure 2. The DTL features were extracted using the DenseNet169 model, which performed best in DTL. The ultrasound radiomics features were extracted using the Extra Trees model, which showed the best performance in ML (the detailed training process can be found in the Appendix 1). The filtered clinical and ultrasound features were then combined with the handcrafted radiomics features and DTL features to create the final predictive model. The performance of the integrated model was evaluated by comparing its performance on a testing set with that of the handcrafted radiomics model and the DTL model. In addition, the gradient-weighted class activation mapping (Grad-CAM) was utilized to generate saliency heatmaps for the DL model, providing a visual explanation method for the DL model, thereby enhancing its clinical interpretability.
Statistical analysis
In this study, the patient’s age and the maximum diameter of the thyroid nodules were presented as means and standard deviations. Comparisons between categorical variables were conducted using the Chi-squared test or Fisher’s test, while comparisons between continuous variables were conducted using the Mann-Whitney U testing or independent t-test. The performance of the predictive model was evaluated using the area under the receiver operating characteristic (ROC) curve, accuracy, sensitivity, and specificity. The DeLong test was used for comparative analysis of the area under the curve (AUC), with parameter estimates including a 95% confidence interval (CI). A P value <0.05 was considered statistically significant. All statistical analyses were conducted using Python software (version 3.7.12).
Results
Patient demographics and ultrasound characteristics
The characteristics of CSTN patients in the training and testing cohorts are shown in Table 1. The training cohort included 194 patients (30 males and 164 females) with an average age of 44.43±12.97 years. The testing cohort comprised 84 patients (10 males and 74 females) with an average age of 44.60±14.34 years. No significant differences were found between the two datasets (see Table S1).
Table 1
| Characteristics and US features | Train cohort | Test cohort | |||||||
|---|---|---|---|---|---|---|---|---|---|
| Total (n=194) | Benign (n=174) | Malignant (n=20) | P value | Total (n=84) | Benign (n=76) | Malignant (n=8) | P value | ||
| Age (years) | 44.43±12.97 | 45.32±12.89 | 36.65±11.24 | 0.003 | 44.60±14.34 | 45.53±14.28 | 35.75±12.41 | 0.06 | |
| Size (mm) | 31.98±11.59 | 32.91±11.24 | 23.95±11.77 | <0.001 | 30.54±12.30 | 31.96±11.95 | 17.00±5.61 | <0.001 | |
| Gender | 0.12 | 0.60 | |||||||
| Male | 30 (15.46) | 24 (13.79) | 6 (30.00) | 10 (11.90) | 10 (13.16) | 0 | |||
| Female | 164 (84.54) | 150 (86.21) | 14 (70.00) | 74 (88.10) | 66 (86.84) | 8 (100.00) | |||
| Location | 0.77 | 0.31 | |||||||
| Upper | 11 (5.67) | 9 (5.17) | 2 (10.00) | 7 (8.33) | 5 (6.58) | 2 (25.00) | |||
| Mid | 122 (62.89) | 109 (62.64) | 13 (65.00) | 45 (53.57) | 42 (55.26) | 3 (37.50) | |||
| Lower | 60 (30.93) | 55 (31.61) | 5 (25.00) | 30 (35.71) | 27 (35.53) | 3 (37.50) | |||
| Sthmus | 1 (0.52) | 1 (0.57) | 0 | 2 (2.38) | 2 (2.63) | 0 | |||
| Composition | 0.16 | 0.41 | |||||||
| Predominately solid | 157 (80.93) | 138 (79.31) | 19 (95.00) | 70 (83.33) | 62 (81.58) | 8 (100.00) | |||
| Predominately cystic | 37 (19.07) | 36 (20.69) | 1 (5.00) | 14 (16.67) | 14 (18.42) | 0 | |||
| Echogenicity | <0.001 | <0.001 | |||||||
| Hypoechoic | 40 (20.62) | 23 (13.22) | 17 (85.00) | 13 (15.48) | 6 (7.89) | 7 (87.50) | |||
| Isoechoic | 149 (76.80) | 146 (83.91) | 3 (15.00) | 69 (82.14) | 68 (89.47) | 1 (12.50) | |||
| Hyperechoic | 5 (2.58) | 5 (2.87) | 0 | 2 (2.38) | 2 (2.63) | 0 | |||
| Echotexture | 0.78 | >0.99 | |||||||
| Homogeneous | 7 (3.61) | 7 (4.02) | 0 | 5 (5.95) | 5 (6.58) | 0 | |||
| Heterogeneous | 187 (96.39) | 167 (95.98) | 20 (100.00) | 79 (94.05) | 71 (93.42) | 8 (100.00) | |||
| Orientation | <0.001 | 0.001 | |||||||
| Horizontal | 190 (97.94) | 174 (100.00) | 16 (80.00) | 82 (97.62) | 76 (100.00) | 6 (75.00) | |||
| Vertical | 4 (2.06) | 0 | 4 (20.00) | 2 (2.38) | 0 | 2 (25.00) | |||
| Echogenic_foc | <0.001 | <0.001 | |||||||
| No | 164 (84.54) | 162 (93.10) | 2 (10.00) | 70 (83.33) | 69 (90.79) | 1 (12.50) | |||
| Microcalcifications | 22 (11.34) | 5 (2.87) | 17 (85.00) | 10 (11.90) | 3 (3.95) | 7 (87.50) | |||
| Macrocalcifications | 8 (4.12) | 7 (4.02) | 1 (5.00) | 4 (4.76) | 4 (5.26) | 0 | |||
| Margin | <0.001 | <0.001 | |||||||
| Circumscribed | 150 (77.32) | 147 (84.48) | 3 (15.00) | 64 (76.19) | 64 (84.21) | 0 | |||
| Ill-defined | 16 (8.25) | 9 (5.17) | 7 (35.00) | 5 (5.95) | 1 (1.32) | 4 (50.00) | |||
| Irregular margin | 28 (14.43) | 18 (10.34) | 10 (50.00) | 15 (17.86) | 11 (14.47) | 4 (50.00) | |||
| Halo | 0.009 | 0.10 | |||||||
| Present halo | 77 (39.69) | 75 (43.10) | 2 (10.00) | 27 (32.14) | 27 (35.53) | 0 | |||
| Absent halo | 117 (60.31) | 99 (56.90) | 18 (90.00) | 57 (67.86) | 49 (64.47) | 8 (100.00) | |||
| Acute_angle | <0.001 | <0.001 | |||||||
| No | 171 (88.14) | 168 (96.55) | 3 (15.00) | 75 (89.29) | 73 (96.05) | 2 (25.00) | |||
| Yes | 23 (11.86) | 6 (3.45) | 17 (85.00) | 9 (10.71) | 3 (3.95) | 6 (75.00) | |||
| CDFI | 0.81 | 0.054 | |||||||
| Peripheral vascularity | 145 (74.74) | 131 (75.29) | 14 (70.00) | 61 (72.62) | 58 (76.32) | 3 (37.50) | |||
| Mixed vascularity | 49 (25.26) | 43 (24.71) | 6 (30.00) | 23 (27.38) | 18 (23.68) | 5 (62.50) | |||
Data are presented as mean ± standard deviation or n (%). CDFI, color Doppler flow imaging; US, ultrasound.
To better understand the relationship between the malignancy risk of CSTN and clinical and ultrasound features, univariate and multivariate logistic regression analyses were conducted on the included clinical and ultrasound characteristics. The results indicated that solid hypoechoic features (P=0.009) and solid partially eccentric acute angles (P<0.001) were independent risk factors for the malignancy risk of CSTN.
Diagnostic performance of the CSTN malignancy risk prediction model
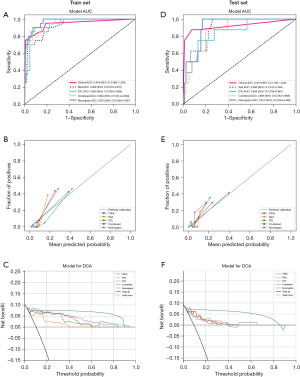
A radiomics model, a DTL model, a clinical model, and an integrated model were successfully constructed. In the testing set, our results revealed that the DTL model exhibited higher specificity compared to the radiomics model (86.80% vs. 76.30%), but lower sensitivity (62.50% vs. 87.50%) (Table 2). The integrated model combined the radiomics, DTL, and clinical models, demonstrating good predictive performance, with a sensitivity and specificity of 87.50% and 82.90%, respectively. Additionally, the AUC of the integrated model in the testing set was 0.913, which was higher than that of the radiomics model (0.913 vs. 0.898, P=0.67) and the DTL model (0.913 vs. 0.848, P=0.38). In the training set, the AUC was 0.973, surpassing that of the radiomics model (0.973 vs. 0.926, P=0.09) and the DTL model (0.973 vs. 0.943, P=0.01) (Table 2 and Figure 3). These findings suggest that the integrated model outperforms individual models.
Table 2
| Signature | Accuracy | AUC (95% CI) | Sensitivity | Specificity | PPV | NPV |
|---|---|---|---|---|---|---|
| Train cohort | ||||||
| Clinical | 0.840 | 0.944 (0.882–1.000) | 0.850 | 0.839 | 0.378 | 0.980 |
| Rad | 0.794 | 0.926 (0.875–0.977) | 0.850 | 0.787 | 0.315 | 0.979 |
| DTL | 0.902 | 0.943 (0.903–0.984) | 0.850 | 0.908 | 0.515 | 0.981 |
| Combined | 0.851 | 0.954 (0.914–0.993) | 0.850 | 0.851 | 0.395 | 0.980 |
| Nomogram | 0.845 | 0.973 (0.949–0.997) | 0.950 | 0.833 | 0.396 | 0.993 |
| Test cohort | ||||||
| Clinical | 0.964 | 0.919 (0.782–1.000) | 0.750 | 0.987 | 0.857 | 0.974 |
| Rad | 0.774 | 0.898 (0.812–0.984) | 0.875 | 0.763 | 0.280 | 0.983 |
| DTL | 0.845 | 0.848 (0.709–0.987) | 0.625 | 0.868 | 0.333 | 0.957 |
| Combined | 0.798 | 0.865 (0.762–0.968) | 0.750 | 0.803 | 0.286 | 0.968 |
| Nomogram | 0.833 | 0.913 (0.844–0.982) | 0.875 | 0.829 | 0.350 | 0.984 |
AUC, area under the curve; CI, confidence interval; Combined, deep transfer leaning + radiomics; DTL, deep transfer leaning; NPV, negative predictive value; PPV, positive predictive value; Rad, radiomics.
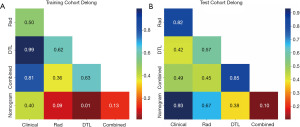
Evaluation of the nomogram
In the training cohort, an AUC of 0.944 (95% CI: 0.882–1.000) was noted for clinical features, 0.926 (95% CI: 0.875–0.977) for radiomics features, 0.943 (95% CI: 0.903–0.984) for DTL features, and 0.954 (95% CI: 0.914–0.993) for DTL and radiomics (DTLR) features. In the testing cohort, an AUC of 0.919 (95% CI: 0.782–1.000) was achieved for the clinical features, 0.898 (95% CI: 0.812–0.984) for radiomics features, 0.848 (95% CI: 0.709–0.987) for DTL features, and 0.865 (95% CI: 0.762–0.968) for DTLR features. A logistic regression algorithm was used to construct the DTL radiomics clinical nomogram (DTLRN), which combined clinical features with the DTL and radiomics model features, demonstrating the best performance with AUCs of 0.973 (95% CI: 0.949–0.997) in the training set and 0.913 (95% CI: 0.844–0.982) in the testing set. Table 2 summarizes all the models used to predict the malignancy risk of CSTN. DeLong testing was performed to compare the clinical features, radiomics features, DTL features, DTLR features, and DTLRN features (Figure 3).
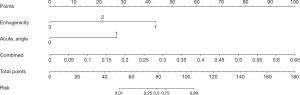
Based on clinical features and DTLR features, a nomogram was constructed to predict the malignancy risk of CSTN (Figure 4). The calibration curve of the nomogram showed a good agreement between the predicted and observed values for CSTN malignancy risk in both the training and testing cohorts.
Each model was evaluated using decision curve analysis (DCA). The DCA for clinical features, radiomics features, DTL features, DTLR features, and the nomogram is illustrated in Figure 5. The use of the integrated model nomogram for preoperative prediction of CSTN malignancy risk demonstrated better clinical utility.
Interpretation of the DL model
To better address the “black box” issue of DL models, Grad-CAM was utilized to identify the regions of interest in the DTL model. Figure 6 presents several representative cases, indicating that the areas of interest are primarily located in the solid portions of the thyroid nodules and the regions where the solid portions come into contact with the cyst wall. This aligns with the areas that radiologists focus on, which are significantly related to the malignancy risk of CSTN.
Discussion
In this study, a comprehensive model DTLRN was developed to predict the malignancy risk of CSTN, which integrates DTL, radiomics, as well as clinical and ultrasound features. In both the training group and the testing group, DTLRN demonstrated good predictive performance, with a sensitivity of 87.50% and a specificity of 82.90%. The AUC in the testing cohort reached 0.913 (95% CI: 0.844–0.982), while the AUC in the training cohort was as high as 0.973 (95% CI: 0.949–0.997). The integrated model was superior to the efficacy of models constructed based on radiomics features, DTL features, or clinical features alone. Therefore, this comprehensive model helps reduce the missed diagnosis rate of malignant risks in CSTN and the incidence of unnecessary fine-needle aspirations, providing a non-invasive preoperative assessment method to predict the risk of malignancy of CSTN. This assessment holds significant clinical implications for decision-making regarding preoperative CSTN management.
Our research findings indicate that both the standalone DTL model and the radiomics model have diagnostic limitations. The DTL model demonstrated higher specificity, while the ultrasound radiomics model exhibited higher sensitivity, indicating a difference between the image characteristics extracted by ultrasound radiomics and those extracted by DTL (15). Compared to models based on ultrasound radiomics or DTL features, the integrated model showed superior performance. Previous studies have focused on traditional radiomics methods to predict the malignancy risk of CSTN and achieved promising results (16,17). Our research also observed that as the performance of the model improves, the model becomes more complex, posing new challenges to its interpretability. In this study, the AUC of the DTLRN model is 0.913, which may seem like a marginal improvement compared to the radiomics model’s 0.898. However, the DTLRN model achieves improved accuracy (0.883 vs. 0.774) and specificity (0.829 vs. 0.763) without compromising sensitivity (0.875 vs. 0.875). This has potential implications in the clinical decision-making process, such as reducing unnecessary thyroid nodule biopsies or improving the accuracy of early diagnosis. Our research findings indicate that the DTLRN model can effectively be used to assess the malignancy risk in CSTN patients. The model promotes more accurate evaluation, thereby enhancing diagnostic confidence.
Similar to previous research findings (17-22), clinical and two-dimensional ultrasound characteristics related to the malignancy risk of CSTN during routine ultrasound diagnostic were analyzed. These factors include the maximum nodule diameter, hypoechogenicity, vertical orientation, microcalcifications, irregular and indistinct margins, and eccentric sharp angles of the solid component. Our multivariate logistic regression analysis suggests that conventional ultrasound hypoechogenicity and the eccentric sharp angles of the solid components are independent risk factors for predicting the malignancy risk of CSTN. Other studies (23,24) have reported that microcalcifications, vertical orientation, and hypoechogenicity are significantly associated with malignant nodules. Our analysis suggests that the hypoechoic characteristics of nodules could be linked to the high density, heterogeneity, and fibrotic response exhibited by malignant cells. Concurrently, the presence of eccentric sharp angles within the solid components may be indicative of the aggressive growth patterns typical of malignant CSTN. Furthermore, these solid components typically emerge from the cyst wall and are situated at the base, displaying variable growth rates. These factors were incorporated into our comprehensive model, and its overall effectiveness has been significantly enhanced. Interestingly, our research findings indicate that the comprehensive model primarily focuses on the solid components of CSTN and their relationship with the cyst wall, aligning with the key areas of interest for clinicians in assessing the malignancy risk of CSTN. These results suggest that the ROI in the comprehensive model that includes clinical and ultrasound features is generally consistent with the judgments of physicians, thereby conferring a certain level of clinical interpretability to the model.
Our research findings reveal that the inclusion of ultrasound radiomics features has a limited contribution to the construction of our integrated model. Nonetheless, radiomics features play a central role in comprehensive models based on computed tomography (CT) images (25). This discrepancy may be attributed to the fact that certain features extracted from ultrasound images through handcrafted radiomics, such as shape, gray level, and texture, can also be obtained using DTL methods. Moreover, during the ultrasound imaging process, variations in signal intensity can produce noise, which reduces the quality of ultrasound images and affects the accuracy of extraction of certain hand-made radiomics characteristics. Therefore, these adverse factors also partially explain why the manually crafted radiomics features have a limited contribution to the integrated model in this study. Compared to models that include only DTL features and DTLR features, nomograms that incorporate traditional ultrasound features demonstrate better predictive performance. Ultrasound features should be analyzed alongside radiomics parameters to enhance the diagnostic capability for predicting the malignancy risk of CSTN.
Nevertheless, the limitations of the present study should be acknowledged. First, contrast-enhanced ultrasound was not incorporated to analyze the malignancy risk of CSTN. Our future research will include contrast-enhanced ultrasound for further analysis. Second, selection bias was inevitable due to the retrospective design of this study and the data collection being completed within one year, future prospective studies are required to control for confounding variables. Finally, our study is a single-center investigation lacking external validation. Our next step is to increase the sample size and collect external data for validation.
Conclusions
In summary, compared to models based solely on ultrasound radiomics or DTL features, the integrated model that combines clinical and ultrasound characteristics demonstrates superior predictive performance. The application of the integrated model can reduce the rate of missed diagnoses of malignant CSTN and unnecessary biopsy procedures, thereby improving clinicians’ acceptance of DL-assisted ultrasound diagnostics.
Acknowledgments
We thank the Laboratory of Guangxi Zhuang Autonomous Region Engineering Research Center for Artificial Intelligence Analysis of Multimodal Tumor Images, Key Laboratory of Ultrasonic Molecular Imaging and Artificial Intelligence for their support of this study.
Footnote
Reporting Checklist: The authors have completed the TRIPOD reporting checklist. Available at https://gs.amegroups.com/article/view/10.21037/gs-2024-551/rc
Data Sharing Statement: Available at https://gs.amegroups.com/article/view/10.21037/gs-2024-551/dss
Peer Review File: Available at https://gs.amegroups.com/article/view/10.21037/gs-2024-551/prf
Funding: None.
Conflicts of Interest: All authors have completed the ICMJE uniform disclosure form (available at https://gs.amegroups.com/article/view/10.21037/gs-2024-551/coif). The authors have no conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved. This study was conducted in accordance with the Declaration of Helsinki and its subsequent amendments. This retrospective study was approved by the Ethics Committee of the First Affiliated Hospital of Guangxi Medical University (No. 2024-E568-01). Informed consent was not required for this study due to the retrospective nature.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Burman KD, Wartofsky L. CLINICAL PRACTICE. Thyroid Nodules. N Engl J Med 2015;373:2347-56. [Crossref] [PubMed]
- Haugen BR, Alexander EK, Bible KC, et al. 2015 American Thyroid Association Management Guidelines for Adult Patients with Thyroid Nodules and Differentiated Thyroid Cancer: The American Thyroid Association Guidelines Task Force on Thyroid Nodules and Differentiated Thyroid Cancer. Thyroid 2016;26:1-133. [Crossref] [PubMed]
- Shi X, Liu R, Gao L, et al. Diagnostic Value of Sonographic Features in Distinguishing Malignant Partially Cystic Thyroid Nodules: A Systematic Review and Meta-Analysis. Front Endocrinol (Lausanne) 2021;12:624409. [Crossref] [PubMed]
- Li W, Zhu Q, Jiang Y, et al. Partially cystic thyroid nodules in ultrasound-guided fine needle aspiration: Prevalence of thyroid carcinoma and ultrasound features. Medicine (Baltimore) 2017;96:e8689. [Crossref] [PubMed]
- Zhou J, Song Y, Zhan W, et al. Thyroid imaging reporting and data system (TIRADS) for ultrasound features of nodules: multicentric retrospective study in China. Endocrine 2021;72:157-70. [Crossref] [PubMed]
- Ha EJ, Chung SR, Na DG, et al. 2021 Korean Thyroid Imaging Reporting and Data System and Imaging-Based Management of Thyroid Nodules: Korean Society of Thyroid Radiology Consensus Statement and Recommendations. Korean J Radiol 2021;22:2094-123. [Crossref] [PubMed]
- Tessler FN, Middleton WD, Grant EG. Thyroid Imaging Reporting and Data System (TI-RADS): A User's Guide. Radiology 2018;287:29-36. [Crossref] [PubMed]
- Tessler FN, Middleton WD, Grant EG, et al. ACR Thyroid Imaging, Reporting and Data System (TI-RADS): White Paper of the ACR TI-RADS Committee. J Am Coll Radiol 2017;14:587-95. [Crossref] [PubMed]
- Russ G, Bonnema SJ, Erdogan MF, et al. European Thyroid Association Guidelines for Ultrasound Malignancy Risk Stratification of Thyroid Nodules in Adults: The EU-TIRADS. Eur Thyroid J 2017;6:225-37. [Crossref] [PubMed]
- Gharib H, Papini E, Garber JR, et al. American Association Of Clinical Endocrinologists, American College Of Endocrinology, And Associazione Medici Endocrinologi Medical Guidelines For Clinical Practice For The Diagnosis And Management Of Thyroid Nodules--2016 Update. Endocr Pract 2016;22:622-39. [Crossref] [PubMed]
- Zhou J, Yin L, Wei X, et al. 2020 Chinese guidelines for ultrasound malignancy risk stratification of thyroid nodules: the C-TIRADS. Endocrine 2020;70:256-79. [Crossref] [PubMed]
- Peng S, Liu Y, Lv W, et al. Deep learning-based artificial intelligence model to assist thyroid nodule diagnosis and management: a multicentre diagnostic study. Lancet Digit Health 2021;3:e250-9. [Crossref] [PubMed]
- Li X, Zhang S, Zhang Q, et al. Diagnosis of thyroid cancer using deep convolutional neural network models applied to sonographic images: a retrospective, multicohort, diagnostic study. Lancet Oncol 2019;20:193-201. [Crossref] [PubMed]
- Choi YJ, Baek JH, Park HS, et al. A Computer-Aided Diagnosis System Using Artificial Intelligence for the Diagnosis and Characterization of Thyroid Nodules on Ultrasound: Initial Clinical Assessment. Thyroid 2017;27:546-52. [Crossref] [PubMed]
- Gao Y, Wang W, Yang Y, et al. An integrated model incorporating deep learning, hand-crafted radiomics and clinical and US features to diagnose central lymph node metastasis in patients with papillary thyroid cancer. BMC Cancer 2024;24:69. [Crossref] [PubMed]
- Zhou T, Hu T, Ni Z, et al. Comparative analysis of machine learning-based ultrasound radiomics in predicting malignancy of partially cystic thyroid nodules. Endocrine 2024;83:118-26. [Crossref] [PubMed]
- Zhao HN, Liu JY, Lin QZ, et al. Partially cystic thyroid cancer on conventional and elastographic ultrasound: a retrospective study and a machine learning-assisted system. Ann Transl Med 2020;8:495. [Crossref] [PubMed]
- Zhou T, Huang H, Dong H, et al. Ultrasound-Based Risk Stratification System for the Assessment of Partially Cystic Thyroid Nodules. Endocr Pract 2023;29:428-35. [Crossref] [PubMed]
- Wang CY, Li Y, Zhang MM, et al. Analysis of Differential Diagnosis of Benign and Malignant Partially Cystic Thyroid Nodules Based on Ultrasound Characterization With a TIRADS Grade-4a or Higher Nodules. Front Endocrinol (Lausanne) 2022;13:861070. [Crossref] [PubMed]
- Song Q, Tian X, Jiao Z, et al. Value of Conventional Ultrasonography with Contrast-Enhanced Ultrasonography in the Differential Diagnosis of Partial Cystic Thyroid Nodules. Ultrasound Med Biol 2021;47:2494-501. [Crossref] [PubMed]
- Fang F, Gong Y, Liao L, et al. Value of Contrast-Enhanced Ultrasound in Partially Cystic Papillary Thyroid Carcinomas. Front Endocrinol (Lausanne) 2021;12:783670. [Crossref] [PubMed]
- Lee YJ, Kim JY, Na DG, et al. Malignancy risk of thyroid nodules with minimal cystic changes: a multicenter retrospective study. Ultrasonography 2022;41:670-7. [Crossref] [PubMed]
- Liu Y, Zhao Y, Fu J, et al. Ultrasonographic differentiation and Ultrasound-based management of partially cystic thyroid nodules. Arch Endocrinol Metab 2021;65:336-41. [Crossref] [PubMed]
- Shi YZ, Jin Y, Zheng L. Partially cystic thyroid nodules on ultrasound: The associated factors for malignancy. Clin Hemorheol Microcirc 2020;74:373-81. [Crossref] [PubMed]
- Hu X, Gong J, Zhou W, et al. Computer-aided diagnosis of ground glass pulmonary nodule by fusing deep learning and radiomics features. Phys Med Biol 2021;66:065015. [Crossref] [PubMed]




