
The role of robot-assisted mastectomy: promise, challenges, and evidence gaps
Introduction
Robot-assisted mastectomy was first introduced by Toesca et al. in 2017 (1), marking a significant milestone in minimally invasive breast surgery. Robot-assisted nipple-sparing mastectomy (RNSM) has since emerged as a potential alternative to conventional nipple-sparing mastectomy (CNSM) with the potential benefits of minimal scarring hidden in the axillary region. Systems like the multi-port Da Vinci Xi and the single-port Da Vinci SP have been utilized, with the latter offering improved ergonomics for small-cavity procedures. The Da Vinci SP system, equipped with an articulating robotic camera and three 6-mm, double-jointed, articulated instruments, enables precise visualization of the surgical dissection field and enhances accuracy and efficiency across various specialties (2).
Robotic technology, with its high-resolution three-dimensional (3D) imaging and precise, flexible instruments, was naturally extended to breast cancer surgery after its success in urology, gynecology, and colorectal surgery. It was anticipated to offer minimal scarring and precise flap dissection along the superficial fascial layer through a remote incision. However, as the breast is predominantly superficial and lacks the superficial fascia in more than 40% of cases, unique challenges arise (3). Achieving consistent skin flap thickness is particularly difficult in larger breasts due to variations in tissue density and volume. Robotic tools and indirect access may complicate implant symmetry, nipple-areola complex (NAC) positioning, and adaptation to complex anatomies. Conversely, CNSM provides greater tactile control and flexibility, though it may result in more visible scars and variable flap quality. The aesthetically pleasing periareolar incision used in CNSM provides adequate access to all regions of the breast and axilla (4). However, robot-assisted mastectomy may enhance access to the upper pole of the breast when using inframammary incisions, particularly in larger breasts where traditional retractors are less effective. This potential benefit of RNSM warrants validation in future studies.
Current evidence
Evidence supporting RNSM remains sparse and inconclusive. The only published randomized controlled trial (RCT) to date, involving 69 patients with breast cancer, reported no significant difference in complications but found higher patient satisfaction scores for RNSM. However, operative times were extended by 78 minutes compared to CNSM (5). The small sample size limits the ability to draw meaningful conclusions.
Concerns about oncological safety arise from longer operative durations (5), which could influence tumor microenvironment dynamics and promote adverse outcomes. Patient-reported satisfaction scores, without blinding, are prone to biases such as expectation bias, the novelty effect, and self-justification. A prospectively designed multicenter trial involving 73 RNSM procedures demonstrated that RNSM was a safe alternative to CNSM in terms of perioperative morbidities, with a trend toward improved wound healing (6). Patient-reported outcomes regarding aesthetics and quality of life indicated parity between the approaches, though RNSM incurred higher costs, averaging $4,000 more per procedure (6).
Notably, the study (6) used unconventional incisions for CNSM, predominantly along the lateral breast, differing from standard practices in advanced centers (4).
A meta-analysis of 13,866 RNSM cases found no significant difference in postoperative complications compared to CNSM, including wound infection, hematoma, skin and nipple necrosis, and implant loss (7). A smaller meta-analysis confirmed similar findings but noted longer hospitalization, reduced blood loss, and a lower incidence of nipple necrosis in RNSM. However, the authors observed a trend toward a higher incidence of positive surgical margins in RNSM (8). In centers with advanced expertise, nipple necrosis rates in CNSM are significantly lower than the reported (14.2%) in this meta-analysis. We previously reported a 0% incidence of nipple necrosis and a 3.2% overall complication rate in CNSM with immediate implant-based reconstruction using a hemi periareolar incision (4). Patients reported satisfaction scores with the aesthetic outcomes were excellent (4). Figure 1 showcases examples of postoperative outcomes of CNSM at various stages of follow-up in patients with differing breast sizes. The results highlight excellent symmetry and aesthetically pleasing periareolar scars, even when positioned on the dome of the breast (9).
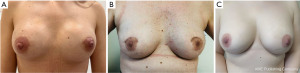
The current evidence, drawn from a single RCT and meta-analyses of retrospective and prospective studies, suggests that RNSM offers no clear advantages over CNSM regarding postoperative complications. This is despite longer operating times, higher costs, and limited oncological follow-up (typically 2–3 years) (5). The predominance of younger women with smaller cup sizes and early-stage disease in RNSM groups introduces selection bias, potentially skewing outcomes. Additionally, the majority of RNSM studies have been conducted in South Korea and Taiwan, where surgeons have pioneered the technique and accumulated substantial experience with robotic platforms. This concentration of expertise within specific regions may limit the generalizability of findings to diverse healthcare settings (8).
Challenges and limitations
RNSM has yet to demonstrate clear advantages over CNSM. The procedure entails higher costs due to the expensive robotic systems and disposable instruments (6). Cost-effectiveness is an increasingly critical consideration for healthcare systems striving for financial sustainability. Extended operative times increase risks associated with prolonged anesthesia, including potential complications and adverse oncological outcomes (8). Prolonged operative times increase surgical stress, potentially triggering systemic inflammation that can contribute to tumor progression and recurrence. This stress can directly alter the tumor microenvironment, accelerating micro metastatic growth. Additionally, prolonged surgery can enhance tumor cell dissemination and immune evasion, facilitating metastasis. Minimizing operative times and surgical stress is crucial to reducing these risks, though further research is needed to clarify the mechanisms and develop mitigation strategies (10,11). However, this underscores the need for long term oncological safety data for RNSM prior to routine adoption.
While minimal scarring is an advantage, challenges with symmetry and the viability of NAC positioning may reduce patient satisfaction, especially in patients with larger breasts. RNSM in such patients requires meticulous planning and potentially modified techniques to mitigate risks. Figure 2 presents the early postoperative photograph of a patient who underwent robot-assisted mastectomy, as reported in academic publications. The image demonstrates good symmetry and the absence of visible surgical scars on the breast (12). Moreover, the lateral component of the dissection is often performed blindly through the axillary incision, using scissors or finger dissection, raising concerns regarding precision. The most important the sensory supplied to the nipple travels from the lateral aspect of the breast via the facial plane.
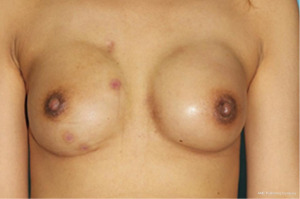
The learning curve for RNSM is steep, with significant improvements in operative time and complication rates observed after 10–15 cases. This curve is influenced by factors such as the surgeon’s familiarity with robotic systems and experience with traditional mastectomy techniques (13).
Two ongoing RCTs are designed to further assess the performance of RNSM in comparison to CNSM. NCT05720039 is a multi-center, two-arm, prospective study comparing RSNM to open nipple-sparing mastectomy (NSM). This investigation focuses on the oncological safety, surgical outcomes, and patient-reported experiences with the da Vinci SP Surgical System in 150 participants (14). Another study, NCT03440398, titled “Robotic vs Conventional Nipple-Sparing Mastectomy” is a prospective randomized trial aimed at evaluating patient satisfaction while comparing RNSM with immediate robotic breast reconstruction to the traditional open approach (15).
While promising, the small sample sizes of these trials emphasize the need for larger, high-quality studies. Selection criteria, perception bias due to non-blinding and variability in surgeon experience remain inherent limitations. Furthermore, the procedure is the focus of several ongoing prospective registry studies. The Mastectomy with Reconstruction Including Robotic Endoscopic Surgery (MARRES) study is a multi-institutional cohort that prospectively collects data on patients undergoing mastectomy and reconstruction (16).
Ethical and practical concerns
The lack of evidence supporting long-term oncological safety and clear benefits over CNSM raises ethical concerns about promoting RNSM for its aesthetic appeal. Axillary incisions marketed as “hidden” are anatomically part of the torso and may still be visible. Periareolar scars from CNSM are typically minimal and well-tolerated, as patients prioritize outcomes like breast contour and symmetry over minor scarring (4). RNSM adoption must be guided by robust evidence especially regarding long-term oncological safety, which is currently lacking.
Ongoing prospective registry trials (14-16) face limitations such as selection bias, heterogeneity in techniques, and incomplete data capture, often focusing on short-term outcomes while neglecting long-term oncological safety. There are concerns about surgeons performing RNSM outside clinical trials without a Food and Drug Administration (FDA)-approved Investigational Device Exemption (IDE). These issues, compounded by inferior outcomes in minimally invasive radical hysterectomy for cervical cancer (17), prompted the FDA to issue safety communications cautioning against robotic platforms for mastectomy outside research settings. In parallel, on 17 April 2025, the National Institute for Health and Care Excellence (NICE) published new early value assessment guidance recommending that robotic-assisted surgery for soft tissue procedures such as RNSM, be adopted within structured evidence-generation frameworks. Although we support the FDA and NICE position statements, we believe that RNSM may be a viable option for risk-reducing surgery in breast cancer-naive women with high-penetrance pathogenic mutations and those diagnosed with non-invasive breast cancer, provided it is performed by experienced surgeons and patients make an informed decision, accepting the longer operative times and higher costs involved (9).
Endoscopic-assisted mastectomy is another form of minimally invasive surgery that has been in use longer than robot-assisted mastectomy and is currently the focus of prospective studies such as MARRES. This study aims to provide evidence on surgical and oncologic outcomes, as well as patient satisfaction, comparing RNSM and endoscopic NSM with CNSM (18).
Conclusions
Robot-assisted mastectomy is a promising yet experimental surgical option, offering satisfactory short-term outcomes. Limited evidence supports its clinical benefits and oncological safety, while high costs, prolonged operative times, and aesthetic challenges hinder its routine adoption. Until robust data from large-scale trials confirm its long-term oncological safety and cost-effectiveness, therapeutic RNSM for breast cancer should be restricted to research settings. CNSM remains the gold standard, delivering excellent oncological and aesthetic outcomes backed by decades of evidence-based practice.
Acknowledgments
None.
Footnote
Provenance and Peer Review: This article was a standard submission to the journal. The article has undergone external peer review.
Peer Review File: Available at https://gs.amegroups.com/article/view/10.21037/gs-2025-16/prf
Funding: None.
Conflicts of Interest: Both authors have completed the ICMJE uniform disclosure form (available at https://gs.amegroups.com/article/view/10.21037/gs-2025-16/coif). K.M. received research grant provided by Breast Cancer Charity, consulting fees from QMedical and Sebbin, and honoraria for offering academic and clinical advice to Merit Medical and QMedical corporations. He is a fractional shareholder of Datar Genetics stock. Furthermore, he owns shares in HCA Healthcare UK and OncoBotanica. The other author has no conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved. Patients provided consent for the use of clinical photographs.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Toesca A, Peradze N, Galimberti V, et al. Robotic Nipple-sparing Mastectomy and Immediate Breast Reconstruction With Implant: First Report of Surgical Technique. Ann Surg 2017;266:e28-30. [Crossref] [PubMed]
- Lee SB, Kim J, Chung IY, et al. Use of the Da Vinci SP surgical system in robot-assisted nipple-sparing mastectomy: a single-center, retrospective study. Sci Rep 2025;15:12. [Crossref] [PubMed]
- Beer GM, Varga Z, Budi S, et al. Incidence of the superficial fascia and its relevance in skin-sparing mastectomy. Cancer 2002;94:1619-25. [Crossref] [PubMed]
- El Hage Chehade H, Headon H, Wazir U, et al. Nipple-sparing mastectomy using a hemi-periareolar incision with or without minimal medial-lateral extensions; clinical outcome and patient satisfaction: A single centre prospective observational study. Am J Surg 2017;213:1116-24. [Crossref] [PubMed]
- Toesca A, Sangalli C, Maisonneuve P, et al. A Randomized Trial of Robotic Mastectomy Versus Open Surgery in Women With Breast Cancer or BrCA Mutation. Ann Surg 2022;276:11-9. [Crossref] [PubMed]
- Lai HW, Chen DR, Liu LC, et al. Robotic Versus Conventional or Endoscopic-assisted Nipple-sparing Mastectomy and Immediate Prosthesis Breast Reconstruction in the Management of Breast Cancer: A Prospectively Designed Multicenter Trial Comparing Clinical Outcomes, Medical Cost, and Patient-reported Outcomes (RCENSM-P). Ann Surg 2024;279:138-46. [Crossref] [PubMed]
- Filipe MD, de Bock E, Postma EL, et al. Robotic nipple-sparing mastectomy complication rate compared to traditional nipple-sparing mastectomy: a systematic review and meta-analysis. J Robot Surg 2022;16:265-72. [Crossref] [PubMed]
- Nessa A, Shaikh S, Fuller M, et al. Postoperative complications and surgical outcomes of robotic versus conventional nipple-sparing mastectomy in breast cancer: meta-analysis. Br J Surg 2024;111:znad336. [Crossref] [PubMed]
- Rizk M, Mokbel K. Robotic Nipple-Sparing Mastectomy: A Critical Appraisal. Clin Breast Cancer 2025;25:e328-9. [Crossref] [PubMed]
- Tohme S, Simmons RL, Tsung A. Surgery for Cancer: A Trigger for Metastases. Cancer Res 2017;77:1548-52. [Crossref] [PubMed]
- Onuma AE, Zhang H, Gil L, et al. Surgical Stress Promotes Tumor Progression: A Focus on the Impact of the Immune Response. J Clin Med 2020;9:4096. [Crossref] [PubMed]
- Park HS, Lee J, Lee H, et al. Development of Robotic Mastectomy Using a Single-Port Surgical Robot System. J Breast Cancer 2020;23:107-12. [Crossref] [PubMed]
- Lai HW, Wang CC, Lai YC, et al. The learning curve of robotic nipple sparing mastectomy for breast cancer: An analysis of consecutive 39 procedures with cumulative sum plot. Eur J Surg Oncol 2019;45:125-33. [Crossref] [PubMed]
- ClinicalTrials.gov. Robotic-assisted nipple-sparing mastectomy trial. Available online: https://clinicaltrials.gov/ct2/show/NCT05720039 [accessed 10 January 2025].
- ClinicalTrials.gov. Robotic vs conventional nipple-sparing mastectomy. Available online: https://clinicaltrials.gov/study/NCT03440398 [accessed 10 January].
- ClinicalTrials.gov. Prospective study of mastectomy with reconstruction including robot endoscopic surgery (MARRES). Available online: https://clinicaltrials.gov/ct2/show/NCT04585074 [accessed 10 January 2025].
- Ramirez PT, Frumovitz M, Pareja R, et al. Minimally Invasive versus Abdominal Radical Hysterectomy for Cervical Cancer. N Engl J Med 2018;379:1895-904. [Crossref] [PubMed]
- Ryu JM, Lee J, Lee J, et al. Mastectomy with Reconstruction Including Robotic Endoscopic Surgery (MARRES): a prospective cohort study of the Korea Robot-Endoscopy Minimal Access Breast Surgery Study Group (KoREa-BSG) and Korean Breast Cancer Study Group (KBCSG). BMC Cancer 2023;23:571. [Crossref] [PubMed]


