
Serum thyroid-stimulating hormone as a diagnostic marker for cancer in atypia of undetermined significance/follicular lesion of undetermined significance nodules
Highlight box
Key findings
• The preoperative level of thyroid-stimulating hormone (TSH) may be a useful diagnostic marker for ruling in rather than ruling out malignancy in thyroid nodules diagnosed as atypia of undetermined significance/follicular lesion of undetermined significance (AUS/FLUS).
What is known and what is new?
• The current literature has conflicting reports regarding the utility of TSH levels for predicting cancer in AUS/FLUS nodules.
• The current study confirmed that elevated TSH levels were associated with a higher risk of malignancy and can be used as a predictive marker.
What is the implication, and what should change now?
• Measuring preoperative TSH levels is a simple adjunct test that may help guide endocrine surgeons in developing a personalized treatment approach for patients with AUS/FLUS thyroid nodules.
Introduction
Background
The prevalence of thyroid cancer (TC) has markedly increased in recent years. This malignancy can manifest clinically as a nodule, which cannot be differentiated from benign lesions (1). In addition to clinical and radiological parameters, the management of thyroid nodules (TNs) is based on cytological assessments, as outlined in the Bethesda System for Reporting Thyroid Cytopathology (TBSRTC) (2). Although fine-needle aspiration cytology (FNAC) is a safe and informative technique for TN assessment, some FNACs have uncertain cytology, and choosing the most appropriate treatment remains challenging (3).
The risk of malignancy (ROM) in TNs diagnosed as atypia of undetermined significance/follicular lesion of undetermined significance (AUS/FLUS), also known as Bethesda III, has been reported to be between 5% and 15% (4). However, the ROM ranges between 6% and 76% in surgically resected nodules (1). Most AUS/FLUS nodules are excised solely in the presence of concerning clinical or sonographic characteristics, an aberrant result on repeated aspiration, or an unfavorable outcome on molecular testing. In contrast, AUS/FLUS nodules that show a benign result on repeated FNAC and/or a benign outcome on molecular testing are not subjected to surgical removal (2).
Research is ongoing to identify a simple adjunct test for stratifying the ROM in cytologically indeterminate thyroid nodules (CITNs). Thyroid-stimulating hormone (TSH), which regulates thyroid function and hormone production, is of particular interest. Measuring serum TSH levels is essential for the biochemical evaluation of individuals with TNs as it is a valuable marker for detecting thyroid dysfunction (5). High TSH levels are known to cause the initiation and progression of differentiated TC, even if the levels are within the normal range (6-8). This may be mediated by TSH receptors, which can be found on cancer cell membranes and activate pathophysiological processes, stimulating TC growth (9).
Rationale and knowledge gap
Few studies have examined the role of TSH as a potential indicator of TC in CITNs, demonstrating the usefulness of TSH as a simple adjunct test while attempting to determine an appropriate cutoff value. Such a threshold can be utilized to predict the ROM in patients with TNs that have undergone surgical intervention (1,9-12). In contrast, other studies have found no correlation between TSH levels and malignant TNs (13-15). In addition to TSH levels, other biomarkers such as anti-thyroid antibodies (thyroid peroxidase and thyroglobulin antibodies) have been utilized as diagnostic and prognostic tools in CITNs (11).
Objective
This study aimed to ascertain whether serum TSH levels may serve as a potential indicator for TC in AUS/FLUS TNs. We present this article in accordance with the STARD reporting checklist (available at https://gs.amegroups.com/article/view/10.21037/gs-2024-520/rc).
Methods
This study is a retrospective analysis of the patients included in our previous report (16); patients with a primary cytological diagnosis of AUS/FLUS who underwent thyroidectomy between January 2011 and December 2014 at King Faisal Specialist Hospital & Research Centre, Riyadh, Saudi Arabia were enrolled. Exclusion criteria included a final diagnosis of lymphoma (n=2) and unavailable preoperative TSH levels (n=4), resulting in a final cohort of 109 patients. Clinical and radiological data were collected from the medical records, and several factors were assessed, including age, sex, ultrasound features, preoperative serum TSH levels, and final pathological results (benign vs. differentiated TC). The final pathological diagnosis was determined based on histopathological examination of surgical specimens using the World Health Organization criteria. The study was conducted in accordance with the Declaration of Helsinki and its subsequent amendments and approved by the Office of Research Affairs at King Faisal Specialist Hospital & Research Centre, Riyadh, Saudi Arabia (No. 2245263; date of approval, 29 May 2024). Patient consent was waived due to anonymous collection of patient data.
TSH levels
TSH levels were measured preoperatively with an electrochemiluminescence immunoassay (Roche Corporation, Indianapolis, IN, USA). The assay was performed according to the manufacturer’s instructions, and TSH levels were categorized into four quartiles: 0.02–0.96, 0.97–2.0, 2.01–3.0, and 3.01–4.59 mIU/L.
Statistical analysis
We used SPSS (version 26; IBM, Armonk, NY, USA) for all calculations. All statistical analyses were two-tailed, with P<0.05 considered significant. Categorical variables are described as frequencies with percentages, whereas quantitative data are displayed as mean ± standard deviation or median and interquartile range (Q1–Q3), based on the distribution of the data. The normality of the distribution was determined using the Shapiro-Wilk test. The study groups (benign vs. malignant nodules) were compared using Fisher’s exact, Student’s t-, and the Chi-squared tests for normally distributed quantitative data and the Mann-Whitney U test for skewed quantitative data. Receiver operating characteristic (ROC) curves were constructed, and the area under the ROC curve (AUC), accuracy, specificity, sensitivity, and positive and negative predictive values were calculated to evaluate the performance of TSH as a diagnostic marker. The optimal diagnostic cutoff for TSH levels was determined using the Youden index, which maximizes sensitivity and specificity. Multiple logistic regression was utilized to identify predictors of TC, expressed as adjusted odds ratios (ORs) and 95% confidence intervals (CIs).
The clinical information and reference standard results were available to the performers/readers of the index test.
Results
Of the 109 patients that met the inclusion criteria, 48 (44%) had malignant pathology; 45 were diagnosed with papillary TC (PTC) and 3 with follicular TC (Figure 1). Table 1 lists the demographic, clinical, and ultrasound characteristics; only sex, the presence of a peripheral halo, and TSH levels were significantly different between the benign and malignant groups. In total, 70.8% and 83.6% of the malignant and benign nodules, respectively, were diagnosed in women (P=0.05). Additionally, 60.4% of malignant nodules had a peripheral halo, whereas only 47.5% of benign nodules showed this characteristic (P=0.05). The median TSH level was 2.32 (range, 0.02–16.1) mIU/L for patients with malignant nodules and 1.60 (range, 0.02–31.2) mIU/L for patients with benign nodules (P=0.04).
Table 1
| Factor | Total | Pathology | P value | |
|---|---|---|---|---|
| Benign | Malignant | |||
| Age (years), mean ± SD [range] | 41.2±11.6 [15–71] | 41.9±11.3 [16–68] | 40.3±12.0 [15–71] | 0.46† |
| Sex | 0.05* | |||
| Male | 24 (22.0) | 10 (16.4) | 14 (29.2) | |
| Female | 85 (78.0) | 51 (83.6) | 34 (70.8) | |
| Content | 0.52‡ | |||
| Solid | 74 (67.9) | 40 (65.6) | 34 (70.8) | |
| Predominantly solid (>50% solid) | 27 (24.8) | 15 (24.6) | 12 (25.0) | |
| Predominantly cyst-like (>50% cystic) | 8 (7.3) | 6 (9.8) | 2 (4.2) | |
| Shape | 0.77 | |||
| Ovoid | 71 (65.1) | 39 (63.9) | 32 (66.7) | |
| Irregular | 38 (34.9) | 22 (36.1) | 16 (33.3) | |
| Margins | 0.44 | |||
| Smooth | 66 (60.6) | 35 (57.4) | 31 (64.6) | |
| Ill-defined | 43 (39.4) | 26 (42.6) | 17 (35.4) | |
| Size (mm) | 0.95† | |||
| Range | 6–130 | 6–100 | 7–130 | |
| Mean ± SD | 34.5±23.0 | 34.4±21.1 | 34.6±25.4 | |
| Echogenicity | 0.28 | |||
| Hypoechoic | 60 (55.0) | 34 (55.7) | 26 (54.2) | |
| Isoechoic | 17 (15.6) | 12 (19.7) | 5 (10.4) | |
| Hyperechoic | 32 (29.4) | 15 (24.6) | 17 (35.4) | |
| Calcification | 0.50 | |||
| Yes | 24 (22.0) | 12 (19.7) | 12 (25.0) | |
| No | 85 (78.0) | 49 (80.3) | 36 (75.0) | |
| Echotexture | 0.21 | |||
| Homogenous | 45 (41.3) | 22 (36.1) | 23 (47.9) | |
| Heterogenous | 64 (58.7) | 39 (63.9) | 25 (52.1) | |
| Vascularity | 0.96 | |||
| Hypervascular | 61 (56.0) | 34 (55.7) | 27 (56.3) | |
| Hypovascular | 48 (44.0) | 27 (44.3) | 21 (43.8) | |
| Lymphadenopathy | 0.72‡ | |||
| Yes | 8 (7.3) | 4 (6.6) | 4 (8.3) | |
| No | 101 (92.7) | 57 (93.4) | 44 (91.7) | |
| Peripheral halo | 0.05* | |||
| Yes | 58 (53.2) | 29 (47.5) | 29 (60.4) | |
| No | 51 (46.8) | 32 (52.5) | 19 (39.6) | |
| TSH (mIU/L), median (range) | 2.03 (0.02–31.2) | 1.60 (0.02–31.2) | 2.32 (0.02–16.1) | 0.04*§ |
Data are presented as n (%) unless otherwise specified. *, P<0.05. P values were calculated using the Pearson χ2 test, unless otherwise noted. †, independent samples t-test; ‡, exact probability test; §, Mann-Whitney U test. AUS/FLUS, atypia of undetermined significance/follicular lesion of undetermined significance; SD, standard deviation; TSH, thyroid-stimulating hormone.
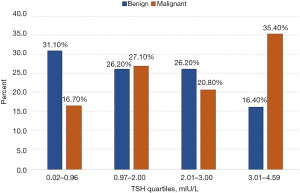
Figure 2 displays the distribution of TSH quartiles based on nodule pathology. Patients with malignant TNs were significantly more likely to have TSH levels in the highest quartile than those with benign pathology; patients with benign nodules were significantly more likely to have serum TSH levels in the lowest quartile than those with malignant pathology (P=0.05).
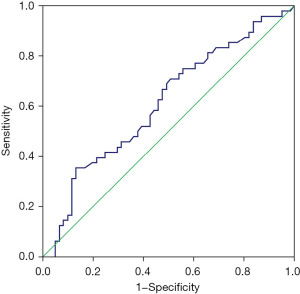
As shown in Table 2 and Figure 3, the ROC analysis demonstrated that TSH levels showed average potential as a marker for the presence of TC, with an AUC of 0.61 (95% CI: 0.51–0.72; P=0.04). The optimal diagnostic cut-off value was ≥3.06 mIU/L, with a sensitivity of 35% and specificity of 87%, indicating that TSH was more useful as a marker to rule in rather than rule out TC.
Table 2
| ROC statistics | Values |
|---|---|
| AUC (95% CI) | 0.61 (0.51–0.72) |
| P value | 0.04* |
| Youden index cut-off | ≥3.06 mIU/L |
| Sensitivity | 35% |
| Specificity | 87% |
| PPV | 84% |
| NPV | 41% |
*, P<0.05. AUC, area under the curve; CI, confidence interval; NPV, negative predictive value; PPV, positive predictive value; ROC, receiver operating characteristic; TSH, thyroid-stimulating hormone.
Multiple logistic regression analysis was performed to determine predictors of TC in AUS/FLUS TNs (Table 3). Of the parameters considered, only TSH levels were associated with the ROM, with a 12% increase in risk at higher TSH levels (OR, 1.12; 95% CI: 1.014–2.047; P=0.05).
Table 3
| Factor | P value | AOR | 95% CI | |
|---|---|---|---|---|
| Lower | Upper | |||
| Age (years) | 0.28 | 0.979 | 0.942 | 1.017 |
| Female | 0.15 | 0.470 | 0.168 | 1.312 |
| TSH | 0.049* | 1.12 | 1.014 | 2.047 |
| Size (mm) | 0.84 | 1.002 | 0.984 | 1.021 |
| Predominantly solid | 0.67 | 1.251 | 0.441 | 3.548 |
| Predominantly cyst-like | 0.66 | 0.660 | 0.107 | 4.086 |
| Irregular shape | 0.69 | 1.268 | 0.401 | 4.015 |
| Ill-defined margin | 0.50 | 0.683 | 0.228 | 2.047 |
| Hyperechogenic echogenicity | 0.68 | 1.113 | 0.671 | 1.847 |
| Calcification | 0.27 | 1.800 | 0.634 | 5.115 |
| Heterogenous echotexture | 0.22 | 0.543 | 0.206 | 1.427 |
| Hypervascularity | 0.49 | 1.363 | 0.564 | 3.293 |
| Lymphadenopathy | 0.72 | 1.328 | 0.281 | 6.285 |
| Peripheral halo | 0.62 | 1.271 | 0.487 | 3.318 |
*, P<0.05. AOR, adjusted odds ratio; AUS/FLUS, atypia of undetermined significance/follicular lesion of undetermined significance; CI, confidence interval; TSH, thyroid-stimulating hormone.
Discussion
Key findings
In our previous studies, we found that clinical and radiological characteristics were ineffective predictors of cancer in AUS/FLUS nodules (16,17). Therefore, this study aimed to examine the utility of TSH levels as a simple biochemical marker for predicting TC in AUS/FLUS nodules. Our findings demonstrated that TSH levels were moderately effective as a diagnostic marker, with greater utility in ruling in malignancy than in ruling it out.
Strengths and limitations
One of the strengths of our study is that all the included patients had a final pathological diagnosis (benign vs. differentiated TC). Additionally, it is one of the few studies to explore the effectiveness of TSH levels as a cancer predictor in AUS/FLUS nodules. However, its limitations should be acknowledged and include the retrospective methodology and small sample size. Furthermore, as a single-institution study where only surgically resected nodules were included, selection bias was unavoidable. Lastly, our study did not include data on cancer stage, disease-free survival, or anti-thyroid antibody levels.
Comparison with similar researches
A prospective study demonstrated that, in conjunction with an individual’s age, sex, and specific type of goiter, the TSH level at the time of diagnosis was an independent predictor of TC, even if it fell within the normal range (18). Another study involving 236 patients without thyroid dysfunction revealed that mean TSH levels were lower in individuals with benign lesions than in those with lesions diagnosed as differentiated TC (5). Correspondingly, TSH suppression has been shown to be beneficial for individuals with differentiated TC (10). In addition, patients with higher TSH levels, even within the normal range, were found to have a heightened risk of developing differentiated TC; therefore, FNAC was recommended for TNs that fell within the biopsy threshold when TSH levels were elevated within the normal range (5).
Cappelli et al. found a correlation between TSH levels in the upper normal range and heightened ROM in patients with CITNs. They concluded that measuring serum TSH levels is a simple supplementary test to support decision-making in individuals with indeterminate nodules (10). Similarly, Amado et al. found a correlation between elevated TSH levels in CITNs and increased ROM (1). Therefore, TSH levels have the potential to become a useful diagnostic tool for stratifying ROM and assisting in the management of these nodules. In 2020, Adhami et al. reported that a TSH level ≥1 mIU/L and high levels of anti-thyroid antibodies were both associated with heightened ROM in patients with CITNs (11). In addition, TSH levels >2.185 mIU/mL have been reported as a reliable indicator of TC in CITNs. Our results identified a cut-off value of ≥3.06 mIU/L, which is higher than those previously reported. Table 4 compares the cut-off values for TSH found in different studies on CITNs.
Table 4
| Study | Cut-off value for TSH (mIU/L) | Cytological diagnosis |
|---|---|---|
| Cappelli et al. [2020] (10) | ≥2.7 | TIR3A and TIR3B |
| Adhami et al. [2020] (11) | ≥1 | Bethesda III, IV, or V |
| Amado et al. [2022] (1) | ≥2.68 | Bethesda III |
| Kaliszewski et al. [2022] (9) | 2.5 | Bethesda III |
| Vinod et al. [2022] (12) | >2.185 | Bethesda III |
| Present study [2024] | ≥3.06 | Bethesda III |
TSH, thyroid-stimulating hormone.
Conversely, Gudmundsson et al. showed that low TSH levels may elevate the risk of malignant cell transformation in the context of three distinct genetic variations found at 2q35, 8p12, and 14q13.3 (19). Furthermore, Castro et al. (20) showed that TSH levels did not predict ROM in cytologically suspicious TNs, although the ROM was elevated among patients who received thyroid hormone replacement therapy.
Explanations of findings
TSH is widely recognized to have a substantial influence on the proliferation of thyroid cells, and thyrotropin-activated signaling pathways are known to affect TC occurrence. Notably, a high TSH level, even if within the normal range, is correlated with an increased ROM (9). A study involving 27,914 patients found a strong correlation between the prevalence of PTC and serum TSH levels, with a lower frequency of PTC in individuals with TSH levels <0.4 µU/mL than in those with TSH levels >3.4 µU/mL (21). Furthermore, high TSH levels have been observed in advanced stages of TC (9) and correlate with poor disease-free survival in individuals with PTC (22). A recent study showed that patients who had TSH levels >4.5 mIU/L, together with specific ultrasound findings, demonstrated elevated ROM (23). Although the results obtained in this study did not reach statistical significance (23), similar findings have been reported in other studies (24,25).
Implications and actions needed
One of the challenges associated with ambiguous nodules is the potential for patients with benign conditions to undergo unnecessary surgical procedures, whereas patients with malignant conditions may not receive timely and optimal therapy (12). Repeated FNAC is recommended for CITNs in the updated versions of TBSRTC (2,26), and Jooya et al. demonstrated that repeated FNAC in CITNs can result in more definite categorization (27). The updated guidelines from the American Thyroid Association (2) and the latest version of TBSRTC identify molecular testing as a useful alternative (26); however, the limited accessibility of molecular testing in most facilities restricts its use.
The current literature remains contentious regarding the appropriate degree of thyroidectomy, highlighting the potential risks of both under- and over-treatment (3). Some investigators believe that total thyroidectomy is the best option for CITNs because it enables a comprehensive assessment of the entire thyroid gland. Conversely, other researchers argue that lobectomy alone is satisfactory for such patients (3). Despite this ongoing debate, adopting personalized approaches for therapy is generally advised, incorporating TSH levels with demographic, radiological, pathological, and molecular data (9,23,28).
For surgeons, the challenge of decision-making in the context of AUS/FLUS may be effectively addressed by assessing TSH levels. This simple measurement can provide valuable insights into the likelihood of malignancy and offers a cost-effective alternative to more expensive molecular testing methods, particularly when used in conjunction with clinical evaluations and ultrasound findings (12).
Importantly, the purpose of investigating the relationship between TSH and malignancy was not to use TSH levels as the only diagnostic indicator to determine the optimal therapeutic strategy for patients with AUS/FLUS nodules. Rather, together with patient characteristics, cytological findings, and ultrasound data, including TSH levels in the assessment process may allow for the classification of patients into high- or low-risk categories. This classification can help prevent unnecessary thyroidectomies and guide clinical decision-making.
Conclusions
In conclusion, elevated TSH levels are a moderately effective diagnostic marker for ROM in AUS/FLUS nodules. Therefore, other factors, including clinical evaluation, ultrasound findings, and cytological assessment, should also be considered. Evaluating additional biochemical tests, such as those for thyroid antibodies, may also be beneficial for the ongoing care of patients with these nodules. Furthermore, prospective multicenter studies are imperative to definitively identify TSH levels as a useful indicator for cancer and define the role of this analyte in the clinical management of CITNs.
Acknowledgments
The authors would like to thank the Deanship of Postgraduate Studies and Scientific Research at Majmaah University for supporting this work under the project number R-2025-1674. The research work should be credited to the Department of Surgery, King Faisal Specialist Hospital & Research Center, Riyadh, Saudi Arabia.
Footnote
Reporting Checklist: The authors have completed STARD reporting checklist. Available at https://gs.amegroups.com/article/view/10.21037/gs-2024-520/rc
Data Sharing Statement: Available at https://gs.amegroups.com/article/view/10.21037/gs-2024-520/dss
Peer Review File: Available at https://gs.amegroups.com/article/view/10.21037/gs-2024-520/prf
Funding: None.
Conflicts of Interest: All authors have completed the ICMJE uniform disclosure form (available at https://gs.amegroups.com/article/view/10.21037/gs-2024-520/coif). The authors have no conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved. The study was conducted in accordance with the Declaration of Helsinki and its subsequent amendments and approved by the Office of Research Affairs at King Faisal Specialist Hospital & Research Centre, Riyadh, Saudi Arabia (No. 2245263; date of approval, 29 May 2024). Patient consent was waived due to anonymous collection of patient data.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Amado A, Castro B, Torre AP, et al. Serum TSH as a predictor of malignancy in indeterminate thyroid nodules. Ann R Coll Surg Engl 2022;104:380-4. [Crossref] [PubMed]
- Cibas ES, Ali SZ. The 2017 Bethesda System for Reporting Thyroid Cytopathology. Thyroid 2017;27:1341-6. [Crossref] [PubMed]
- Alqahtani SM. Current controversies in the management of patients with indeterminate thyroid nodules. Saudi Med J 2023;44:633-9. [Crossref] [PubMed]
- Cibas ES, Ali SZ. The Bethesda System for Reporting Thyroid Cytopathology. Thyroid 2009;19:1159-65. [Crossref] [PubMed]
- Duccini K, de Souza MVL, Delfim R, et al. High Serum Thyrotropin Concentrations within the Reference Range: A Predictor of Malignancy in Nodular Thyroid Disease. Med Princ Pract 2018;27:272-7. [Crossref] [PubMed]
- Haymart MR, Repplinger DJ, Leverson GE, et al. Higher serum thyroid stimulating hormone level in thyroid nodule patients is associated with greater risks of differentiated thyroid cancer and advanced tumor stage. J Clin Endocrinol Metab 2008;93:809-14. [Crossref] [PubMed]
- Golbert L, de Cristo AP, Faccin CS, et al. Serum TSH levels as a predictor of malignancy in thyroid nodules: A prospective study. PLoS One 2017;12:e0188123. [Crossref] [PubMed]
- Rowe CW, Paul JW, Gedye C, et al. Targeting the TSH receptor in thyroid cancer. Endocr Relat Cancer 2017;24:R191-202. [Crossref] [PubMed]
- Kaliszewski K, Diakowska D, Rzeszutko M, et al. Assessment of Preoperative TSH Serum Level and Thyroid Cancer Occurrence in Patients with AUS/FLUS Thyroid Nodule Diagnosis. Biomedicines 2022;10:1916. [Crossref] [PubMed]
- Cappelli C, Pirola I, Gandossi E, et al. Could Serum TSH Levels Predict Malignancy in Euthyroid Patients Affected by Thyroid Nodules with Indeterminate Cytology? Int J Endocrinol 2020;2020:7543930. [Crossref] [PubMed]
- Adhami M, Michail P, Rao A, et al. Anti-Thyroid Antibodies and TSH as Potential Markers of Thyroid Carcinoma and Aggressive Behavior in Patients with Indeterminate Fine-Needle Aspiration Cytology. World J Surg 2020;44:363-70. [Crossref] [PubMed]
- Vinod A, Ramachandran R, Pillai AV, et al. Serum TSH Level as a Simple Efficient Tool to Assess the Risk of Thyroid Malignancy in Euthyroid Patients with Indeterminate Cytology - A Cohort Study. Indian J Endocrinol Metab 2022;26:446-52. [Crossref] [PubMed]
- Oh EM, Chung YS, Song WJ, et al. The utility of clinical findings including serum TSH and neck ultrasonography for predicting thyroid malignancy in atypia of undetermined significance/follicular lesions of undetermined significance. Korean J Endocr Surg 2013;13:144-50. [Crossref]
- Kuzu F, Arpaci D, Cakmak GK, et al. The value of blood cell markers in patients with thyroid nodules including atypia of undetermined significance/follicular lesion of undetermined significance cytology. Med-Science 2018;7:386-90. [Crossref]
- Al-Hakami HA, Altayyeb JF, Alsharif SM, et al. Preoperative Thyroid-Stimulating Hormone Levels and Risk of Thyroid Cancer in Post-thyroidectomy Patients for Thyroid Nodules: A Study From a Tertiary Hospital in Western Saudi Arabia. Cureus 2023;15:e47622. [Crossref] [PubMed]
- Alqahtani S, Alsobhi S, Alsalloum RI, et al. Surgical outcome of thyroid nodules with atypia of undetermined significance and follicular lesion of undetermined significance in fine needle aspiration biopsy. World J Endoc Surg 2017;9:100-3. [Crossref]
- Alqahtani SM, Al-Sobhi SS, Alturiqy MA, et al. The impact of thyroid imaging reporting and data system on the management of Bethesda III thyroid nodules. J Taibah Univ Med Sci 2023;18:506-11. [Crossref] [PubMed]
- Boelaert K, Horacek J, Holder RL, et al. Serum thyrotropin concentration as a novel predictor of malignancy in thyroid nodules investigated by fine-needle aspiration. J Clin Endocrinol Metab 2006;91:4295-301. [Crossref] [PubMed]
- Gudmundsson J, Sulem P, Gudbjartsson DF, et al. Discovery of common variants associated with low TSH levels and thyroid cancer risk. Nat Genet 2012;44:319-22. [Crossref] [PubMed]
- Castro MR, Espiritu RP, Bahn RS, et al. Predictors of malignancy in patients with cytologically suspicious thyroid nodules. Thyroid 2011;21:1191-8. [Crossref] [PubMed]
- Fiore E, Rago T, Provenzale MA, et al. L-thyroxine-treated patients with nodular goiter have lower serum TSH and lower frequency of papillary thyroid cancer: results of a cross-sectional study on 27 914 patients. Endocr Relat Cancer 2010;17:231-9. [Crossref] [PubMed]
- Xiang Y, Xu Y, Bhandari A, et al. Serum TSH levels are associated with postoperative recurrence and lymph node metastasis of papillary thyroid carcinoma. Am J Transl Res 2021;13:6108-16. [PubMed]
- Al Dawish M, Alwin Robert A, Al Shehri K, et al. Risk Stratification of Thyroid Nodules with Bethesda III Category: The Experience of a Territorial Healthcare Hospital. Cureus 2020;12:e8202. [Crossref] [PubMed]
- Zafon C, Obiols G, Baena JA, et al. Preoperative thyrotropin serum concentrations gradually increase from benign thyroid nodules to papillary thyroid microcarcinomas then to papillary thyroid cancers of larger size. J Thyroid Res 2012;2012:530721. [Crossref] [PubMed]
- Al Dawish MA, Alwin Robert A, Thabet MA, et al. Thyroid Nodule Management: Thyroid-Stimulating Hormone, Ultrasound, and Cytological Classification System for Predicting Malignancy. Cancer Inform 2018;17:1176935118765132. [Crossref] [PubMed]
- Ali SZ, Baloch ZW, Cochand-Priollet B, et al. The 2023 Bethesda System for Reporting Thyroid Cytopathology. Thyroid 2023;33:1039-44. [PubMed]
- Jooya A, Saliba J, Blackburn A, et al. The role of repeat fine needle aspiration in the management of indeterminate thyroid nodules. J Otolaryngol Head Neck Surg 2016;45:51. [Crossref] [PubMed]
- Pusztaszeri M, Rossi ED, Auger M, et al. The Bethesda System for Reporting Thyroid Cytopathology: Proposed Modifications and Updates for the Second Edition from an International Panel. Acta Cytol 2016;60:399-405.



