
A web-based predictive model for secondary skin infections in breast cancer patients undergoing reconstruction
Highlight box
Key findings
• In a retrospective study of 166 breast cancer patients undergoing implant-based reconstruction, body mass index (BMI), chemotherapy, and prosthesis thickness were identified as independent risk factors for postoperative skin infections. A predictive model was developed, showing strong discriminative ability with area under the curve (AUC) values of 0.87 and 0.812 in training and validation cohorts, respectively. A web-based calculator was created to facilitate easy risk assessment for clinicians.
What is known and what is new?
• Complications, including infections, can occur after implant-based breast reconstruction, influenced by factors such as age, BMI, smoking, and adjuvant therapies.
• This study introduces a novel predictive model specifically targeting postoperative skin infections, quantifying the impact of key risk factors and providing a practical tool for personalized risk assessment.
What is the implication, and what should change now?
• The predictive model enables clinicians to assess individual infection risk, potentially improving patient counseling and tailoring surgical and postoperative care. Integration of the web-based calculator into clinical practice could enhance decision-making. Further validation in larger, multi-center studies is recommended to confirm the model’s applicability across diverse populations. Clinicians should consider incorporating this risk assessment tool into preoperative evaluations to optimize patient outcomes.
Introduction
Breast cancer (BC) remains one of the most prevalent malignancies affecting women globally, with various treatment modalities available depending on the stage and characteristics of the disease (1,2). While targeted therapies, endocrine treatments, and chemotherapy have advanced significantly in recent years, surgical intervention remains the cornerstone of BC management (2-4). Surgical options primarily include breast-conserving surgery (lumpectomy) and mastectomy, with the choice often influenced by tumor size, location, and patient preference (5). Among these, mastectomy, the complete removal of the breast, is frequently employed to achieve local control of the disease. Following mastectomy, breast reconstruction, particularly prosthetic implant-based reconstruction, plays a vital role in restoring the physical appearance of the breast and enhancing the psychological well-being of patients (6,7). Although this method offers the advantage of immediate breast contour restoration and involves a less complex surgical procedure, the variability in outcomes due to patient-specific factors underscores the importance of understanding and predicting the risks associated with implant-based reconstruction (7,8).
Clinical and patient-specific factors significantly affect the success of breast implant surgery. Age, body mass index (BMI), comorbidities like diabetes, and lifestyle choices such as smoking increase the risk of complications like infections, delayed healing, and implant failure (9-11). Tumor characteristics and adjuvant therapies, such as chemotherapy or radiotherapy, also influence outcomes (12,13). Radiation therapy increases the risk of capsular contracture, where scar tissue forms around the implant, causing discomfort and aesthetic issues (14,15). Other common complications include seroma, implant displacement, and rupture, which can impact physical outcomes and patient satisfaction. These complexities highlight the need for thorough preoperative evaluations to minimize risks and create personalized treatment plans for better surgical results.
This study aims to address this gap by analyzing various clinical factors associated with BC patients undergoing prosthetic implant-based reconstruction. By utilizing demographic, oncological, and treatment-related data, we developed a predictive model to assess the likelihood of complications following implant surgery. This model provides personalized risk assessments, enabling clinicians to make more informed decisions and optimize surgical outcomes. Ultimately, this approach aims to improve patient care by minimizing complications and enhancing the overall success of breast reconstruction procedures for BC survivors. We present this article in accordance with the TRIPOD reporting checklist (available at https://gs.amegroups.com/article/view/10.21037/gs-24-470/rc).
Methods
Study patients and data collection
A retrospective study was conducted involving Chinese female BC patients treated in the Breast Surgery Department of The First Affiliated Hospital of Wenzhou Medical University between 2015 and 2021. The inclusion criteria were as follows: (I) BC patients aged 18 years or older; (II) patients who underwent unilateral mastectomy; (III) patients who received breast implant reconstruction following surgery. Exclusion criteria included: (I) patients with distant metastasis; (II) those who had undergone radiotherapy, chemotherapy, or endocrine therapy prior to surgery; (III) patients with incomplete or missing specific medical data; (IV) patients with concurrent malignant tumors. A total of 207 patients were initially considered for the study. After applying the inclusion and exclusion criteria, 166 patients were ultimately enrolled in the final cohort (Figure 1). The study was conducted in accordance with the Declaration of Helsinki and its subsequent amendments. Ethics approval was obtained from the Ethics Committee of the Breast Surgery Department at The First Affiliated Hospital of Wenzhou Medical University (No. KY2024-R27). The requirement for written informed consent was waived due to the retrospective nature of the study.
Data collection
Pathological information collected from each patient included the following: duration of hospitalization (in days), number of dressing changes, clinical staging, patient age (in years), BMI (kg/m2), histology type, lymph node metastasis count, chemotherapy, radiotherapy, targeted therapy, endocrine therapy, prosthesis volume (cm3), prosthesis thickness, use of mesh, use of tissue expander, postoperative inflammatory lymph nodes, smoking status, preoperative white blood cell count (WBC)/postoperative WBC, preoperative neutrophil/postoperative neutrophil, and skin infection status. Clinical staging was classified according to the American Joint Committee on Cancer tumor-node-metastasis (TNM) staging system. The pathological types of BC were classified into invasive ductal carcinoma (IDC), ductal carcinoma in situ (DCIS), and others. All BC patients received radiotherapy using intensity-modulated radiation therapy (IMRT). In our study, implant thickness was measured intraoperatively using a sterile digital caliper, and the measurement refers to the maximum anterior-posterior height (in cm) of the implant, as provided by the manufacturer and confirmed in the operating room (Figure S1). Postoperative skin infection was defined as a clinically diagnosed infectious complication localized to the surgical site following implant-based breast reconstruction. This includes confirmed local signs of infection such as erythema with purulent drainage, wound dehiscence with positive microbial culture, abscess formation, and implant exposure secondary to infection. Minor complications such as localized seroma without signs of infection, mild erythema resolving without antibiotics, or non-infectious itching were explicitly excluded from this definition.
Statistical analysis
Statistical analyses were conducted using IBM SPSS Statistics version 25.0 and R software version 4.1.2. Continuous variables were reported as means with standard deviations (SDs) or as medians with interquartile ranges, depending on the data distribution, while categorical data were presented as frequencies and percentages. To compare baseline characteristics between patient groups, the Mann-Whitney U test was applied for continuous variables, and the Pearson χ2 test was used for categorical variables. In the univariate analysis, variables with a P value of less than 0.05 were considered statistically significant and were subsequently included in a multivariate logistic regression model. This analysis aimed to identify independent risk factors for postoperative skin infection in BC patients who underwent prosthetic implantation. Significant variables from the multivariate analysis (P<0.05) were then selected to construct a nomogram using R software. The predictive accuracy and calibration of the nomogram were evaluated through receiver operating characteristic (ROC) curves, which assessed the model’s discriminative ability, and calibration curves, which tested the agreement between predicted and observed outcomes. To further assess the clinical utility of the nomogram, decision curve analysis (DCA) was employed, quantifying the net benefit across various probability thresholds. Finally, to simplify the practical application of the nomogram, a web-based dynamic calculator was developed, providing an accessible tool for clinicians to predict the likelihood of postoperative skin infections based on individual patient characteristics.
Results
Baseline characteristics of the patients
Baseline clinical characteristics of the patients are listed in Tables 1,2. Among the 166 patients included in the study, 30 patients (18.07%) developed postoperative skin infections, while the remaining 136 patients (81.93%) did not experience any infection. Patients with skin infections had a mean age of 44.6 years (SD: 9.69), compared to a mean age of 42.4 years (SD: 8.04) in those without infections. The BMI was also higher in the infected group, with a mean of 24.1 kg/m2 (SD: 2.69) versus 21.9 kg/m2 (SD: 2.35) in the non-infected group. Notably, patients with skin infections had a higher mean lymph node metastasis count [1.50 (range, 0–9)] compared to those without infections [0.816 (range, 0–17)]. Furthermore, a higher proportion of patients in the infected group received chemotherapy (80%) compared to the non-infected group (39.7%). Similarly, 60% of the patients with infections had undergone radiotherapy, whereas only 16.9% of those without infections received radiotherapy. These findings suggest that older age, higher BMI, a greater number of lymph node metastases, and the receipt of chemotherapy and radiotherapy are more common among patients who developed postoperative skin infections.
Table 1
| Variables | Infection status (no) (N=136) | Infection status (yes) (N=30) | Overall (N=166) |
|---|---|---|---|
| Duration of hospitalization (days) | |||
| Mean ± SD | 7.82±4.25 | 7.87±5.99 | 7.83±4.59 |
| Median [Min, Max] | 7.00 [2.00, 20.0] | 5.50 [2.00, 28.0] | 6.00 [2.00, 28.0] |
| Number of dressing changes | |||
| Mean ± SD | 2.41±1.49 | 3.13±3.50 | 2.54±2.01 |
| Median [Min, Max] | 2.00 [1.00, 8.00] | 2.00 [1.00, 17.0] | 2.00 [1.00, 17.0] |
| Clinical staging, n (%) | |||
| III | 13 (9.6) | 2 (6.7) | 15 (9.0) |
| IVA | 41 (30.1) | 12 (40.0) | 53 (31.9) |
| IVB | 52 (38.2) | 5 (16.7) | 57 (34.3) |
| IVC | 25 (18.4) | 10 (33.3) | 35 (21.1) |
| V | 5 (3.7) | 1 (3.3) | 6 (3.6) |
| Patient age (years) | |||
| Mean ± SD | 42.4±8.04 | 44.6±9.69 | 42.8±8.38 |
| Median [Min, Max] | 43.0 [25.0, 63.0] | 44.5 [28.0, 70.0] | 43.0 [25.0, 70.0] |
| Body mass index (kg/m2) | |||
| Mean ± SD | 21.9±2.35 | 24.1±2.69 | 22.3±2.55 |
| Median [Min, Max] | 21.7 [16.4, 32.1] | 23.7 [19.4, 29.9] | 22.2 [16.4, 32.1] |
| Histology type, n (%) | |||
| DCIS | 42 (30.9) | 6 (20.0) | 48 (28.9) |
| IDC | 85 (62.5) | 19 (63.3) | 104 (62.7) |
| Other | 9 (6.6) | 5 (16.7) | 14 (8.4) |
| Lymph node metastasis count | |||
| Mean ± SD | 0.816±2.06 | 1.50±2.16 | 0.940±2.09 |
| Median [Min, Max] | 0 [0, 17.0] | 0 [0, 9.00] | 0 [0, 17.0] |
| Chemotherapy, n (%) | |||
| No | 54 (39.7) | 4 (13.3) | 58 (34.9) |
| Yes | 82 (60.3) | 26 (86.7) | 108 (65.1) |
| Radiotherapy, n (%) | |||
| No | 113 (83.1) | 18 (60.0) | 131 (78.9) |
| Yes | 23 (16.9) | 12 (40.0) | 35 (21.1) |
| Targeted therapy, n (%) | |||
| No | 112 (82.4) | 26 (86.7) | 138 (83.1) |
| Yes | 24 (17.6) | 4 (13.3) | 28 (16.9) |
| Endocrine therapy, n (%) | |||
| No | 37 (27.2) | 11 (36.7) | 48 (28.9) |
| Yes | 99 (72.8) | 19 (63.3) | 118 (71.1) |
| Prosthesis volume (cm3) | |||
| Mean ± SD | 202±61.1 | 222±48.3 | 205±59.4 |
| Median [Min, Max] | 190 [100, 440] | 215 [155, 340] | 190 [100, 440] |
| Prosthesis thickness (mm) | |||
| Mean ± SD | 45.3±6.57 | 52.9±7.83 | 46.7±7.39 |
| Median [Min, Max] | 44.0 [30.0, 69.0] | 50.5 [41.0, 72.0] | 46.5 [30.0, 72.0] |
| Use of mesh, n (%) | |||
| No | 111 (81.6) | 19 (63.3) | 130 (78.3) |
| Yes | 25 (18.4) | 11 (36.7) | 36 (21.7) |
| Use of tissue expander, n (%) | |||
| No | 52 (38.2) | 13 (43.3) | 65 (39.2) |
| Yes | 84 (61.8) | 17 (56.7) | 101 (60.8) |
| Postoperative inflammatory lymph nodes, n (%) | |||
| No | 86 (63.2) | 14 (46.7) | 100 (60.2) |
| Yes | 50 (36.8) | 16 (53.3) | 66 (39.8) |
| Smoking status, n (%) | |||
| No | 112 (82.4) | 21 (70.0) | 133 (80.1) |
| Yes | 24 (17.6) | 9 (30.0) | 33 (19.9) |
| Preoperative WBC/postoperative WBC | |||
| Mean ± SD | 1.12±0.467 | 1.08±0.504 | 1.12±0.473 |
| Median [Min, Max] | 1.02 [0.510, 4.34] | 0.960 [0.232, 2.65] | 1.02 [0.232, 4.34] |
| Preoperative neutrophil/postoperative neutrophil | |||
| Mean ± SD | 1.46±2.48 | 1.20±1.01 | 1.41±2.29 |
| Median [Min, Max] | 1.09 [0.304, 29.0] | 1.01 [0.103, 5.93] | 1.06 [0.103, 29.0] |
DCIS, ductal carcinoma in situ; IDC, invasive ductal carcinoma; SD, standard deviation; WBC, white blood cell count.
Table 2
| Variables | Training (N=101) | Validation (N=65) | Overall (N=166) | P value |
|---|---|---|---|---|
| Duration of hospitalization (days) | 0.55 | |||
| Mean ± SD | 7.45±3.95 | 8.42±5.43 | 7.83±4.59 | |
| Median [Min, Max] | 6.00 [2.00, 18.0] | 7.00 [2.00, 28.0] | 6.00 [2.00, 28.0] | |
| Number of dressing changes | 0.39 | |||
| Mean ± SD | 2.29±1.31 | 2.94±2.73 | 2.54±2.01 | |
| Median [Min, Max] | 2.00 [1.00, 7.00] | 2.00 [1.00, 17.0] | 2.00 [1.00, 17.0] | |
| Clinical staging, n (%) | 0.27 | |||
| III | 7 (6.9) | 8 (12.3) | 15 (9.0) | |
| IVA | 35 (34.7) | 18 (27.7) | 53 (31.9) | |
| IVB | 30 (29.7) | 27 (41.5) | 57 (34.3) | |
| IVC | 25 (24.8) | 10 (15.4) | 35 (21.1) | |
| V | 4 (4.0) | 2 (3.1) | 6 (3.6) | |
| Patient age (years) | 0.06 | |||
| Mean ± SD | 41.9±8.09 | 44.1±8.70 | 42.8±8.38 | |
| Median [Min, Max] | 43.0 [25.0, 60.0] | 44.0 [28.0, 70.0] | 43.0 [25.0, 70.0] | |
| Body mass index (kg/m2) | 0.95 | |||
| Mean ± SD | 22.5±2.79 | 22.1±2.11 | 22.3±2.55 | |
| Median [Min, Max] | 22.2 [16.4, 32.1] | 22.0 [17.8, 27.7] | 22.2 [16.4, 32.1] | |
| Histology type, n (%) | 0.22 | |||
| DCIS | 33 (32.7) | 15 (23.1) | 48 (28.9) | |
| IDC | 58 (57.4) | 46 (70.8) | 104 (62.7) | |
| Other | 10 (9.9) | 4 (6.2) | 14 (8.4) | |
| Lymph node metastasis count | 0.99 | |||
| Mean ± SD | 0.802±1.57 | 1.15±2.70 | 0.940±2.09 | |
| Median [Min, Max] | 0 [0, 9.00] | 0 [0, 17.0] | 0 [0, 17.0] | |
| Chemotherapy, n (%) | >0.99 | |||
| No | 35 (34.7) | 23 (35.4) | 58 (34.9) | |
| Yes | 66 (65.3) | 42 (64.6) | 108 (65.1) | |
| Radiotherapy, n (%) | 0.76 | |||
| No | 81 (80.2) | 50 (76.9) | 131 (78.9) | |
| Yes | 20 (19.8) | 15 (23.1) | 35 (21.1) | |
| Targeted therapy, n (%) | 0.84 | |||
| No | 83 (82.2) | 55 (84.6) | 138 (83.1) | |
| Yes | 18 (17.8) | 10 (15.4) | 28 (16.9) | |
| Endocrine therapy, n (%) | 0.80 | |||
| No | 28 (27.7) | 20 (30.8) | 48 (28.9) | |
| Yes | 73 (72.3) | 45 (69.2) | 118 (71.1) | |
| Prosthesis volume (cm3) | 0.73 | |||
| Mean ± SD | 214±62.8 | 192±51.4 | 205±59.4 | |
| Median [Min, Max] | 190 [100, 440] | 180 [100, 340] | 190 [100, 440] | |
| Prosthesis thickness (mm) | 0.45 | |||
| Mean ± SD | 47.3±7.44 | 45.8±7.29 | 46.7±7.39 | |
| Median [Min, Max] | 48.0 [30.0, 72.0] | 44.0 [32.0, 72.0] | 46.5 [30.0, 72.0] | |
| Use of mesh, n (%) | 0.17 | |||
| No | 75 (74.3) | 55 (84.6) | 130 (78.3) | |
| Yes | 26 (25.7) | 10 (15.4) | 36 (21.7) | |
| Use of tissue expander, n (%) | 0.50 | |||
| No | 37 (36.6) | 28 (43.1) | 65 (39.2) | |
| Yes | 64 (63.4) | 37 (56.9) | 101 (60.8) | |
| Postoperative inflammatory lymph nodes, n (%) | 0.66 | |||
| No | 59 (58.4) | 41 (63.1) | 100 (60.2) | |
| Yes | 42 (41.6) | 24 (36.9) | 66 (39.8) | |
| Smoking status, n (%) | 0.57 | |||
| No | 79 (78.2) | 54 (83.1) | 133 (80.1) | |
| Yes | 22 (21.8) | 11 (16.9) | 33 (19.9) | |
| Preoperative WBC/postoperative WBC | 0.98 | |||
| Mean ± SD | 1.12±0.505 | 1.11±0.422 | 1.12±0.473 | |
| Median [Min, Max] | 1.01 [0.232, 4.34] | 1.02 [0.572, 2.88] | 1.02 [0.232, 4.34] | |
| Preoperative neutrophil/postoperative neutrophil | 0.97 | |||
| Mean ± SD | 1.48±2.84 | 1.31±0.959 | 1.41±2.29 | |
| Median [Min, Max] | 1.07 [0.103, 29.0] | 1.06 [0.489, 5.93] | 1.06 [0.103, 29.0] | |
| Infection status, n (%) | 0.18 | |||
| No | 79 (78.2) | 57 (87.7) | 136 (81.9) | |
| Yes | 22 (21.8) | 8 (12.3) | 30 (18.1) |
DCIS, ductal carcinoma in situ; IDC, invasive ductal carcinoma; SD, standard deviation; WBC, white blood cell count.
In the study, the patients were randomly divided into a training group (60%) and a validation group (40%). Statistical analyses, including the Chi-squared test, Fisher’s exact test, and Mann-Whitney U test, were conducted to compare the baseline characteristics between the two groups. No significant differences were found between the training and validation groups for any of the variables. This indicates that the random grouping was reliable, with no apparent bias in the distribution of baseline characteristics, ensuring the validity of the training and validation phases. The key variables were well balanced between the groups. Therefore, the randomization process effectively minimized potential confounding factors and supported the robustness of the model validation.
Logistic regression analysis results in the training group
In the univariate logistic regression analysis (Table 3), several factors were found to be significantly associated with postoperative skin infections in BC patients undergoing implant reconstruction. BMI was significantly associated with the risk of infection, with an odds ratio (OR) of 1.3072 [95% confidence interval (CI): 1.0744–1.5904, P=0.007], indicating that a higher BMI increased the likelihood of infection. Chemotherapy also showed a significant association with postoperative infections, with an OR of 9.8462 (95% CI: 1.2452–77.8541, P=0.03), suggesting that patients who received chemotherapy were at a greater risk. Radiotherapy was another significant factor, with an OR of 3.8231 (95% CI: 1.2319–11.8646, P=0.02). Additionally, prosthesis thickness showed a significant relationship with infections, with an OR of 1.1277 (95% CI: 1.0452–1.2167, P=0.002). In contrast, other factors, such as duration of hospitalization, number of dressing changes, clinical staging, histology type, lymph node metastasis count, targeted therapy, endocrine therapy, prosthesis volume, use of mesh, use of tissue expander, smoking status, and preoperative and postoperative WBC/neutrophil counts, were not statistically significant (P>0.05) in the univariate analysis.
Table 3
| Clinical characteristics | Univariable | Multivariable | |||||
|---|---|---|---|---|---|---|---|
| OR | 95% CI | P value | OR | 95% CI | P value | ||
| Duration of hospitalization (days) | 0.9559 | 0.8431–1.0837 | 0.48 | ||||
| Number of dressing changes | 1.0796 | 0.8704–1.339 | 0.49 | ||||
| Clinical staging | |||||||
| III | |||||||
| IVA | 1.7778 | 0.1856–17.0243 | 0.62 | ||||
| IVB | 0.7742 | 0.0707–8.4742 | 0.83 | ||||
| IVC | 3.2 | 0.3259–31.4212 | 0.32 | ||||
| V | 2.6667 | 0.1234–57.6233 | 0.53 | ||||
| Patient age (years) | 1.0315 | 0.9701–1.0969 | 0.32 | ||||
| Body mass index (kg/m2) | 1.3072 | 1.0744–1.5904 | 0.007* | 1.3329 | 1.0398–1.7087 | 0.02* | |
| Histology type | |||||||
| DCIS | |||||||
| IDC | 1.4 | 0.4017–4.8788 | 0.60 | ||||
| Other | 3.5 | 0.616–19.888 | 0.16 | ||||
| Lymph node metastasis count | 1.029 | 0.8297–1.2761 | 0.79 | ||||
| Chemotherapy | |||||||
| No | |||||||
| Yes | 9.8462 | 1.2452–77.8541 | 0.03* | 34.0813 | 2.1166–548.7675 | 0.01* | |
| Radiotherapy | |||||||
| No | |||||||
| Yes | 3.8231 | 1.2319–11.8646 | 0.02* | 3.482 | 0.8058–15.0459 | 0.09 | |
| Targeted therapy | |||||||
| No | |||||||
| Yes | 1.4221 | 0.3511–5.7602 | 0.62 | ||||
| Endocrine therapy | |||||||
| No | |||||||
| Yes | 0.6913 | 0.2291–2.0856 | 0.51 | ||||
| Prosthesis volume (cm3) | 1.0066 | 0.9976–1.0157 | 0.15 | ||||
| Prosthesis thickness (mm) | 1.1277 | 1.0452–1.2167 | 0.002* | 1.1622 | 1.0431–1.2949 | 0.006* | |
| Use of mesh | |||||||
| No | |||||||
| Yes | 1.6422 | 0.509–5.2975 | 0.41 | ||||
| Use of tissue expander | |||||||
| No | |||||||
| Yes | 1.7386 | 0.6095–4.9596 | 0.30 | ||||
| Postoperative inflammatory lymph nodes | |||||||
| No | |||||||
| Yes | 0.8437 | 0.2965–2.4011 | 0.75 | ||||
| Smoking status | |||||||
| No | |||||||
| Yes | 1.9167 | 0.587–6.2583 | 0.28 | ||||
| Preoperative WBC/postoperative WBC | 0.5352 | 0.1273–2.2505 | 0.39 | ||||
| Preoperative neutrophil/postoperative neutrophil | 0.8973 | 0.4729–1.7023 | 0.74 | ||||
*, denotes statistically significant values with a P value of <0.05. CI, confidence interval; DCIS, ductal carcinoma in situ; IDC, invasive ductal carcinoma; OR, odds ratio; WBC, white blood cell count.
In the multivariate logistic regression analysis, variables with a P value of less than 0.05 in the univariate analysis were included to adjust for potential confounders. BMI remained a significant predictor of postoperative infection, with an adjusted OR of 1.3329 (95% CI: 1.0398–1.7087, P=0.02). Chemotherapy continued to show a strong association with infection, with an adjusted OR of 34.0813 (95% CI: 2.1166–548.7675, P=0.01). Although radiotherapy approached significance, it did not meet the threshold for statistical significance in the multivariate analysis (OR: 3.482, 95% CI: 0.8058–15.0459, P=0.09). Prosthesis thickness remained a significant factor, with an adjusted OR of 1.1622 (95% CI: 1.0431–1.2949, P=0.006). These results indicate that higher BMI, chemotherapy and increased prosthesis thickness are independent risk factors for the development of postoperative skin infections in this patient population.
Establishment and prediction of the predictive model
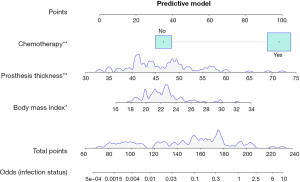
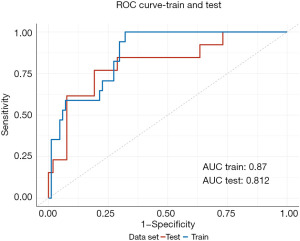
To enhance the interpretability of our predictive model, we constructed a nomogram that encompasses all statistically significant risk factors from the multivariate logistic regression model (BMI, chemotherapy, prosthesis thickness) to assist clinicians in evaluating the risk of skin infection after implant-based reconstruction in BC patients. Based on the regression coefficients, a score is assigned to each variable by drawing a straight line above the reference line, and a total score is obtained by summing the scores for the 3 predictive variables. The corresponding risk can then be estimated by drawing a line labeled “Risk” on the axis of the nomogram (Figure 2). ROC analysis was conducted for both the training and validation cohorts to assess the diagnostic accuracy of the model (Figure 3). The ROC curve demonstrated that the model had a high efficiency in predicting survival outcomes, with area under the curve (AUC) values of 0.87 (95% CI: 0.7944–0.9465) and 0.872 (95% CI: 0.6719–0.9523) for the training and validation cohorts, respectively. These results indicate that the model exhibits good discriminatory ability in distinguishing the presence or absence of skin infection risk following implant-based reconstruction in BC patients, as evidenced by the high AUC values.
Furthermore, calibration curves were plotted to evaluate the model’s performance in the training and validation cohorts. The predicted values showed good agreement with the observed values in both the training cohort (mean absolute error =0.028) and the validation cohort (mean absolute error =0.031). The calibration curves demonstrate that the model has good calibration ability (Figure 4).
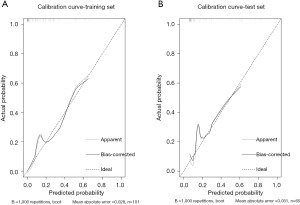
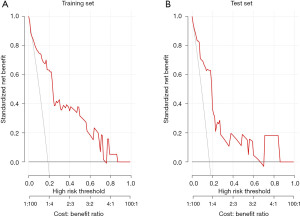
Figure 5 shows DCA for the training set and training set. The red line represents the model’s net benefit across different high-risk thresholds, compared to the gray “treat all” line. The model provides positive net benefit at certain thresholds, indicating potential clinical value in risk prediction.
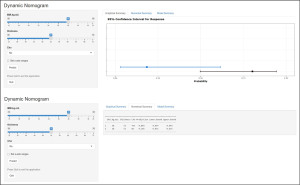
To simplify the use of the nomogram, we created a web-based dynamic calculator, accessible at https://kevinpan.shinyapps.io/InfectionStatus/. By entering specific clinical characteristics, users can calculate the predicted infection probability along with its 95% CI. For example, a BC patient with a BMI of 28 kg/m2, a prosthesis thickness of 52 mm, and no adjuvant chemotherapy has an estimated 18.5% risk of developing ipsilateral skin infection, with a 95% CI ranging from 0.031 to 0.616, as demonstrated in Figure 6.
Discussion
BC is one of the most prevalent malignancies affecting women worldwide. While advancements in targeted therapies, chemotherapy, and endocrine treatments have greatly improved patient outcomes, surgery remains a cornerstone of BC treatment. Mastectomy followed by implant-based reconstruction is a common approach that restores breast contour and enhances psychological well-being. However, postoperative complications, particularly skin infections, can adversely affect both the clinical outcome and patient satisfaction. This study aimed to identify clinical factors associated with infection risk in BC patients undergoing implant-based reconstruction and to develop a predictive model to assist clinicians in assessing these risks. In our analysis, three factors—BMI, prosthesis thickness, and adjuvant chemotherapy—emerged as significant predictors of postoperative skin infection. Based on these three factors, we developed a predictive model to estimate the risk of postoperative skin infection in BC patients undergoing implant-based reconstruction. The model was validated using both training and validation cohorts, and a web-based dynamic calculator was subsequently created to provide clinicians with an easy-to-use tool for individualized risk prediction.
Several studies have investigated the role of BMI in predicting complications following breast reconstruction, but the findings have been mixed (9,10,16-18). In a retrospective study by Leitner et al., 196 breast reconstructions from 134 patients were analyzed to assess the impact of BMI on postoperative outcomes after implant-based reconstruction. The study found that while BMI was not significantly associated with increased complication rates, the overall complication rate was 30.5%, with common issues including impaired wound healing, seroma, and infection. Additionally, longer operative times were linked to higher complication rates and extended hospital stays (10). However, in a study by McCarthy et al., 1,170 expander/implant reconstructions were performed in 884 patients, revealing that smoking, obesity, hypertension, and age over 65 years were significant independent risk factors for complications. The study found that smokers had 2.2 times greater odds of developing complications and a five-fold higher risk of reconstructive failure, while obese patients faced nearly double the odds of complications and a seven-fold increase in failure risk. These findings underscore the importance of evaluating individual risk factors to personalize reconstructive plans and minimize complications (9). Based on our study, BMI emerged as an independent risk factor for postoperative complications in implant-based breast reconstruction. Higher BMI was linked to increased risks of skin infections and impaired wound healing. Several mechanisms may explain this association: excess adipose tissue in individuals with higher BMI has reduced vascularity, impairing oxygen delivery and delaying wound healing. Additionally, the increased mechanical stress on the incision and implant can lead to complications such as wound dehiscence and seroma. Obesity is also associated with a chronic pro-inflammatory state and metabolic conditions like insulin resistance, which further impair immune response and healing, increasing the risk of complications (19,20). These findings underscore the importance of closely monitoring BMI as part of the preoperative assessment for breast reconstruction. Implementing strategies such as weight management before surgery may help reduce the risk of postoperative complications and improve overall surgical outcomes in this patient population.
The study by Yin et al. also identified prosthesis thickness as a risk factor for complications in breast reconstruction (21). The results showed that, compared to medium-thickness acellular dermal matrix (ADM), the use of thicker ADM was significantly associated with higher rates of infection (P<0.04) and wound complications (P<0.001). A thicker prosthesis may exert additional pressure on the overlying skin, increasing the risk of mechanical stress and tension, which can lead to skin breakdown and infection. This underscores the importance of carefully selecting prosthesis size during reconstruction to balance aesthetic outcomes while minimizing complications. Surgeons should take into account the patient’s tissue characteristics when choosing the implant to prevent excessive tension and reduce the risk of infection.
Lee et al. conducted a study involving 602 cases across 568 patients, with a mean follow-up period of 58.5 months (22). The study demonstrated that adjuvant chemotherapy significantly increased the rates of overall complications in two-stage implant-based breast reconstruction, including infections (OR 4.239, 95% CI: 1.059–16.970, P<0.05), severe capsular contracture (OR 2.107, 95% CI: 1.067–4.159, P<0.05), and reconstruction failures (OR 12.754, 95% CI: 1.587–102.481, P<0.05). Furthermore, specific chemotherapy regimens, such as sequential anthracycline/cyclophosphamide and taxane, were associated with higher risks of adverse outcomes compared to patients who did not receive chemotherapy, while other regimens, such as anthracycline/cyclophosphamide alone, were not linked to these increased risks. The results of this study indicate that patients receiving chemotherapy are more likely to develop postoperative skin infections, possibly due to the immunosuppressive effects of chemotherapy, which can impair the body’s ability to heal wounds and fight infections. Similarly, other studies have shown that neoadjuvant chemotherapy does not increase the risk of complications following breast reconstruction (12,13). Therefore, this study only included patients undergoing implant-based breast reconstruction who did not receive neoadjuvant treatment. In addition, radiotherapy was not included as a significant variable in our model. Traditionally, radiotherapy has been associated with an increased risk of complications, such as capsular contracture, delayed wound healing, and infections, especially in the context of breast reconstruction (23). This may be attributed to advances in radiotherapy techniques that have reduced the incidence of these complications. Modern techniques, such as IMRT, allow for more precise targeting of cancerous tissues while sparing surrounding healthy tissue, thereby minimizing the adverse effects traditionally associated with radiotherapy.
Despite the valuable insights gained from this study, several limitations should be acknowledged. The retrospective design inherently carries the risk of selection bias, and the sample size, while sufficient for initial analysis, may not fully capture the variability present in larger or more diverse populations. Since the study was conducted at a single institution, the results may not be directly applicable to other healthcare settings or patient populations, limiting the external validity of the findings. Future large-scale, multi-center studies are warranted to validate and refine this model to ensure its broader applicability across diverse clinical settings and populations. Nonetheless, this study offers significant strengths, including the development of a simple and effective predictive model based on easily accessible clinical factors. The nomogram and web-based calculator provide clinicians with a practical tool for assessing the individualized risk of postoperative skin infections. The model demonstrated high accuracy and reliability, as reflected by the strong performance in both the training and validation cohorts.
Conclusions
In conclusion, BMI, prosthesis thickness, and chemotherapy are critical factors influencing the risk of postoperative skin infections in BC patients undergoing implant-based reconstruction. By applying our predictive model, clinicians can better assess risk, personalize treatment plans, and ultimately improve patient outcomes. Further studies with larger cohorts and multi-center validation are needed to confirm these findings and refine the model for broader clinical use.
Acknowledgments
None.
Footnote
Reporting Checklist: The authors have completed the TRIPOD reporting checklist. Available at https://gs.amegroups.com/article/view/10.21037/gs-24-470/rc
Data Sharing Statement: Available at https://gs.amegroups.com/article/view/10.21037/gs-24-470/dss
Peer Review File: Available at https://gs.amegroups.com/article/view/10.21037/gs-24-470/prf
Funding: The study was supported by
Conflicts of Interest: All authors have completed the ICMJE uniform disclosure form (available at https://gs.amegroups.com/article/view/10.21037/gs-24-470/coif). The authors have no conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved. The study was conducted in accordance with the Declaration of Helsinki and its subsequent amendments. Ethics approval was obtained from the Ethics Committee of the Breast Surgery Department at The First Affiliated Hospital of Wenzhou Medical University (No. KY2024-R27). The requirement for written informed consent was waived due to the retrospective nature of the study.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Sung H, Ferlay J, Siegel RL, et al. Global Cancer Statistics 2020: GLOBOCAN Estimates of Incidence and Mortality Worldwide for 36 Cancers in 185 Countries. CA Cancer J Clin 2021;71:209-49. [Crossref] [PubMed]
- Loibl S, Poortmans P, Morrow M, et al. Breast cancer. Lancet 2021;397:1750-69. [Crossref] [PubMed]
- Kerr AJ, Dodwell D, McGale P, et al. Adjuvant and neoadjuvant breast cancer treatments: A systematic review of their effects on mortality. Cancer Treat Rev 2022;105:102375. [Crossref] [PubMed]
- Prat A, Pineda E, Adamo B, et al. Clinical implications of the intrinsic molecular subtypes of breast cancer. Breast 2015;24:S26-35. [Crossref] [PubMed]
- Zehra S, Doyle F, Barry M, et al. Health-related quality of life following breast reconstruction compared to total mastectomy and breast-conserving surgery among breast cancer survivors: a systematic review and meta-analysis. Breast Cancer 2020;27:534-66. [Crossref] [PubMed]
- Scomacao I, AlHilli Z, Schwarz G. The Role of Oncoplastic Surgery for Breast Cancer. Curr Treat Options Oncol 2020;21:94. [Crossref] [PubMed]
- Prasad K, Zhou R, Zhou R, et al. Cosmetic reconstruction in breast cancer patients: Opportunities for nanocomposite materials. Acta Biomater 2019;86:41-65. [Crossref] [PubMed]
- Momoh AO, Ahmed R, Kelley BP, et al. A systematic review of complications of implant-based breast reconstruction with prereconstruction and postreconstruction radiotherapy. Ann Surg Oncol 2014;21:118-24. [Crossref] [PubMed]
- McCarthy CM, Mehrara BJ, Riedel E, et al. Predicting complications following expander/implant breast reconstruction: an outcomes analysis based on preoperative clinical risk. Plast Reconstr Surg 2008;121:1886-92. [Crossref] [PubMed]
- Leitner HS, Pauzenberger R, Ederer IA, et al. BMI Specific Complications Following Implant-Based Breast Reconstruction after Mastectomy. J Clin Med 2021;10:5665. [Crossref] [PubMed]
- Rifkin WJ, Kantar RS, Cammarata MJ, et al. Impact of Diabetes on 30-Day Complications in Mastectomy and Implant-Based Breast Reconstruction. J Surg Res 2019;235:148-59. [Crossref] [PubMed]
- Nag S, Berlin L, Hunter K, et al. Effects of Neoadjuvant Chemotherapy on Autologous and Implant-Based Breast Reconstruction: A Systematic Review and Meta-Analysis of the Literature. Clin Breast Cancer 2024;24:184-90. [Crossref] [PubMed]
- Sabitovic A, Trøstrup H, Damsgaard TE. The impact of neoadjuvant chemotherapy on surgical outcomes following autologous and implant-based immediate breast reconstruction: A systematic review and meta-analysis. J Plast Reconstr Aesthet Surg 2023;87:17-23. [Crossref] [PubMed]
- de Faria Castro Fleury E, Jasmin Huanca Bernal K, Lucena Miranda Madeiro A, et al. Side effects in breast implants related to radiotherapy in breast cancer reconstructive surgery. Tech Innov Patient Support Radiat Oncol 2021;18:8-11. [Crossref] [PubMed]
- Mark RJ, Zimmerman RP, Greif JM. Capsular contracture after lumpectomy and radiation therapy in patients who have undergone uncomplicated bilateral augmentation mammoplasty. Radiology 1996;200:621-5. [Crossref] [PubMed]
- Fischer JP, Nelson JA, Kovach SJ, et al. Impact of obesity on outcomes in breast reconstruction: analysis of 15,937 patients from the ACS-NSQIP datasets. J Am Coll Surg 2013;217:656-64. [Crossref] [PubMed]
- Nguyen KT, Hanwright PJ, Smetona JT, et al. Body mass index as a continuous predictor of outcomes after expander-implant breast reconstruction. Ann Plast Surg 2014;73:19-24. [Crossref] [PubMed]
- Gabriel A, Sigalove S, Storm-Dickerson TL, et al. Dual-Plane versus Prepectoral Breast Reconstruction in High-Body Mass Index Patients. Plast Reconstr Surg 2020;145:1357-65. [Crossref] [PubMed]
- Cox AJ, West NP, Cripps AW. Obesity, inflammation, and the gut microbiota. Lancet Diabetes Endocrinol 2015;3:207-15. [Crossref] [PubMed]
- Snider AP, Wood JR. Obesity induces ovarian inflammation and reduces oocyte quality. Reproduction 2019;158:R79-90. [Crossref] [PubMed]
- Yin Z, Wang H, Liu Y, et al. Single-Institution Algorithm for Prevention and Management of Complications in Direct-to-Implant Breast Reconstruction. Plast Reconstr Surg 2022;150:48S-60S. [Crossref] [PubMed]
- Lee KT, Bae J, Jeon BJ, et al. Adjuvant Chemotherapy in Two-Stage Tissue Expander/Implant Breast Reconstruction: Does it Affect Final Outcomes? Ann Surg Oncol 2021;28:2191-8. [Crossref] [PubMed]
- Rocco N, Catanuto G, Nava MB. Radiotherapy and breast reconstruction. Minerva Chir 2018;73:322-8. [Crossref] [PubMed]


