
Recent changes in surgical outcomes and preoperative biliary drainage with the increased use of neoadjuvant chemotherapy in pancreatic cancer patients undergoing pancreatoduodenectomy: a single-center retrospective study
Highlight box
Key findings
• In pancreatic ductal adenocarcinoma (PDAC) patients who underwent pancreatoduodenectomy (PD), there was no significant difference in surgical outcomes between patients who underwent neoadjuvant chemotherapy (NAC) and those who underwent upfront surgery. Preoperative biliary drainage (PBD) in NAC patients was more frequently performed with metallic stent (MS) placement, and PBD-related adverse events decreased. MS placement was associated with longer patency than plastic stent placement.
What is known and what is new?
• Although the number of patients undergoing NAC increased the selection of MS placement, MS placement had a longer patency period and no increase in PBD-related adverse events or postoperative complications. In the present study, we clarify the preoperative management of PBD and postoperative outcomes of PD during transitional period when NAC is increasingly performed for PDAC, based on actual clinical data.
• This study adds to the knowledge that MS placement is useful as a PBD for PDAC patients undergoing PD after NAC.
What is the implication, and what should change now?
• For PBD in PDAC patients undergoing PD after NAC, MS placement is currently the most appropriate. However, MS placement is associated with a certain incidence of PBD-related adverse events, including delay in the surgical schedule, and future studies on better drainage are expected.
Introduction
Pancreatoduodenectomy (PD) is widely performed worldwide as a standard surgical procedure for the treatment of pancreatic cancer and other pancreatic head malignancies. Although the safety of PD has improved in the past, it remains one of the most invasive medical procedures, with a complication rate of 40% or more (1). Preoperative biliary drainage (PBD) is performed in patients with biliary obstruction to improve the safety of PD as much as possible. PBD is expected to improve cholangitis, liver function, and immunity (2). However, the pros and cons of PBD are still controversial, as an increase in biliary infections after PBD is associated with postoperative infectious complications (3).
The treatment of pancreatic ductal adenocarcinoma (PDAC) has changed over the past few decades. Several effective chemotherapies for pancreatic cancer have emerged, and the efficacy of postoperative adjuvant chemotherapy and even preoperative neoadjuvant chemotherapy (NAC) has been reported, rendering surgery and adjuvant therapy the most effective treatment options (4-6). NAC is increasingly used when PD is planned for PDAC, and changes have been observed in the extension of the preoperative period and in preoperative patient management.
There is debate about whether PBD should be performed, but even in medical institutions that do not recommend it, there are cases where PBD must be performed in patients with severe jaundice or cholangitis. There are several approaches to PBD, including transpapillary methods using endoscopic retrograde cholangiopancreatography (ERCP), such as plastic stent (PS) placement, metallic stent (MS) placement, or endoscopic nasobiliary drainage (ENBD). Historically, percutaneous transhepatic biliary drainage (PTBD) has been used, but more recently, biliary drainage methods using endoscopic ultrasonography (EUS) have also been reported. Among these, endoscopic transpapillary drainage is widely used, and the benefit of MS placement in PDAC patients undergoing NAC has been reported in a meta-analysis (7) and is also mentioned in guidelines (6,8,9).
In actual clinical practice, we are in a transitional period where NAC is more commonly used than upfront surgery (US), so how have biliary drainage and surgical outcomes changed, and what is needed in the future? To address this clinical question, we designed this retrospective study. To evaluate the change in clinical practice in perioperative management, including PBD, and surgical outcomes for PDAC at a single referral center in a rural area of Japan, we conducted this study to compare the NAC group with the US group and to examine the implications and problems of PBD in an era when NAC has become the standard treatment for PDAC. We also discuss the optimal PBD method for PDAC patients undergoing NAC. Although this was a retrospective study at a single institution, University of Miyazaki Hospital strives to provide standard preoperative management, treatment, and surgery according to clinical guidelines. As standard treatment evolves to include multimodal therapy, we believe these data can be used as reference data for other institutions. We present this article in accordance with the STROBE reporting checklist (available at https://gs.amegroups.com/article/view/10.21037/gs-2024-507/rc).
Methods
Patient selection
From 2013 to 2021, 143 consecutive patients with PDAC were treated at Division of Hepato-Biliary-Pancreas Surgery, Department of Surgery, University of Miyazaki Faculty of Medicine. Among these patients, 99 patients who underwent PD were included in this study; 40 patients who underwent distal pancreatectomy and four patients who underwent simultaneous resection of cancers of other organs were excluded. Among the 99 patients who underwent PD for PDAC, 40 patients who underwent NAC and 59 patients who underwent US were compared in terms of background, PBD, and perioperative outcomes, and the characteristics of these clinical factors in patients who underwent NAC were retrospectively analyzed via medical records.
In addition, the clinical and perioperative factors were compared between the 27 patients in the NAC group and the 33 patients in the US group, including only those patients who underwent PBD (Figure 1). Furthermore, we evaluated the optimal PBD method for preoperative PDAC patients, we investigated the outcomes, patency, and adverse events of a total of 128 PBD procedures, including 45 PBD procedures for NAC patients and 83 PBD procedures for US patients.
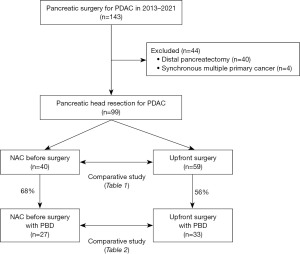
The study was designed to include patients from a period when the number of patients in both groups was comparable, during the transition from US to NAC. Although the sample size setting may not have been ideal, consecutive patients from a defined period were included in the study because it was a retrospective study.
This study was approved by the ethics committee of the University of Miyazaki Faculty of Medicine (No. O-1128), and conducted in accordance with the Declaration of Helsinki and its subsequent amendments. Opt-out patient consent was obtained for the study on the basis of retrospective medical information.
Data collection
Patient data, including age, gender, body mass index (BMI), diabetes mellitus, preoperative albumin (ALB) level, bilirubin level, C-reactive protein (CRP) level, resectability classification, operative procedure, operative time and blood loss, pancreatic texture and duct diameter, and postoperative complications, were retrospectively collected. In addition, in a detailed study of PBD, drainage procedure, initial and final drainage procedure, number of PBD procedures, duration of PBD, and PBD-related adverse events were retrospectively collected. In addition, in the results of each PBD procedure were examined by collecting data on procedural outcomes, adverse events, and duration of patency.
Preoperative patient management and PBD
Patients who came to University of Miyazaki Hospital with suspected PDAC were evaluated by computed tomography (CT) and other imaging examinations for local extension and distant metastasis, and histological examinations such as EUS-fine needle aspiration (FNA) were performed to make a diagnosis. The resectability of PDAC was classified based on the Japanese diagnostic criteria and guidelines (5,6,8).
The following is our policy for PBD. All patients with obstructive jaundice and cholangitis were indicated for PBD. Endoscopic procedures were the first choice of treatment, with priority given to internal drainage. The drainage method, such as the type of stent (MS, PS, ENBD), was selected by the endoscopist after reviewing the radiographic images during the examination. Among the PS used, 7 Fr straight stents were most commonly used, but 8.5 and 10 Fr straight stents and 7 Fr double pigtail stents were also used; among the MS, 10-mm-diameter fully covered self-expandable MSs were used in most cases. When recurrent biliary obstruction occurred, additional endoscopic procedures such as stent exchange were performed. Surgery was performed after the cholangitis improved and the serum bilirubin level decreased to less than 5 mg/dL.
NAC
NAC was administered for 6 weeks with gemcitabine and S-1 for resectable pancreatic cancer and for 8–12 weeks with gemcitabine and nab-paclitaxel for borderline resectable pancreatic cancer. The need for additional chemotherapy depends on the tumor status and tumor marker levels. In patients who could not continue chemotherapy due to side effects, surgery was performed if NAC could not be completed. Surgery was performed after a few weeks of withdrawal from NAC and a period of evaluation of the patient’s condition.
Surgical procedure and postoperative patient management
Surgery was performed for PD with regional lymph node dissection, and reconstruction was performed via the modified child method. Historically, we have performed pyloric preserving PD (PPPD) in many cases; however, in recent years, subtotal stomach preserving PD (SSPPD) has been our first choice to reduce delayed gastric emptying. Depending on the degree of pancreatic cancer progression, plexus dissection or portal vein resection was performed. Two closed drains were placed around the pancreatic and biliary anastomoses.
Prophylactic antibiotics were usually administered for 3 days, including the day of surgery. The patients were given epidural anesthesia for 3–4 days postoperatively and/or adequate analgesia, and early ambulation was encouraged. We made an early effort to remove the drain [usually on postoperative day (POD) 5] and to begin food intake (usually on POD 4).
Pancreatic fistula was defined and graded according to the International Study Groups on Pancreatic Fistula (ISGPF) definition, and a clinical pancreatic fistula was defined as grade B or C (10). Surgical site infection (SSI) was defined according to the three defined levels of the Centers for Disease Control and Prevention: superficial incisional, deep incisional, and organ or space infection (11). Postoperative complications were graded according to the Clavien-Dindo classification (12). Mortality was defined as patient death occurring until POD 30.
Statistical analysis
Data were expressed as numbers (percentages) or medians [interquartile ranges (IQRs)]. For comparisons between patients who received NAC and those who received US, categorical variables were compared with the Chi-squared test or Fisher’s exact test, and quantitative variables were compared with the Mann-Whitney U test. A parametric or nonparametric test was selected to test the variance between the two groups. Baseline data for age, sex, and BMI were similar between the two groups, and no statistically adjusted analyses were performed. To compare the duration of patency for each drainage method, the log-rank test was performed via the Kaplan-Meier method. The significance level was set at P<0.05 using a two-tailed test.
Results
Patient characteristics and perioperative factors
A comparison of patient characteristics and perioperative factors between the NAC and US groups is shown in Table 1. Regarding patient characteristics, there were no differences in age, sex, BMI, diabetes mellitus status, preoperative bilirubin level, or CRP level. However, preoperative ALB levels were higher in the NAC group than in the US group (median 3.73 vs. 3.47 g/dL, P=0.002). Regarding the resectability classification, the NAC group had an equal number of resectable and borderline resectable cancers, whereas the US group had mostly resectable cancers. Among the regimens of NAC, gemcitabine and S-1 were the most common. The number of patients with NAC has increased significantly in recent years, and SSPPD is the most frequently selected surgical procedure. The operative findings revealed less blood loss in the NAC group than in the US group (median 895 vs. 1,250 mL, P=0.02). There was no difference in the occurrence of postoperative complications between the two groups (NAC vs. US, 35% vs. 46%, respectively, P=0.29), but the postoperative hospital stay was significantly shorter in the NAC group than in the US group (median 17 vs. 28 days, P<0.001).
Table 1
| Variables | NAC group (n=40) | US group (n=59) | P value |
|---|---|---|---|
| Age (years) | 69 [61–77] | 69 [61–76] | 0.99 |
| Gender (male) | 19 [48] | 33 [56] | 0.41 |
| BMI (kg/m2) | 21.1 [19.8–22.5] | 20.9 [19.0–23.0] | 0.73 |
| Diabetes mellitus | 20 [50] | 19 [32] | 0.10 |
| Preoperative ALB (g/dL) | 3.73 [3.45–3.94] | 3.47 [3.11–3.65] | 0.002 |
| Preoperative bilirubin (mg/dL) | 0.5 [0.5–0.7] | 0.6 [0.5–1.4] | 0.06 |
| Preoperative CRP (mg/dL) | 0.19 [0.05–0.28] | 0.10 [0.04–0.29] | 0.42 |
| Resectability classification | <0.001 | ||
| Resectable | 19 [48] | 53 [90] | |
| Borderline resectable | 19 [48] | 5 [8] | |
| Locally advanced | 2 [5] | 1 [2] | |
| NAC regimen | – | ||
| Gemcitabine + nab-paclitaxel | 15 [38] | – | |
| Gemcitabine + S-1 | 22 [55] | – | |
| Gemcitabine | 2 [5] | – | |
| S-1 | 1 [3] | – | |
| Duration of NAC (days) | 43 [35–63] | – | – |
| Operative year | <0.001 | ||
| 2013 | 4 | 10 | |
| 2014 | 2 | 14 | |
| 2015 | 2 | 9 | |
| 2016 | 1 | 8 | |
| 2017 | 3 | 6 | |
| 2018 | 3 | 3 | |
| 2019 | 3 | 8 | |
| 2020 | 6 | 1 | |
| 2021 | 16 | 0 | |
| PBD | 27 [68] | 33 [56] | 0.25 |
| Operative procedure | 0.02 | ||
| Pylorus-preserving PD | 2 [5] | 16 [27] | |
| Subtotal stomach-preserving PD | 31 [78] | 36 [61] | |
| PD (including in distal gastrectomy) | 2 [5] | 3 [5] | |
| Total pancreatectomy | 5 [13] | 4 [7] | |
| Operative findings | |||
| Operative time (minutes) | 589 [480–686] | 619 [532–681] | 0.38 |
| Operative blood loss (mL) | 895 [490–1,550] | 1,250 [950–1,795] | 0.02 |
| Soft pancreas | 8 [20] | 14 [24] | 0.78 |
| Main pancreatic duct diameter (mm) | 5 [3–7] | 5 [3–6] | 0.29 |
| Portal vein resection | 21 [53] | 16 [27] | 0.01 |
| Blood transfusion | 14 [35] | 32 [54] | 0.06 |
| Postoperative complications | 14 [35] | 27 [46] | 0.29 |
| Clavien-Dindo grade III or over | 4 [10] | 11 [19] | 0.24 |
| POPF (clinically relevant) | 1 [3] | 6 [10] | 0.14 |
| Organ/space SSI | 3 [8] | 6 [10] | 0.65 |
| Superficial/deep SSI | 1 [3] | 7 [12] | 0.09 |
| DGE | 3 [8] | 6 [10] | 0.65 |
| PPH | 2 [5] | 1 [2] | 0.35 |
| Bile leakage | 0 | 2 [3] | 0.24 |
| Cholangitis | 2 [5] | 2 [3] | 0.69 |
| Pneumonia | 1 [3] | 3 [5] | 0.52 |
| Thrombosis | 3 [8] | 2 [3] | 0.36 |
| Reoperation | 1 [3] | 1 [2] | 0.78 |
| Mortality | 0 | 0 | – |
| Postoperative hospital stay (days) | 17 [15–28] | 28 [18–37] | <0.001 |
Data were presented as median [IQR], number [%], or number. ALB, albumin; BMI, body mass index; NAC, neoadjuvant chemotherapy; CRP, C-reactive protein; PD, pancreatoduodenectomy; POPF, postoperative pancreatic fistula; SSI, surgical site infection; DGE, delayed gastric emptying; PPH, postpancreatectomy hemorrhage; IQR, interquartile range; PBD, preoperative biliary drainage; US, upfront surgery.
Comparison of PBD between NAC and US patients
A comparison of 27 NAC patients with PBD and 33 US patients with PBD is shown in Table 2. For recurrent biliary obstruction, the same patient may undergo several different biliary drainage procedures, and the total number of patients who underwent each PBD procedure is shown in the table. The PBD period in the NAC group was significantly longer than that in the US group (median 101 vs. 46 days, P<0.001). In the NAC group, MS placement were more frequently, and MS were also selected more frequently as the initial procedure (NAC vs. US, 52% vs. 15%, respectively, P=0.009). However, PS placement was most frequently in the US group. PBD-related adverse events were observed in 33% of patients in the NAC group, whereas they were significantly different in 61% of patients in the US group (P=0.04). In addition, the number of drainage procedures was lower in the NAC group. Cholangitis was the most common PBD-related adverse events. The incidence of cholangitis was significantly lower in the NAC group than in the US group (22% vs. 48%, P=0.04). Other PBD-related adverse events included stent dislodgment, pancreatitis, and cholecystitis. The incidence of recurrent biliary obstruction was the same between the NAC and US groups among patients with MS placement (NAC vs. US, 11% vs. 9%, respectively, P=0.83) and was greater in the US group than in the NAC group among patients with PS placement, but the difference was not significant (NAC vs. US, 26% vs. 58%, respectively, P=0.07). Among the patients with PBD-related adverse events, 2 patients in the MS group (7%) and 4 patients in the US group (12%) had to postpone their scheduled surgery.
Table 2
| Variables | NAC with PBD group (n=27) | US with PBD group (n=33) | P value |
|---|---|---|---|
| Procedure of biliary drainage | 0.13 | ||
| MS placement | 20 [74] | 17 [52] | |
| PS placement | 11 [41] | 21 [64] | |
| PTBD | 1 [4] | 3 [9] | |
| EUS-guided biliary drainage | 1 [4] | 0 | |
| Initially, drainage procedure | 0.009 | ||
| MS | 14 [52] | 5 [15] | |
| PS | 6 [22] | 14 [42] | |
| ENBD | 5 [19] | 11 [33] | |
| Final drainage procedure | 0.05 | ||
| MS | 18 [67] | 14 [42] | |
| PS | 4 [15] | 7 [21] | |
| ENBD | 3 [11] | 9 [27] | |
| Number of PBD procedure | 2 [1–3] | 2 [2–4] | 0.02 |
| Duration of PBD (days) | 101 [83–119] | 46 [26–75] | <0.001 |
| PBD-related adverse events | 9 [33] | 20 [61] | 0.04 |
| Cholangitis | 6 [22] | 16 [48] | 0.04 |
| Dislodgment | 2 [7] | 2 [6] | 0.84 |
| Pancreatitis | 1 [4] | 2 [6] | 0.68 |
| Cholecystitis | 1 [4] | 0 | 0.27 |
| Recurrent biliary obstruction for MS | 3 [11] | 3 [9] | 0.83 |
| Recurrent biliary obstruction for PS | 7 [26] | 19 [58] | 0.07 |
| Delay of surgery schedule related to PBD | 2 [7] | 4 [12] | 0.55 |
| Age (years) | 68 [59–77] | 70 [61–76] | 0.87 |
| Gender (male) | 15 [56] | 22 [67] | 0.38 |
| BMI (kg/m2) | 21.0 [19.7–22.3] | 21.3 [19.8–23.9] | 0.30 |
| Diabetes mellitus | 12 [44] | 11 [33] | 0.38 |
| Preoperative ALB (g/dL) | 3.55 [3.39–3.92] | 3.33 [3.05–3.55] | <0.001 |
| Preoperative bilirubin (mg/dL) | 0.5 [0.4–0.9] | 1.0 [0.6–2.9] | 0.002 |
| Preoperative CRP (mg/dL) | 0.20 [0.05–0.44] | 0.15 [0.07–0.42] | 0.99 |
| Operative findings | |||
| Operative time (minutes) | 591 [483–696] | 628 [555–666] | 0.80 |
| Operative blood loss (mL) | 1,020 [460–1,650] | 1,460 [1,073–2,040] | 0.02 |
| Portal vein resection | 11 [41] | 8 [24] | 0.17 |
| Postoperative complication | 8 [30] | 15 [45] | 0.21 |
| Clavien-Dindo grade III or over | 2 [7] | 8 [24] | 0.08 |
| POPF (clinical relevant) | 0 | 4 [12] | 0.12 |
| Organ/space SSI | 3 [11] | 4 [12] | 0.90 |
| Superficial/deep SSI | 0 | 4 [12] | 0.12 |
| DGE | 3 [11] | 4 [12] | 0.90 |
| PPH | 1 [4] | 1 [3] | 0.89 |
| Postoperative hospital stay (days) | 16 [14–25] | 27 [20–39] | 0.002 |
Data were presented as median [IQR] or number [%]. In PBD-related adverse events, one case of cholangitis and dislodgment overlapped. ALB, albumin; BMI, body mass index; CRP, C-reactive protein; DGE, delayed gastric emptying; ENBD, endoscopic nasobiliary drainage; EUS, endoscopic ultrasonography; IQR, interquartile range; MS, metallic stent; NAC, neoadjuvant chemotherapy; PBD, preoperative biliary drainage; POPF, postoperative pancreatic fistula; PPH, postpancreatectomy hemorrhage; PS, plastic stent; PTBD, percutaneous transhepatic biliary drainage; SSI, surgical site infection; US, upfront surgery.
In the comparison of patient characteristics and perioperative factors for the 60 patients in both groups in whom PBD was performed, similar to the results of all 99 patients, the preoperative ALB level was significantly greater in the NAC group than in the US group (median 3.55 vs. 3.33 g/dL, P<0.001). Preoperative bilirubin levels were significantly lower in the NAC group than in the US group in patients who underwent PBD (median 0.5 vs. 1.0 mg/dL, P=0.002). The surgical results revealed that there were no differences in operative time between the two groups, but there was significantly less blood loss in the NAC group than in the US group (median 1,020 vs. 1,460 mL, P=0.02). Postoperative complications did not differ between the two groups (NAC vs. US, 30% vs. 45%, respectively, P=0.21). The complication rate in patients who underwent PBD was similar to that in the entire patient cohort, but the postoperative hospital stay was significantly shorter in the NAC group than in the US group (16 vs. 27 days, P=0.002).
Outcomes by drainage procedure
A total of 128 PBD procedures were performed on these patients, including 27 in the NAC group and 33 in the US group. Since there were cases of different PBD procedures being performed on the same patient, a total of 128 outcomes for each PBD procedure were investigated and are shown in Table 3. The 128 PBD procedures included MS placement 36 times, PS placement 48 times, and ENBD 44 times. After MS placement, the most common outcome was sustained patency until surgery (80%), whereas after PS placement, the most common outcome was drainage failure, such as cholangitis or obstruction (55%). MS placement and PS placement were equally likely to require a delay in the surgical schedule due to PBD-related adverse events (MS vs. PS, 6% vs. 6%, respectively, P>0.99). In University of Miyazaki Hospital, ENBD is most frequently placed temporarily during cholangitis, and the most common outcome after ENBD placement was scheduled exchange for internal drainage after the improvement of cholangitis (57%).
Table 3
| Variables | MS (n=36) | PS (n=48) | ENBD (n=44) | P value |
|---|---|---|---|---|
| Outcome | <0.001 | |||
| Sustained patency | 29 [81] | 10 [21] | 14 [32] | |
| Planning exchange | 1 [3] | 10 [21] | 25 [57] | |
| Recurrent biliary obstruction | 6 [17] | 26 [54] | 3 [7] | |
| Obstruction from debris | 5 [14] | 26 [54] | 3 [7] | |
| Obstruction from granulation | 1 [3] | 0 | 0 | |
| Dislodgment | 0 | 2 [4] | 2 [5] | |
| PBD-related adverse events | <0.001 | |||
| Cholangitis | 6 [17] | 20 [42] | 2 [5] | |
| Cholecystitis | 1 [3] | 0 | 0 | |
| Pancreatitis | 2 [6] | 1 [2] | 0 | |
| Delayed surgery schedule due to PBD-related adverse events | 2 [6] | 3 [6] | – | >0.99 |
| Duration of patency (days) | <0.001 | |||
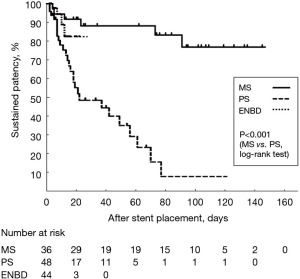
| Median [IQR] | 68 [21–103] | 15 [8–35] | 7 [6–12] | |
| Mean ± SD | 64±46 | 24±24 | 9±5 |
Data were presented as number [%], unless otherwise stated. In the cases of delayed surgery schedule, one case was a PTBD-related adverse event and was excluded from this table. ENBD, endoscopic nasobiliary drainage; IQR, interquartile range; MS, metallic stent; PBD, preoperative biliary drainage; PS, plastic stent; PTBD, percutaneous transhepatic biliary drainage; SD, standard deviation.
The duration of patency for each drainage procedure is also listed in Table 3. MS placement was associated with the longest duration of patency, with a median of 68 days and a mean of 64 days. However, compared with MS placement, PS placement resulted in a shorter patency period, with a median of 15 days and a mean of 24 days, indicating a significant difference (log-rank test, P<0.001). The Kaplan-Meier curves for the duration of patency for each drainage procedure are shown in Figure 2.
Discussion
In the present study, PDAC patients who underwent PD after NAC had fewer PBD-related adverse events compared with those who underwent US, although the preoperative waiting and treatment periods were longer. MS was more frequently placed in patients who underwent NAC, and MS placement had a longer patency period in the drainage procedure-specific study, suggesting that the choice of MS placement may be a factor in the reduction in PBD-related adverse events. There was no difference in postoperative complications between the NAC and US groups, but there was significantly less blood loss in the NAC group, which had fewer PBD-related adverse events.
PDAC is a cancer with a poor prognosis, and surgery and adjuvant chemotherapy or chemoradiotherapy before and after surgery are considered useful for controlling systemic metastasis. The usefulness of pre- and postoperative adjuvant chemotherapy has been reported not only for borderline resectable pancreatic cancer but also for resectable pancreatic cancer, and preoperative NAC for PDAC has been proposed in guidelines and is becoming a standard treatment (6,8,9). The historical bias in this study has led to the application of NAC to resectable cancers as well.
Previous studies have reported that NAC does not have any adverse effects, such as complications, compared with US in patients with PDAC (4,6,8,13). In the patients in this study, preoperative ALB levels were greater, and intraoperative blood loss was lower in the NAC group. This was a retrospective study, and historical bias may have influenced the outcomes of the NAC and US groups. Although it is difficult to prove a causal relationship, the following points may be related to recent improvements in surgical outcomes. Understanding of surgical anatomy and the standardization of surgical procedures may have contributed to the decrease in blood loss. In addition, attention has been given to preoperative nutritional therapy and rehabilitation, which may be related to the maintenance of ALB levels in NAC patients.
It has been reported that PBD is associated with increased biliary tract infections and postoperative infectious complications and should not be performed routinely (3). PBD is still controversial. Several studies have reported that PBD-related adverse events worsen short- and long-term outcomes (14,15), and increased blood loss and operative time (16). However, in previous studies examining the effects of PBD on PD, many of the background diseases included a wide variety of diseases (pancreatic cancer, biliary tract cancer, duodenal cancer, and other diseases). The treatment strategy for pancreatic cancer has changed since NAC has been increasingly used, and it is necessary to discuss the appropriate PBD for each disease even when the same PD is performed. Accordingly, the following discussion focuses on PBD in PDAC.
In recent years, there have been many reports that MS placement is effective in PBD for PDAC patients who undergo PD after NAC (7,17-27). Many studies have demonstrated the efficacy of adjuvant therapy for PDAC, and since NAC has become the standard of treatment and the preoperative period has increased, stable and durable PBD for a longer period is now needed. Most of these studies, including meta-analyses (7), reported longer patency and fewer PBD-related adverse events with MS placement than with PS placement. According to these reports, the recurrent biliary obstruction rate is reported to be significantly higher with PS placement (40–90%) than with MS placement (10–30%) (17-27). In addition, medical costs may also be lower with MS placement because of reduced endoscopic re-interventions (17). Ballard et al. reported the safety and efficacy of MS placement in the same session after EUS-FNA and onsite cytology (18). In the present study, patients who underwent MS placement had longer patency and fewer PBD-related adverse events, suggesting that MS placement is useful as a PBD for patients with PDAC. MS placement is considered a useful drainage method that is consistent with the requirement for stable and durable biliary drainage in the era of prolonged preoperative treatment with NAC for patients with PDAC. A consensus is emerging regarding the use of MSs for PBD for obstructive jaundice in patients with PDAC. Recent guidelines recommend the use of MS for PBD in PDAC patients (6,8,9).
However, there are still problems with MS placement. First, the dilatational force of the MS may cause inflammation of the surrounding tissues, leading to intraoperative adhesions and increased blood loss. The impact of preoperative MS placement on surgery has not been fully elucidated. Although many studies have reported that MS placement does not increase postoperative complications, Mandai reported that fully covered MSs had a lower rate of endoscopic re-intervention but resulted in more intraoperative blood loss, a higher incidence of surgical morbidity, and a longer postoperative hospital stay in a multicenter cohort study (19). Second, specific complications of cholecystitis and pancreatitis may occur due to MS obstruction of the cystic duct and pancreatic duct (7,20,28). In the present study, we also experienced cases of biliary obstruction and cholangitis due to inflammatory granulation and kink associated with MS placement, as well as cholecystitis and pancreatitis. Furthermore, among MS placement, there is still some debate about the condition of the stent cover and the optimal inner diameter of the stent, for example (28). Currently, there is no stent that satisfies all functions, and endoscopic techniques require expertise. Improvements in endoscopic techniques related to the choice of diameter and length of stents and their placement position, as well as improvements in medical techniques related to the materials and shapes of stents, are expected to improve MS and reduce adverse events. Further research will be needed to validate the optimal drainage method.
There are several limitations of this study. This was a retrospective study of a small number of cases at a single institution, and there may be bias in the drainage method, operative procedures, and other factors, with historical bias in treatment selection and management. Further well-designed studies that can collect and analyze large-scale data are needed.
Conclusions
In conclusion, the results of a retrospective study at University of Miyazaki Hospital revealed that MS placement was more common in PDAC patients scheduled for PD after NAC, and MS placement was considered useful as a drainage method with longer patency and fewer PBD-related adverse events compared with PS placement. However, even when the MS is placed, the treatment schedule may be delayed due to PBD-related adverse events, and further research is needed to identify the ideal drainage method and stent.
Acknowledgments
None.
Footnote
Reporting Checklist: The authors have completed the STROBE reporting checklist. Available at https://gs.amegroups.com/article/view/10.21037/gs-2024-507/rc
Data Sharing Statement: Available at https://gs.amegroups.com/article/view/10.21037/gs-2024-507/dss
Peer Review File: Available at https://gs.amegroups.com/article/view/10.21037/gs-2024-507/prf
Funding: None.
Conflicts of Interest: All authors have completed the ICMJE uniform disclosure form (available at https://gs.amegroups.com/article/view/10.21037/gs-2024-507/coif). The authors have no conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved. This study was approved by the ethics committee of the University of Miyazaki Faculty of Medicine (No. O-1128), and conducted in accordance with the Declaration of Helsinki and its subsequent amendments. Opt-out patient consent was obtained for the study on the basis of retrospective medical information.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Miura F, Yamamoto M, Gotoh M, et al. Validation of the board certification system for expert surgeons (hepato-biliary-pancreatic field) using the data of the National Clinical Database of Japan: part 2 - Pancreatoduodenectomy. J Hepatobiliary Pancreat Sci 2016;23:353-63. [Crossref] [PubMed]
- Wu R, Zhang Y, Cheng Q, et al. The effect of biliary obstruction, biliary drainage and bile reinfusion on bile acid metabolism and gut microbiota in mice. Liver Int 2022;42:135-48. [Crossref] [PubMed]
- van der Gaag NA, Rauws EA, van Eijck CH, et al. Preoperative biliary drainage for cancer of the head of the pancreas. N Engl J Med 2010;362:129-37. [Crossref] [PubMed]
- Motoi F, Kosuge T, Ueno H, et al. Randomized phase II/III trial of neoadjuvant chemotherapy with gemcitabine and S-1 versus upfront surgery for resectable pancreatic cancer (Prep-02/JSAP05). Jpn J Clin Oncol 2019;49:190-4. [Crossref] [PubMed]
- Japan Pancreatic Society. General rules for the study of pancreatic cancer. 7th ed. Tokyo: Kanehara & Co., Ltd.; 2016.
- Japan Pancreas Society. Clinical practice guidelines for pancreatic cancer 2019. Tokyo: Kanehara & Co., Ltd.; 2019.
- Endo Y, Tanaka M, Kitago M, et al. Comparison Between Plastic and Metallic Biliary Stent Placement for Preoperative Patients with Pancreatic Head Cancer: A Systematic Review and Meta-Analysis. Ann Surg Oncol 2024;31:1319-27. [Crossref] [PubMed]
- Okusaka T, Nakamura M, Yoshida M, et al. Clinical Practice Guidelines for Pancreatic Cancer 2019 From the Japan Pancreas Society: A Synopsis. Pancreas 2020;49:326-35. [Crossref] [PubMed]
- National Comprehensive Cancer Network. Pancreatic Cancer, NCCN clinical practice guideline in oncology. Pancreatic adenocarcinoma. Version 2. 2024. Available online: https://www.nccn.org/professionals/physician_gls/pdf/pancreatic.pdf
- Bassi C, Marchegiani G, Dervenis C, et al. The 2016 update of the International Study Group (ISGPS) definition and grading of postoperative pancreatic fistula: 11 Years After. Surgery 2017;161:584-91. [Crossref] [PubMed]
- National Healthcare Safety Network. Surgical Site Infection Event (SSI). Accessed January 2024. Available online: http://www.cdc.gov/nhsn/pdfs/pscmanual/9pscssicurrent.pdf
- Dindo D, Demartines N, Clavien PA. Classification of surgical complications: a new proposal with evaluation in a cohort of 6336 patients and results of a survey. Ann Surg 2004;240:205-13. [Crossref] [PubMed]
- Krell RW, McNeil LR, Yanala UR, et al. Neoadjuvant Therapy for Pancreatic Ductal Adenocarcinoma: Propensity-Matched Analysis of Postoperative Complications Using ACS-NSQIP. Ann Surg Oncol 2021;28:3810-22. [Crossref] [PubMed]
- Darnell EP, Wang TJ, Lumish MA, et al. Preoperative cholangitis is an independent risk factor for mortality in patients after pancreatoduodenectomy for pancreatic cancer. Am J Surg 2021;221:134-40. [Crossref] [PubMed]
- Gong L, Huang X, Wang L, et al. The effect of preoperative biliary stents on outcomes after pancreaticoduodenectomy: A meta-analysis. Medicine (Baltimore) 2020;99:e22714. [Crossref] [PubMed]
- Rystedt J, Tingstedt B, Ansorge C, et al. Major intraoperative bleeding during pancreatoduodenectomy - preoperative biliary drainage is the only modifiable risk factor. HPB (Oxford) 2019;21:268-74. [Crossref] [PubMed]
- Kobayashi K, Kobara H, Kamada H, et al. Comparison of plastic stent versus metal stent in preoperative biliary drainage for pancreatic head cancer with neoadjuvant chemoradiotherapy. J Hepatobiliary Pancreat Sci 2021;28:856-63. [Crossref] [PubMed]
- Ballard DD, Rahman S, Ginnebaugh B, et al. Safety and efficacy of self-expanding metal stents for biliary drainage in patients receiving neoadjuvant therapy for pancreatic cancer. Endosc Int Open 2018;6:E714-21. [Crossref] [PubMed]
- Mandai K, Tsuchiya T, Kawakami H, et al. Fully covered metal stents vs plastic stents for preoperative biliary drainage in patients with resectable pancreatic cancer without neoadjuvant chemotherapy: A multicenter, prospective, randomized controlled trial. J Hepatobiliary Pancreat Sci 2022;29:1185-94. [Crossref] [PubMed]
- Kozakai F, Ogawa T, Koshita S, et al. Fully covered self-expandable metallic stents versus plastic stents for preoperative biliary drainage in patients with pancreatic head cancer and the risk factors for post-endoscopic retrograde cholangiopancreatography pancreatitis. DEN Open 2024;4:e263. [Crossref] [PubMed]
- Ichikawa H, Iwashita T, Iwasa Y, et al. Covered self-expandable metallic stent versus plastic stent for preoperative endoscopic biliary drainage in patients with pancreatic cancer: a multi-center retrospective cohort study. Scand J Gastroenterol 2022;57:493-500. [Crossref] [PubMed]
- Saffo S, Peng C, Salem R, et al. Impact of Neoadjuvant Chemotherapy and Pretreatment Biliary Drainage for Pancreatic Head Ductal Adenocarcinoma. Dig Dis Sci 2022;67:1409-16. [Crossref] [PubMed]
- Hasegawa S, Kubota K, Yagi S, et al. Covered metallic stent placement for biliary drainage could be promising in the coming era of neoadjuvant chemo-radiation therapy for all pancreatic cancer. J Hepatobiliary Pancreat Sci 2021;28:617-24. [Crossref] [PubMed]
- Kuwatani M, Nakamura T, Hayashi T, et al. Clinical Outcomes of Biliary Drainage during a Neoadjuvant Therapy for Pancreatic Cancer: Metal versus Plastic Stents. Gut Liver 2020;14:269-73. [Crossref] [PubMed]
- Saito K, Nakai Y, Isayama H, et al. A Prospective Multicenter Study of Partially Covered Metal Stents in Patients Receiving Neoadjuvant Chemotherapy for Resectable and Borderline Resectable Pancreatic Cancer: BTS-NAC Study. Gut Liver 2021;15:135-41. [Crossref] [PubMed]
- Tamura T, Itonaga M, Ashida R, et al. Covered self-expandable metal stents versus plastic stents for preoperative biliary drainage in patient receiving neo-adjuvant chemotherapy for borderline resectable pancreatic cancer: Prospective randomized study. Dig Endosc 2021;33:1170-8. [Crossref] [PubMed]
- Nakamura K, Sho M, Akahori T, et al. A Comparison Between Plastic and Metallic Biliary Stent Placement in Patients Receiving Preoperative Neoadjuvant Chemoradiotherapy for Resectable Pancreatic Cancer. World J Surg 2019;43:642-8. [Crossref] [PubMed]
- Seo DW, Sherman S, Dua KS, et al. Covered and uncovered biliary metal stents provide similar relief of biliary obstruction during neoadjuvant therapy in pancreatic cancer: a randomized trial. Gastrointest Endosc 2019;90:602-612.e4. [Crossref] [PubMed]


