
Correlation study of tumor-infiltrating lymphocytes (TILs) and subtypes analysis before and after neoadjuvant chemotherapy in triple-negative breast cancer
Highlight box
Key findings
• The increase of stromal tumor-infiltrating lymphocytes (sTILs) and CD8 can improve the neoadjuvant efficacy of triple-negative breast cancer (TNBC). The detection of relevant immune markers after neoadjuvant therapy could provide more prognostic and therapeutic information.
What is known and what is new?
• Neoadjuvant chemotherapy (NACT), an important component of comprehensive treatment for TNBC, can individualize the observation of chemotherapy drug response.
• The increase of TILs, CD3, and CD8 immune subsets can improve the predictive efficacy of TNBC NACT.
What is the implication, and what should change now?
• Our study deepens knowledge of the changes of immune components before and after NACT, which can provide more prognostic information for the individualized treatment of TNBC.
Introduction
According to the International Agency for Research on Cancer (IARC) data in 2021, breast cancer has the highest incidence rate compared to other cancers (1). The age of onset is also gradually becoming younger (2). About 10–20% of all breast tumors are triple-negative breast cancers (TNBCs), which are identified by the absence of hormone receptor expression and the absence of overexpression or amplified human epidermal growth factor receptor 2 (HER2) (3). With an elevated likelihood of recurrence, metastatic disease, and a poor prognosis with conventional chemotherapy, it is considered a particularly aggressive subtype of breast cancer (4).
In recent years, immune checkpoint inhibitors (ICIs) have shown potential in neoadjuvant therapy. Sahin et al. (5) have indicated that the neutrophil-to-erythrocyte ratio (NER) can serve as a prognostic marker for TNBC, with high NER levels correlating with poorer survival rates. Caputo et al. (6) have demonstrated efficacy in metastatic TNBC consistent with clinical trials, and its toxicity can be effectively managed through pretreatment. However, ICIs-related adverse events, such as hearing loss, although rare, require attention, especially regarding the impact on quality of life with long-term use (7). Additionally, while the combination of chemotherapy and immunotherapy significantly increases the rate of pathological complete response (pCR), it also raises the rate of treatment discontinuation and the risk of severe adverse events (8). Clinical trials such as KEYNOTE-522 have further confirmed the efficacy of immunotherapy in early-stage TNBC, but identifying the patient population most likely to benefit remains an area for exploration (9).
For TNBC, neoadjuvant chemotherapy (NACT) serves as a routine treatment approach. We can observe pathological, biological and even genomic changes in tumours before and after NACT, which provides an ideal in vivo model for studying tumour sensitivity to chemotherapy (10). NACT can also reduce tumour clinical staging, eliminate micrometastases, and improve breast preservation and surgical resection rates. Patients with pCR can achieve better disease-free survival and overall survival (11). However, the impact on prognosis is contingent upon developing pCR, which is impacted by the quantities of lymphocytes that infiltrate tumors and trigger the immune response against them. Nevertheless, NACT can influence the quality of immune inflammation and response.
More recently, tumour-infiltrating lymphocytes (TILs) have been recognized as a prognostic biomarker, reaching level-1b evidence (12). Additionally, a study by de Moraes et al. (13) has indicated that TILs are a critical prognostic biomarker in TNBC and may facilitate the development of personalized treatment approaches. According to the consensus standards of the International TIL Working Group, the evaluation of TILs is straightforward, affordable, and validated (14). This endorsement by international guidelines further validates the potential of TIL assessment for routine evaluation in TNBC. However, TILs may only provide a basic understanding of immune activation. Therefore, our research focuses on a more comprehensive characterization of the tumour immune microenvironment (TIME) to identify potential biomarkers that could further enhance risk prediction. This study aims to evaluate the TIME, including TILs, CD8, CD4, CD3, FOXP3, CD20, CD163, programmed cell death ligand 1 (PD-L1), and its association with the clinical and pathological response in TNBC patients in stages II–III before and after NACT.
The goal is to explore the role of the immune microenvironment in NACT of TNBC, with the potential to impact clinical practice in breast cancer treatment significantly. We present this article in accordance with the STROBE reporting checklist (available at https://gs.amegroups.com/article/view/10.21037/gs-2024-537/rc).
Methods
Patients and tissue specimens
This study comprised pathological specimens of 71 patients with phase II–III TNBC obtained from Bengbu Medical University’s First Affiliated Hospital between July 2018 and July 2023, both before and after NACT. Clinical examination, mammography, ultrasound, and core biopsy were used to reach the diagnosis. Clinical examination and imaging were utilized to assess the tumor size, which led to the clinical staging (T, N, and M categorization). Before surgery, patients were treated with docetaxel, epirubicin, and cyclophosphamide (TEC). This study was conducted in accordance with the Declaration of Helsinki (as revised in 2013). The study was approved by the Bengbu Medical University’s institutional ethics board (No. [2024] 160) and all patients gave their informed consent.
Hematoxylin-eosin (HE) staining and evaluation
Two pathologists double-blindly reviewed HE staining slides. The 5th Edition World Health Organization (WHO) and tumor-node-metastasis (TNM) staging of breast cancer, created by the American Joint Committee on Cancer (AJCC, 7th edition), is referred to in the histological evaluation standards. The International TILs Working Group’s recommended system (15) and The International Task Force on ImmunoOncology Biomarkers for Breast Cancer report (16) were followed when evaluating TILs on HE-stained representative whole-tissue sections on glass slides.
The type of inflammatory infiltration and the frequency of stromal TILs (sTILs) were assessed by examining stromal sections at low magnification (×4). The fraction of the tumor stroma that is made up of mononuclear cells, such as lymphocytes and plasma cells, is known as sTILs. Within the tumor invasion’s perimeter, scoring is carried out. The examination excludes normal lobules, atypical hyperplasia, and crush artifacts with ductal carcinoma in situ surrounded necrosis, severe hyalinization, and inflammatory reaction. Five high-magnification views (40×) were randomly selected to determine the frequency of positive cells and the mean positivity values. Microscopic analyses were evaluated independently by two investigators without prior knowledge of the clinical data. Discrepancies between the two observers were reviewed jointly with a third trained BC pathologist to reach a consensus.
sTILs were assessed on pre-treatment biopsy samples (pre-NACT TILs) for all patients and on surgical specimens (post-NACT TILs) for patients who did not achieve pCR. We selected 30% as the cut-off value of sTIL.
Immunohistochemistry procedure
In each case, tissue sections that were formalin-fixed paraffin-embedded (FFPE) were used according to a conventional procedure. The Elivision technique was used for immunohistochemistry, and HE-stained slices (4-µm thick) were reexamined to assess the tumor’s histological characteristics. Antibodies estrogen receptor (ER) (monoclonal, cloneSP1), progesterone receptor (PR) (monoclonal, clone1SP2), HER-2 (monoclonal, cloneMXR001), Ki-67 (monoclonal, clone MIB-1), CD3 (monoclonal, cloneSP7), CD4 (monoclonal, cloneSP35), CD8 (monoclonal, cloneSP16), CD20 (monoclonal, cloneL26), FOXP3 (monoclonal, clone236A/E7), CD163 (monoclonal, cloneMX081) were ready for usage and acquired from Maixin Biotech, Inc. (Fuzhou, China). PD-L1 (monoclonal, clone22C3) was obtained from Dako, North America. Positive ER and PR expression was defined as any nuclear labeling of 1% or above (17). HER-2 immunoreactivity was evaluated using a standardized scale from 0 to 3, based on the percentage of invasive tumor cells and the intensity of cell membrane staining. This assessment followed the clinical practice guidelines established by the American Society of Clinical Oncology (ASCO) and the College of American Pathologists (CAP). Intense complete membrane staining observed in over 10% of tumor cells was classified as positive (score, 3+). The fluorescence in situ hybridization (FISH) test was used to further evaluate samples with a score of 2+, while intensity patterns with scores of 0–1+ were regarded as negative. A FISH ratio of 2.0 or higher was regarded as positive for HER-2 gene amplification (18).
TILs in all samples were assessed for markers including CD8, CD4, CD3, FOXP3, CD20, and CD163. Positive cells were quantified per high-power field by manually examining stained sections, focusing on five regions with the highest staining intensity. Initially, each slide was scanned at low magnification (×100) to identify the area with the highest density of positively stained cells (hot spot), which was then selected for detailed analysis. The mean tumour-infiltrating immune cells in these areas for each case were evaluated carefully (17). For statistical analyses, the number of positive cells was divided into lower and higher groups based on cut-off points according to the median.
A combined positive score (CPS) was used to interpret PD-L1 in TNBC. CPS refers to the percentage of tumour cells, lymphocytes and macrophages with partial or complete membrane staining of any strength in all tumour cells (excluding areas of neoplastic necrosis). The positive threshold in clinical use was CPS ≥10 (19).
Evaluation of response to NACT
The pathological response was graded as either residual disease or complete response. The lack of residual invasive cancer in the breast and axilla (ypT0/Tis, ypN0) was referred to as pCR.
Statistical analysis
Statistical analyses were conducted using non-parametric methods after confirming non-normal distributions of immune cell subset counts (Shapiro-Wilk test, P<0.05). Quantitative counting of immune cell subsets was described by median, range and interquartile range (IQR). The contents of each immune index in the pre-NACT biopsy tissue and the residual lesion tissue after NACT were taken as two independent samples for statistical analysis by the Mann-Whitney U test. The Wilcoxon signed-rank difference test was performed to determine the changes in immune indexes in the paired tissues with residual lesions. The binary logistic regression analysis model was used to analyze the predictive value of clinicopathological factors and the content of immune cell subsets in patients with NACT response.
Statistical software
All data were analyzed and graphed using GraphPad Prism 9.0 and IBM SPSS Statistics 26.0 statistical software.
Results
Clinicopathological characteristics of samples
After NACT, 26 patients achieved pCR, and 45 patients achieved non-pCR. Of the 45 non-pCR patients, 6 had invasive cancer components only in the axillary lymph nodes, and 39 had residual invasive cancer lesions in the breast. The clinicopathologic characteristics of patients are presented in Table 1.
Table 1
| Clinical pathological features | Counts | % |
|---|---|---|
| Age (years) | ||
| ≤50 | 40 | 56.3 |
| >50 | 31 | 43.7 |
| Pre-tumor size (cm) | ||
| ≤5.0 (cT2) | 29 | 40.8 |
| >5.0 (cT3–4) | 42 | 59.2 |
| Pre-lymph node status | ||
| cN0 | 21 | 29.6 |
| cN1–2 | 27 | 38.0 |
| cN3 | 23 | 32.4 |
| Pre-clinical TNM classification | ||
| Stage cII | 30 | 42.3 |
| Stage cIII | 41 | 57.7 |
| Pre-NACT grade | ||
| Grade 2 | 24 | 33.8 |
| Grade 3 | 47 | 66.2 |
| Pre-Ki-67 | ||
| <30% | 6 | 8.5 |
| ≥30% | 65 | 91.5 |
| Post-tumor size | ||
| ypT0/Tis | 32 | 45.1 |
| ypT1 | 26 | 36.6 |
| ypT2–4 | 13 | 18.3 |
| Post-lymph node status | ||
| ypN0 | 37 | 52.1 |
| ypN1 | 26 | 36.6 |
| ypN2–3 | 8 | 11.3 |
| Post-TNM classification | ||
| pCR | 26 | 36.6 |
| ypI | 14 | 19.7 |
| ypII | 22 | 31.0 |
| ypIII | 9 | 12.7 |
| Pre-sTILs | ||
| Low | 46 | 64.8 |
| High | 25 | 35.2 |
| Pre-PD-L1 | ||
| Negative | 52 | 73.2 |
| Positive | 19 | 26.8 |
Pre, before NACT; Post, after NACT. NACT, neoadjuvant chemotherapy; pCR, pathological complete response; PD-L1, programmed cell death ligand 1; sTILs, stromal tumor-infiltrating lymphocytes; TNM, tumor-node-metastasis.
Comparison of baseline characteristics between pCR and non-pCR group (Table 2)
Table 2
| Clinical pathological features | pCR (N=26) | Non-pCR (N=45) | χ2 | P | |||
|---|---|---|---|---|---|---|---|
| Count | % | Counts | % | ||||
| Age (years) | 0.031 | 0.94 | |||||
| <50 | 15 | 57.7 | 25 | 55.6 | |||
| ≥50 | 11 | 42.3 | 20 | 44.4 | |||
| Pre-tumor size (cm) | 7.270 | 0.01 | |||||
| ≤5.0 (cT2) | 16 | 61.5 | 13 | 28.9 | |||
| >5.0 (cT3–4) | 10 | 38.5 | 32 | 71.1 | |||
| Pre-lymph node status | 2.148 | 0.34 | |||||
| cN0 | 9 | 34.6 | 12 | 26.7 | |||
| cN1 | 7 | 26.9 | 20 | 44.4 | |||
| cN2 + N3 | 10 | 38.5 | 13 | 28.9 | |||
| Pre-clinical stages | 2.259 | 0.21 | |||||
| Stage cII | 14 | 53.8 | 16 | 35.6 | |||
| Stage cIII | 12 | 46.2 | 29 | 64.4 | |||
| Histological grade | 10.463 | 0.003 | |||||
| Grade 1–2 | 15 | 57.7 | 9 | 20.0 | |||
| Grade 3 | 11 | 42.3 | 36 | 80.0 | |||
| Pre-Ki-67 | 6.162 | 0.041 | |||||
| <30% | 5 | 19.2 | 1 | 2.2 | |||
| ≥30% | 21 | 80.8 | 44 | 97.8 | |||
| Pre-sTILs | 16.371 | <0.001 | |||||
| Low | 9 | 34.6 | 37 | 82.3 | |||
| High | 17 | 65.4 | 8 | 17.7 | |||
| Pre-PD-L1 | 0.336 | 0.76 | |||||
| Negative | 18 | 69.2 | 34 | 75.6 | |||
Pre, before NACT. pCR, pathological complete response; PD-L1, programmed cell death ligand 1; sTILs, stromal tumor-infiltrating lymphocytes.
There were statistically significant differences in tumour size (χ2=7.270, P=0.01), histological grade (χ2=10.463, P=0.003), Ki-67 status (χ2=6.162, P=0.041) and sTILs content (χ2=16.371, P<0.001) in pCR and non-pCR groups before NACT. There were no significant differences in age, lymph node status, clinical stage and PD-L1 expression before NACT.
The relationship between sTILs, PD-L1 and clinicopathological features before NACT
The baseline levels of sTILs and PD-L1 before NACT and their relationship with clinicopathological features are presented in Table 3. Among the 71 patients, there were 46 cases (64.8%) with low sTILs and 25 cases (35.2%) with high sTILs. There were 52 (73.2%) PD-L1 negative group and 19 (26.8%) PD-L1 positive group. Pre-sTILs were positively correlated with histological grade (rs =0.340, P=0.004) and negatively correlated with lymph node status (rs =−0.364, P=0.002). PD-L1 status was correlated with tumor size (χ2=6.740, P=0.009) and lymph node status (χ2=7.395, P=0.03).
Table 3
| Clinical pathological features | Total | Pre-sTILs | PD-L1 | |||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Low | High | rs | P | Negative | Positive | χ2 | P | |||
| Age (years) | −0.114 | 0.34 | 0.145 | 0.70 | ||||||
| <50 | 40 | 24 | 16 | 30 | 10 | |||||
| ≥50 | 31 | 22 | 9 | 22 | 9 | |||||
| Pre-T | 0.133 | 0.27 | 6.740 | 0.009 | ||||||
| cT2 | 29 | 21 | 8 | 26 | 3 | |||||
| cT3–4 | 42 | 25 | 17 | 26 | 16 | |||||
| Pre-N | −0.364 | 0.002 | 7.395 | 0.03 | ||||||
| cN0 | 21 | 8 | 13 | 20 | 1 | |||||
| cN1 | 27 | 18 | 9 | 17 | 10 | |||||
| cN2 + N3 | 23 | 20 | 3 | 15 | 8 | |||||
| Pre-TNM | 0.153 | 0.20 | 1.211 | 0.27 | ||||||
| cII | 30 | 22 | 8 | 23 | 7 | |||||
| cIII | 41 | 24 | 17 | 29 | 12 | |||||
| Histological grade | 0.340 | 0.004 | 0.650 | 0.42 | ||||||
| Grade 1–2 | 24 | 21 | 3 | 19 | 5 | |||||
| Grade 3 | 47 | 25 | 22 | 33 | 14 | |||||
| Pre-Ki-67 | 0.012 | 0.92 | 1.806 | 0.18 | ||||||
| <30% | 6 | 4 | 2 | 3 | 3 | |||||
| ≥30% | 65 | 42 | 23 | 49 | 16 | |||||
Pre, before NACT. NACT, neoadjuvant chemotherapy; PD-L1, programmed cell death ligand 1; sTILs, stromal tumor-infiltrating lymphocytes; TNM, tumor-node-metastasis.
sTILs and PD-L1 status of residual lesions in 39 patients with non-pCR were also detected: 22 cases (56.4%) were in the low-sTILs group, and 17 cases (43.6%) were in the high-sTILs group. There were 21 cases (53.8%) in PD-L1 negative group and 18 cases (46.2%) in PD-L1 positive group. The proportion of high-sTILs and PD-L1 positive group in residual lesions after NACT was significantly increased compared to before treatment.
Levels of immune cell subsets in pre-NACT biopsy and post-NACT residual lesion (Table 4)
Table 4
| Immune subsets | Pre-NACT biopsy | Post-NACT residual lesion | |||||
|---|---|---|---|---|---|---|---|
| Median | Range | IQR | Median | Range | IQR | ||
| CD3 | 122 | 15–390 | 70–183 | 167 | 61–426 | 92–220 | |
| CD163 | 117 | 10–343 | 75–195 | 132 | 41–323 | 91–176 | |
| CD4 | 103 | 5–370 | 65–175 | 124 | 45–375 | 82–182 | |
| CD8 | 85 | 12–320 | 60–135 | 137 | 51–393 | 87–184 | |
| FOXP3 | 47 | 0–145 | 26–77 | 57 | 8–119 | 38–75 | |
| CD20 | 31 | 0–135 | 19–55 | 35 | 8–172 | 25–63 | |
IQR, interquartile range; NACT, neoadjuvant chemotherapy.
The level of different immune subsets in TNBC biopsy and residual lesion was obviously heterogeneous, with the low content being completely zero and the high content reaching more than 400/high power field (HPF). According to the median, the content of pre-NACT immune subsets ranked from high to low as follows: CD3, CD163, CD4, CD8, FOXP3 and CD20.
Immunohistochemical staining
Immunohistochemical staining demonstrates the expression of immune markers, including CD3, CD4, CD8, FOXP3, CD20, and CD163, in tumor tissues. A significant increase in CD3+ and CD8+ T cells is observed in residual lesions after NACT, suggesting that chemotherapy may enhance T lymphocyte infiltration by activating immune responses. Additionally, increased expression of CD4 and CD20 indicates potential roles of helper T cells and B lymphocytes in post-chemotherapy immune modulation. These findings provide valuable insights into the dynamic changes in the tumor immune microenvironment and highlight the potential for immune-based therapeutic strategies in TNBC (as shown in Figure 1).
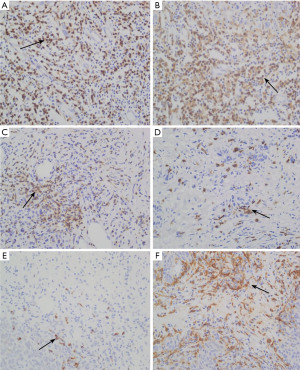
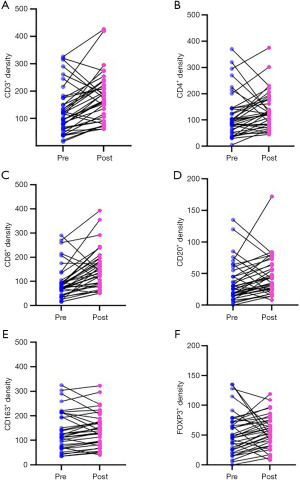
The content of immune cell subsets before and after NACT as independent sample test
Mann-Whitney U test was performed on 71 samples of pre-NACT biopsy and 39 samples of residual lesion, and the content of immune cell subsets increased in different degrees. Statistical analysis showed that the content of CD3 and CD8 positive cells in residual lesion increased significantly. The median CD3 before and after NACT were 122 (IQR, 70–183) and 167 (IQR, 92–220) (Z=−2.365, P=0.02). The median CD8 before and after NACT were 85 (IQR, 60–135) and 137 (IQR, 87–184) (Z=−2.972, P=0.003). The P values of other immune cell subsets were all >0.05, which had no statistical significance (as shown in Figure 2).

The content of immune cell subsets before and after NACT as paired samples
In 39 patients with residual lesions after NACT, compared with their pre-NACT paired tissues, CD3, CD4, CD8, and CD20 contents increased significantly (as shown in Figure 3). The median CD3 before and after NACT were 104 (IQR, 64–175) and 167 (IQR, 92–220) (Z=−3.405, P<0.001). The median CD4 before and after NACT were 93 (IQR, 60–145) and 124 (IQR, 82–182) (Z=−2.463, P=0.01). The median CD8 before and after NACT were 78 (IQR, 59–96) and 137 (IQR, 87–183) (Z=−4.407, P<0.001). The median CD20 before and after NACT were 26 (IQR, 15–45) and 35 (IQR, 25–63) (Z=−2.241, P=0.03).
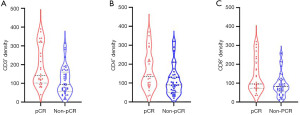
Relationship between immune cell subsets with pCR before NACT
The contents of CD3, CD4 and CD8 before NACT in pCR patients were significantly higher than those in the non-pCR group. The median CD3 in pCR and non-pCR patients were 142 (IQR, 102–320) and 93 (IQR, 57–160) (Z=−3.187, P=0.001). The median CD4 in pCR and non-pCR patients were 134 (IQR, 85–221) and 90 (IQR, 51–136) (Z=−2.262, P=0.02), and the median CD8 in pCR and non-pCR patients were 95 (IQR, 73–212) and 82 (IQR, 55–113) (Z=−2.215, P=0.03). The two groups had no significant difference in other immune cell subsets with P values >0.05 (as shown in Figure 4).
Univariate and multivariate analysis of pCR after NACT (Table 5)
Table 5
| Pathological features and immune subsets | Univariate factor analysis | Multivariate factor analysis | |||||
|---|---|---|---|---|---|---|---|
| HR | 95% CI | P | HR | 95% CI | P | ||
| Size (cT2/cT3–4) | 1.812 | 0.679–4.837 | 0.24 | – | – | – | |
| Histological grade (1–2/3) | 1.384 | 0.503–3.804 | 0.53 | – | – | – | |
| Ki-67 (<30%/≥30%) | 0.320 | 0.035–2.901 | 0.31 | – | – | – | |
| Post-sTILs (low/high) | 0.114 | 0.038–0.348 | 0.001 | 0.194 | 0.039–0.975 | 0.047 | |
| Post-CD3 (low/high) | 0.224 | 0.078–0.643 | 0.005 | 2.645 | 0.327–21.375 | 0.36 | |
| Post-CD4 (low/high) | 0.166 | 0.055–0.498 | 0.001 | 0.433 | 0.068–2.767 | 0.38 | |
| Post-CD8 (low/high) | 0.245 | 0.087–0.689 | 0.008 | 0.240 | 0.060–0.960 | 0.044 | |
Post, after NACT. CI, confidence interval; HR, hazard ratio; NACT, neoadjuvant chemotherapy; pCR, pathological complete response; sTILs, stromal tumor-infiltrating lymphocytes; TNBC, triple-negative breast cancer.
Univariate analysis showed that the contents of post-sTILs, post-CD3, post-CD4 and post-CD8 were correlated with both pCR and non-pCR. Further multivariate regression analysis showed that post-sTILs and post-CD8 were independent predictors of TNBC neoadjuvant efficacy.
Discussion
In recent years, an increasing number of studies have focused on the significant role of the immune microenvironment in the development, suppression, drug resistance, and targeted therapy of tumors. sTILs constitute a critical component of the immune microenvironment in TNBC. sTILs represent a heterogeneous population of lymphocytes predominantly found within the tumor stroma, encompassing T lymphocytes, B lymphocytes, and plasma cells. CD8+ T lymphocytes can directly recognize and eliminate tumor cells via mechanisms including the release of perforin and granzyme, activation of the Fas-FasL pathway, and secretion of cytokines. Those pathways that kill tumor cells can enhance chemotherapy’s efficacy in TNBC. Additionally, chemotherapy drugs not only exert direct cytotoxic effects on tumor cells but also induce the release of tumor antigens and damage-associated molecular patterns (DAMPs), thereby promoting sTIL infiltration and activation, enhancing the anti-tumor immune response and ultimately improving the overall effectiveness of chemotherapy (20,21). In TNBC, the levels and composition of sTILs are strongly associated with chemotherapy response and patient prognosis. Higher sTIL levels generally indicate a more favorable response to chemotherapy and improved clinical outcomes (15,22). The immune cell subsets within the tumor microenvironment (TME) of TNBC encompass T cells, B cells, natural killer (NK) cells, dendritic cells (DCs), and others. Each cell type exerts distinct functions: CD8+ cytotoxic T cells can directly eliminate tumor cells, while CD4+ helper T cells coordinate and enhance immune responses. Regulatory T cells (Tregs) and myeloid-derived suppressor cells (MDSCs), on the other hand, may suppress immune responses and facilitate tumor progression (22). Consequently, the quantity and functional status of sTILs and immune subsets collectively determine the efficacy of immune-mediated tumor clearance, influencing the response to chemotherapy and patient prognosis.
The changes in the TME affect the efficacy of ICIs through multiple mechanisms. Immune cell infiltration and functional status are critical determinants of the efficacy of ICIs. CD8+ T cells, as the primary effector cells in anti-tumor immunity, play a pivotal role in ICIs effectiveness, with their infiltration levels and functional activities directly influencing therapeutic outcomes (23). Tregs and tumor-associated macrophages (TAMs), on the other hand, can undermine the anti-tumor effects of ICIs by secreting immunosuppressive factors such as IL-10 and TGF-β and by recruiting additional immunosuppressive cells. Furthermore, the TME modulates the expression levels of immune checkpoint molecules, including PD-L1, LAG-3, and TIM-3 (24). Elevated PD-L1 expression is generally associated with improved responses to ICIs; however, upregulation of other immune checkpoints may contribute to resistance (25). Furthermore, immunosuppressive factors within the TME, such as metabolic products including lactic acid and adenosine, along with low tumor mutational burden (TMB), can diminish the efficacy of ICIs by inhibiting T-cell activity and limiting neoantigen generation (26). Finally, ICIs treatment can dynamically alter the TME, such as increasing CD8+ T cell infiltration. The TME may also develop resistance through adaptive mechanisms (such as upregulating other immune checkpoints) (27). Therefore, a deeper understanding of the dynamic changes in the immune microenvironment and their regulatory mechanisms is of great significance for optimizing ICIs treatment strategies and developing combination therapies.
NACT, an important means of comprehensive treatment for TNBC, has gradually been used clinically in recent years. The benefits of NACT extend beyond pCR to tumour downstaging, permitting conservative surgical options in the breast and axilla. Assessment of response provides valuable prognostic information to enable escalation and de-escalation of adjuvant therapy to optimise oncological outcomes (28).
With the deepening of research on the TIME, more and more evidence shows that chemotherapy drugs not only kill tumours directly through cytotoxicity but also activate the body’s anti-tumour immune response to achieve the effect of tumour suppression (29). It has been reported that the changes in immune components in the tumour microenvironment before and after NACT can provide predictive indicators for tumour immunotherapy and patient prognosis (30,31).
In this study, we investigated the correlation between sTILs and treatment outcomes in cancer patients undergoing NACT. We found that the level of sTILs in biopsy before NACT was correlated with histological grade (P=0.004) and lymph node metastasis (P=0.002). The pre-sTILs level reflects the immune status of the body. If the body’s immune status is stronger, the ability to limit tumours is naturally more substantial, and the possibility of tumour invasion and metastasis is relatively slight. After NACT, the proportion of the high sTILs group in residual lesions was higher than before. The increase of sTILs in residual lesions is the body’s response to chemotherapy drugs. This also reflects that chemotherapy drugs can not only exert cytotoxicity to kill tumour cells directly but also mobilize the immune state of the body. Binary logistic analysis showed that the level of pre-sTILs was positively correlated with the pCR rate. A study by Dieci et al. (32) confirmed that an increase in sTILs before NACT was associated with a higher pCR rate and that pCR patients had higher levels of sTILs before treatment compared to non-pCR patients. Dieci et al.’s study supports our findings. There is a correlation between the basic immune status of patients and the efficacy of NACT. Luen et al. found that low sTIL levels in the tumours before NACT were associated with large residual tumour lesions and more metastatic lymph nodes, while those with high sTIL levels had a relatively good prognosis (33). We can extrapolate that sTILs in TNBC neoadjuvant therapy have a specific predictive value. When chemotherapy drugs kill the tumour, they make the tumour cells release related antigens and enhance the anti-tumour sensitivity of the body. In patients with high sTILs, in addition to chemotherapy drugs killing tumour cells, the body’s adaptive immunity will also further inhibit tumours. However, patients with low sTILs find it difficult to produce sufficient immune responses themselves. Even if stimulated by tumour-related antigen, it is hard to generate potent immune killing, which means that patients with low sTILs can only rely on the cytotoxic effect of chemotherapy drugs to kill tumours, and it is difficult to achieve ideal pathological remission outcomes.
As immune checkpoint inhibitors and other forms of immunotherapy prove successful in clinical settings, the need for tumour immune markers that are widely applicable, accessible, and reliable in clinics is increasingly emphasized (34). Considering the limited tumour fraction in biopsy and the heterogeneity of TNBC, in this study, we selected six clinically accessible immune markers (CD8, CD4, CD3, FOXP3, CD20, CD163) to characterize the TNBC tumour microenvironment status. The highest content of CD3 and the lowest content of CD20 in sTILs of biopsy before NACT indicated that the immune infiltrating cells before treatment were mainly T lymphocytes and B lymphocytes were relatively few. Statistical results showed that before NACT, the levels of CD3, CD4 and CD8 immune subsets in patients who achieved pCR were significantly higher than those in the non-pCR group. In the independent sample test before and after NACT, the counts of CD3 and CD8 increased significantly and in the paired sample test before and after NACT, the increases of CD3, CD4, CD8, and CD20 in residual lesions were statistically higher than before. Our results confirm that CD8+ T lymphocytes have a predictive value for neoadjuvant therapy similar to that of sTILs and also suggest the potential value of CD3+ and CD4+ T lymphocytes in predicting neoadjuvant therapy for TNBC. Miskad’s study suggested that the number of CD8 subsets was proportional to sTILs and positively correlated with neoadjuvant therapy’s efficacy and prognostic value (35). García-Martínez et al. (36) also showed that high infiltration of CD4+ T lymphocytes was associated with high pCR rates, verified in the public genome dataset. Castaneda et al. (37) found that a higher ratio of CD8/CD4 before NACT was associated with pCR. Similar studies support that appropriate immune subset testing can provide additional information on the efficacy and prognosis of NACT (38,39).
Compared with previous studies by Dieci, García-Martínez, and Castaneda, our research provides several novel insights. First, we comprehensively analyzed multiple immune cell subsets, including CD8, CD4, CD3, FOXP3, CD20, and CD163. This approach offers a more detailed characterization of TIME compared to earlier studies that primarily focused on TILs or specific subsets such as CD4 and CD8 T cells. Second, our study uniquely evaluated the dynamic changes in the immune profile before and after NACT, revealing a significant increase in CD3 and CD8 T cells within residual lesions, underscoring chemotherapy’s immunomodulatory effects. Third, we identified pre-NACT CD8 T cells and sTILs as independent predictors of pCR while highlighting the potential predictive roles of CD3 and CD4 T cells, thereby providing a more nuanced understanding of immune biomarkers in TNBC. Additionally, we observed a significant increase in PD-L1 expression in residual lesions post-NACT, suggesting enhanced potential for immunotherapy in these patients—a finding not fully explored in earlier studies. Finally, we conducted a detailed heterogeneity analysis of immune cell subsets, mainly focusing on different infiltration patterns in residual tumors, offering new insights into the complex interplay between chemotherapy and immune responses. Collectively, these findings advance our understanding of the dynamic nature of TIME in TNBC and provide valuable biomarkers for predicting NACT efficacy and guiding subsequent immunotherapy strategies.
In our group, because of the low content of CD20+ B lymphocytes and the limitation and heterogeneity of biopsy, CD20 has not been confirmed as a predictor in NACT. FOXP3, as a marker of Treg helper T cell, has been suggested to play a negative inhibitory role in the immune microenvironment in literature, and this indicator is associated with poor prognosis in TNBC (40). In our study, we found that FOXP3, as a marker of nuclear antibody, was less sensitive than other membrane-positive antibodies, possibly because antigen information was quickly lost. In actual detection, the number of positive cells was small, so it was unsuitable for biopsy detection. Although CD163 was detected in a large amount in this study, it did not show positive results and obvious predictive value, which may be related to the type of sample, the accompanying reaction, and the sensitivity and specificity of the markers. Thus, the detection of immune subsets in neoadjuvant therapy still needs to explore more specific and stable indicators.
The above experiments confirmed the independent predictive value of pre-sTILs and pre-CD8 for the efficacy of NACT for TNBC. Furthermore, when immune indicators such as CD3, CD4 and CD8 were incorporated into the multivariate model that included tumour size, histological grade, and pre-sTILs, a more comprehensive understanding of tumour immune status was obtained along with additional predictive information. Studies have shown that prior treatment sTIL levels and specific immune cell content predict the response to NACT and have important predictive value for prognosis (41,42). However, the time of neoadjuvant therapy for TNBC in our centre is still short, and no long-term positive end-point events have occurred in our group, so this experiment failed to study the correlation with long-term prognosis, which is also a regret of this study.
While exploring sTILs and immune subsets in pre-NACT biopsy, we also paid attention to the observation of residual lesions after NACT. In the process of practical observation, we found that selecting appropriate regions and accurately identifying relevant sTILs were the two most pivotal challenges. After NACT, tumours may undergo centripetal and centrifugal retraction, accompanied by varying degrees of necrosis, collagenization, foam cell aggregation, etc. These complex changes pose challenges to assessing sTILs in residual tumours following NACT. We observed a significantly higher number of immune cells surrounding the retraction or dispersed near apoptotic tumour cells than those within and distant from the tumour nest. This phenomenon may be attributed to the release of tumour-related antigens during apoptosis or retraction, which attracts a substantial infiltration of immune cells. This change in the content of immune subsets in residual tumours reflects the effect of chemotherapy on the immune microenvironment, and the assessment of immune cell infiltration in the residual tumours may provide further prognostic information.
In the context of immune checkpoint inhibitors application in TNBC patients, to enhance the remission rate of neoadjuvant therapy, certain studies have proposed incorporating immunotherapy either after conventional chemotherapy or concurrently with chemotherapy, aiming to explore the change of TIME following neoadjuvant therapy and further explore its correlation with therapeutic efficacy (43,44). In this study, the expression of PD-L1 (22C3) was also detected in the specimens before and after NACT. The results revealed that prior to NACT, 19 cases (26.8%) exhibited positive PD-L1 expression, which was significantly associated with tumour size (P=0.009) and lymph node metastasis (P=0.03). The positive rate of PD-L1 in residual lesions was 46.2%, which exhibited a significant increase compared to the pre-NACT levels. This finding implies enhanced potential for immunotherapy benefits among patients with residual lesions, highlighting the need for further sequential adjuvant chemotherapy or clinical trials. Moreover, it provides a foundation for exploring immune cell reactivation in residual lesions and its application in immunotherapy.
This study is a relatively comprehensive study exploring sTILs, immune subsets, and PD-L1 changes in the TNBC microenvironment before and after NACT. We meticulously analyzed the specificity and sensitivity of CD8, CD4, CD3, FOXP3, CD20, and CD163 antibodies in biopsy specimens and residual lesions to identify the most reliable indicators for assessing therapeutic efficacy and immune status. The increase of sTILs, CD8, can improve the neoadjuvant efficacy of TNBC. Adding CD3 and CD8 immune subsets can improve the efficacy of TNBC neoadjuvant prediction. Detecting relevant immune markers after neoadjuvant therapy could provide more prognostic and therapeutic information.
When evaluating sTILs in residual lesions after NACT for TNBC, researchers encounter several challenges that significantly affect the interpretation of results. First, the heterogeneous regression patterns of tumor tissue, including centripetal and centrifugal regression, complicate the accurate delineation of the tumor bed, thereby influencing the selection of appropriate regions for sTILs assessment. Second, post-chemotherapy changes in tumor tissue, such as necrosis, collagenization, and foam cell aggregation, can obscure the true distribution of sTILs, complicating their identification and quantification. Moreover, the spatial heterogeneity of sTILs within the TME, characterized by significant variations in density across different regions, increases the risk of misinterpreting sTIL levels if sampling is not representative. These factors collectively pose substantial challenges to the accurate assessment of TILs in residual lesions following neoadjuvant therapy.
The findings of our study carry substantial clinical implications, particularly in guiding the selection of adjuvant chemotherapy or sequential treatment strategies for TNBC patients. The results demonstrate that higher levels of sTILs and CD8+ T cells prior to NACT are independent predictors of pCR. This suggests that these immune markers can serve as indicators to identify patients who are more likely to benefit from NACT. Furthermore, the observed increase in sTILs and PD-L1 expression in residual lesions post-NACT indicates that immunotherapy may offer more significant benefits for patients with residual disease. This underscores the importance of incorporating ICIs or other immunotherapies into the adjuvant setting for such patients. Additionally, the dynamic changes in immune cell subsets, especially the significant increases in CD3, CD4, and CD8 T cells within residual lesions, provide valuable prognostic information and facilitate personalized treatment planning following NACT. These insights not only enhance the accuracy of predicting NACT efficacy but also establish a theoretical foundation for personalized treatment plans, potentially improving the long-term prognosis of TNBC patients.
Our study holds significant potential value in NACT for TNBC, particularly in predicting the efficacy of NACT and exploring the tumor immune microenvironment. The research found that sTILs and specific immune cell subsets (CD3, CD4, CD8) can serve as biomarkers for predicting the efficacy of NACT, which is helpful for the formulation of personalized treatment strategies. Additionally, the study revealed an increase in PD-L1 expression after NACT, suggesting the potential application of immunotherapy in patients with residual disease. However, the study still has some knowledge gaps, such as the lack of long-term follow-up data to verify the prognostic value of these immune markers, and the heterogeneity in the expression of immune markers (such as CD20, FOXP3, CD163) limits their applicability as reliable predictive indicators. Moreover, the specific mechanism by which chemotherapy enhances the immune response has not been fully elucidated. To bridge these gaps, subsequent studies should focus on refining TNBC treatment approaches by implementing longitudinal analyses, identifying new biomarkers, elucidating underlying mechanisms, and evaluating combination therapies in clinical trials. Standardized methods for assessing immune infiltration will also help improve the comparability and reliability of research results.
This study is a relatively comprehensive exploration of the changes in sTILs, immune subgroups and PD-L1 expression in the microenvironment of TNBC after NACT. The limitations of this study include the difficulty in achieving more detailed classification of immune subgroups due to the limitations of clinical immunohistochemical antibodies, and the possibility of overlapping cells identified by different antibodies. The limitations of biopsy samples and tumor heterogeneity are also important issues that cannot be ignored in the counting of TILs and immune subgroups in this study. The center has been conducting NACT for TNBC for a relatively short time, with a limited sample size and a short follow-up period, and has not obtained five-year survival prognosis information. Currently, only the pathological response is used as the short-term endpoint. However, as the most accessible and beneficial method for patients in clinical practice, this study is of great significance. Based on clinical practice, we carefully analyzed the specificity and sensitivity of several antibodies, including CD8, CD4, CD3, FOXP3, CD20, and CD163, in staining of biopsy tissues and residual lesions after neoadjuvant therapy, and selected the indicators that best reflect the treatment effect and immune status. Although the sample size is limited, the combined quantitative analysis of multiple markers involves a large amount of data and work. In the study, two senior breast pathologists selected sufficient fields of view from each slice at different times for evaluation to reduce subjective differences. We will continue to expand the sample size and extend the follow-up period, and incorporate prognosis information to further improve and validate the results of this study. If conditions permit, we will use multiplex immunofluorescence staining or single-cell sequencing methods to further conduct in-depth research on the immune microenvironment of TNBC in the future.
In the next five years, significant progress is expected in the treatment of TNBC, particularly in the combination of NACT and immunotherapy, precision treatment strategies, combined treatment regimens, and the update of clinical guidelines. The combination of NACT and immunotherapy will become a research focus. For instance, the KEYNOTE-522 study demonstrated that this combined treatment strategy not only enhances the direct cytotoxic effect of chemotherapy but also improves efficacy by activating anti-tumor immune responses (9). Immunotherapy will become more precise, with the identification and utilization of specific biomarkers to predict patients’ responses to immunotherapy. Additionally, novel immunotherapy strategies, such as CAR-T cell therapy, vaccine therapy, and antibody-drug conjugates (ADCs), will be further explored and optimized. Combined treatment strategies will become the mainstream direction, such as the application of gossamer in combination with immune checkpoint inhibitors or PARP inhibitor (PARPi) in neoadjuvant treatment of TNBC. Personalized treatment plans will become more widespread, with the precise identification of patients’ immune microenvironment characteristics and molecular features to formulate individualized treatment plans (45).
Conclusions
The increase of sTILs and CD8 can improve the neoadjuvant efficacy of TNBC. Adding CD3 and CD8 immune subsets can also improve the efficacy of TNBC neoadjuvant prediction. Detecting relevant immune markers after neoadjuvant therapy could provide more prognostic and therapeutic information.
Acknowledgments
None.
Footnote
Reporting Checklist: The authors have completed the STROBE reporting checklist. Available at https://gs.amegroups.com/article/view/10.21037/gs-2024-537/rc
Data Sharing Statement: Available at https://gs.amegroups.com/article/view/10.21037/gs-2024-537/dss
Peer Review File: Available at https://gs.amegroups.com/article/view/10.21037/gs-2024-537/prf
Funding: This study was supported by
Conflicts of Interest: All authors have completed the ICMJE uniform disclosure form (available at https://gs.amegroups.com/article/view/10.21037/gs-2024-537/coif). The authors have no conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved. This study was conducted in accordance with the Declaration of Helsinki (as revised in 2013). The study was approved by the Bengbu Medical University’s institutional ethics board (No. [2024] 160) and all patients gave their informed consent.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Sung H, Ferlay J, Siegel RL, et al. Global Cancer Statistics 2020: GLOBOCAN Estimates of Incidence and Mortality Worldwide for 36 Cancers in 185 Countries. CA Cancer J Clin 2021;71:209-49. [Crossref] [PubMed]
- Cao W, Chen HD, Yu YW, et al. Changing profiles of cancer burden worldwide and in China: a secondary analysis of the global cancer statistics 2020. Chin Med J (Engl) 2021;134:783-91. [Crossref] [PubMed]
- Bianchini G, De Angelis C, Licata L, et al. Treatment landscape of triple-negative breast cancer - expanded options, evolving needs. Nat Rev Clin Oncol 2022;19:91-113. [Crossref] [PubMed]
- Thomas A, Reis-Filho JS, Geyer CE Jr, et al. Rare subtypes of triple negative breast cancer: Current understanding and future directions. NPJ Breast Cancer 2023;9:55. [Crossref] [PubMed]
- Sahin TK, Ayasun R, Rizzo A, et al. Prognostic Value of Neutrophil-to-Eosinophil Ratio (NER) in Cancer: A Systematic Review and Meta-Analysis. Cancers (Basel) 2024;16:3689. [Crossref] [PubMed]
- Caputo R, Buono G, Piezzo M, et al. Sacituzumab Govitecan for the treatment of advanced triple negative breast cancer patients: a multi-center real-world analysis. Front Oncol 2024;14:1362641. [Crossref] [PubMed]
- Rizzo A, Schipilliti FM, Di Costanzo F, et al. Discontinuation rate and serious adverse events of chemoimmunotherapy as neoadjuvant treatment for triple-negative breast cancer: a systematic review and meta-analysis. ESMO Open 2023;8:102198. [Crossref] [PubMed]
- Guven DC, Erul E, Kaygusuz Y, et al. Immune checkpoint inhibitor-related hearing loss: a systematic review and analysis of individual patient data. Support Care Cancer 2023;31:624. [Crossref] [PubMed]
- Rizzo A, Cusmai A, Acquafredda S, et al. KEYNOTE-522, IMpassion031 and GeparNUEVO: changing the paradigm of neoadjuvant immune checkpoint inhibitors in early triple-negative breast cancer. Future Oncol 2022;18:2301-9. [Crossref] [PubMed]
- Korde LA, Somerfield MR, Carey LA, et al. Neoadjuvant Chemotherapy, Endocrine Therapy, and Targeted Therapy for Breast Cancer: ASCO Guideline. J Clin Oncol 2021;39:1485-505. [Crossref] [PubMed]
- van den Ende NS, Nguyen AH, Jager A, et al. Triple-Negative Breast Cancer and Predictive Markers of Response to Neoadjuvant Chemotherapy: A Systematic Review. Int J Mol Sci 2023;24:2969. [Crossref] [PubMed]
- Loi S, Michiels S, Adams S, et al. The journey of tumor-infiltrating lymphocytes as a biomarker in breast cancer: clinical utility in an era of checkpoint inhibition. Ann Oncol 2021;32:1236-44. [Crossref] [PubMed]
- de Moraes FCA, Souza MEC, Sano VKT, et al. Association of tumor-infiltrating lymphocytes with clinical outcomes in patients with triple-negative breast cancer receiving neoadjuvant chemotherapy: a systematic review and meta-analysis. Clin Transl Oncol 2025;27:974-87. [Crossref] [PubMed]
- Romagnoli G, Wiedermann M, Hübner F, et al. Morphological Evaluation of Tumor-Infiltrating Lymphocytes (TILs) to Investigate Invasive Breast Cancer Immunogenicity, Reveal Lymphocytic Networks and Help Relapse Prediction: A Retrospective Study. Int J Mol Sci 2017;18:1936. [Crossref] [PubMed]
- Salgado R, Denkert C, Demaria S, et al. The evaluation of tumor-infiltrating lymphocytes (TILs) in breast cancer: recommendations by an International TILs Working Group 2014. Ann Oncol 2015;26:259-71. [Crossref] [PubMed]
- Dieci MV, Radosevic-Robin N, Fineberg S, et al. Update on tumor-infiltrating lymphocytes (TILs) in breast cancer, including recommendations to assess TILs in residual disease after neoadjuvant therapy and in carcinoma in situ: A report of the International Immuno-Oncology Biomarker Working Group on Breast Cancer. Semin Cancer Biol 2018;52:16-25. [Crossref] [PubMed]
- Goldhirsch A, Wood WC, Coates AS, et al. Strategies for subtypes--dealing with the diversity of breast cancer: highlights of the St. Gallen International Expert Consensus on the Primary Therapy of Early Breast Cancer 2011. Ann Oncol 2011;22:1736-47. [Crossref] [PubMed]
- Wolff AC, Hammond MEH, Allison KH, et al. Human Epidermal Growth Factor Receptor 2 Testing in Breast Cancer: American Society of Clinical Oncology/College of American Pathologists Clinical Practice Guideline Focused Update. J Clin Oncol 2018;36:2105-22. [Crossref] [PubMed]
- Pathology Quality Control Center, Chinese Society of Pathology, Pathology Committee of Chinese Society of Clinical Oncology. Consensus on the immunohistochemical tests of PD-L1 in solid tumors (2021 version). Chinese Journal of Pathology 2021;50:710-8. [PubMed]
- Fridman WH, Zitvogel L, Sautès-Fridman C, et al. The immune contexture in cancer prognosis and treatment. Nat Rev Clin Oncol 2017;14:717-34. [Crossref] [PubMed]
- Savas P, Salgado R, Denkert C, et al. Clinical relevance of host immunity in breast cancer: from TILs to the clinic. Nat Rev Clin Oncol 2016;13:228-41. [Crossref] [PubMed]
- Loi S, Drubay D, Adams S, et al. Tumor-Infiltrating Lymphocytes and Prognosis: A Pooled Individual Patient Analysis of Early-Stage Triple-Negative Breast Cancers. J Clin Oncol 2019;37:559-69. [Crossref] [PubMed]
- Blank CU, Haining WN, Held W, et al. Defining 'T cell exhaustion'. Nat Rev Immunol 2019;19:665-74. [Crossref] [PubMed]
- DeNardo DG, Ruffell B. Macrophages as regulators of tumour immunity and immunotherapy. Nat Rev Immunol 2019;19:369-82. [Crossref] [PubMed]
- Sharma P, Hu-Lieskovan S, Wargo JA, et al. Primary, Adaptive, and Acquired Resistance to Cancer Immunotherapy. Cell 2017;168:707-23. [Crossref] [PubMed]
- Leone RD, Powell JD. Metabolism of immune cells in cancer. Nat Rev Cancer 2020;20:516-31. [Crossref] [PubMed]
- Ribas A, Wolchok JD. Cancer immunotherapy using checkpoint blockade. Science 2018;359:1350-5. [Crossref] [PubMed]
- Gluz O, Nitz U, Kolberg-Liedtke C, et al. De-escalated Neoadjuvant Chemotherapy in Early Triple-Negative Breast Cancer (TNBC): Impact of Molecular Markers and Final Survival Analysis of the WSG-ADAPT-TN Trial. Clin Cancer Res 2022;28:4995-5003. [Crossref] [PubMed]
- Pinard C, Debled M, Ben Rejeb H, et al. Residual cancer burden index and tumor-infiltrating lymphocyte subtypes in triple-negative breast cancer after neoadjuvant chemotherapy. Breast Cancer Res Treat 2020;179:11-23. [Crossref] [PubMed]
- Lotfinejad P, Asghari Jafarabadi M, Abdoli Shadbad M, et al. Prognostic Role and Clinical Significance of Tumor-Infiltrating Lymphocyte (TIL) and Programmed Death Ligand 1 (PD-L1) Expression in Triple-Negative Breast Cancer (TNBC): A Systematic Review and Meta-Analysis Study. Diagnostics (Basel) 2020;10:704. [Crossref] [PubMed]
- Lopes AD, Galdino NAL, Figueiredo AB, et al. Systemic immune mediators reflect tumour-infiltrating lymphocyte intensity and predict therapeutic response in triple-negative breast cancer. Immunology 2023;169:229-41. [Crossref] [PubMed]
- Dieci MV, Tsvetkova V, Griguolo G, et al. Integration of tumour infiltrating lymphocytes, programmed cell-death ligand-1, CD8 and FOXP3 in prognostic models for triple-negative breast cancer: Analysis of 244 stage I-III patients treated with standard therapy. Eur J Cancer 2020;136:7-15. [Crossref] [PubMed]
- Luen SJ, Salgado R, Dieci MV, et al. Prognostic implications of residual disease tumor-infiltrating lymphocytes and residual cancer burden in triple-negative breast cancer patients after neoadjuvant chemotherapy. Ann Oncol 2019;30:236-42. [Crossref] [PubMed]
- Hendry S, Salgado R, Gevaert T, et al. Assessing Tumor-infiltrating Lymphocytes in Solid Tumors: A Practical Review for Pathologists and Proposal for a Standardized Method From the International Immunooncology Biomarkers Working Group: Part 1: Assessing the Host Immune Response, TILs in Invasive Breast Carcinoma and Ductal Carcinoma In Situ, Metastatic Tumor Deposits and Areas for Further Research. Adv Anat Pathol 2017;24:235-51. [Crossref] [PubMed]
- Miskad UA, Rifai RA, Masadah R, et al. The value of tumor-infiltrating lymphocytes and CD8 expression as a predictor of response to anthracycline-based neoadjuvant chemotherapy in invasive breast carcinoma of no special type. Breast Dis 2021;40:S9-14. [Crossref] [PubMed]
- García-Martínez E, Gil GL, Benito AC, et al. Tumor-infiltrating immune cell profiles and their change after neoadjuvant chemotherapy predict response and prognosis of breast cancer. Breast Cancer Res 2014;16:488. [Crossref] [PubMed]
- Castaneda CA, Mittendorf E, Casavilca S, et al. Tumor infiltrating lymphocytes in triple negative breast cancer receiving neoadjuvant chemotherapy. World J Clin Oncol 2016;7:387-94. [Crossref] [PubMed]
- De Angelis C, Nagi C, Hoyt CC, et al. Evaluation of the Predictive Role of Tumor Immune Infiltrate in Patients with HER2-Positive Breast Cancer Treated with Neoadjuvant Anti-HER2 Therapy without Chemotherapy. Clin Cancer Res 2020;26:738-45. [Crossref] [PubMed]
- Sarradin V, Lusque A, Filleron T, et al. Immune microenvironment changes induced by neoadjuvant chemotherapy in triple-negative breast cancers: the MIMOSA-1 study. Breast Cancer Res 2021;23:61. [Crossref] [PubMed]
- Koletsa T, Kotoula V, Koliou GA, et al. Prognostic impact of stromal and intratumoral CD3, CD8 and FOXP3 in adjuvantly treated breast cancer: do they add information over stromal tumor-infiltrating lymphocyte density?. Cancer Immunol Immunother 2020;69:1549-64. [Crossref] [PubMed]
- Abdelrahman AE, Rashed HE. Clinicopathological significance of the immunologic signature (PDL1, FOXP3+ Tregs, TILs) in early stage triple-negative breast cancer treated with neoadjuvant chemotherapy. Ann Diagn Pathol 2021;51:151676. [Crossref] [PubMed]
- Kim R, Kawai A, Wakisaka M, et al. Immune correlates of the differing pathological and therapeutic effects of neoadjuvant chemotherapy in breast cancer. Eur J Surg Oncol 2020;46:77-84. [Crossref] [PubMed]
- Wang H, Hu X, Li X, et al. Research progress of immunotherapy for triple-negative breast cancer. Cancer Research on Prevention and Treatment 2022;49:996-1002.
- Loizides S, Constantinidou A. Triple negative breast cancer: Immunogenicity, tumor microenvironment, and immunotherapy. Front Genet 2022;13:1095839. [Crossref] [PubMed]
- Denkert C, von Minckwitz G, Brase JC, et al. Tumor-infiltrating lymphocytes and response to neoadjuvant chemotherapy with or without carboplatin in human epidermal growth factor receptor 2-positive and triple-negative primary breast cancers. J Clin Oncol 2015;33:983-91. [Crossref] [PubMed]


