
Correlation between ultrasound-based hepatic steatosis grade and thyroid hormone levels
Highlight box
Key findings
• Ultrasound attenuation analysis was used to quantitatively evaluate the correlation between liver fat and thyroid hormone.
What is known and what is new?
• The quantification of liver fat degeneration allows for the quantitative and statistical identification of the key factors contributing to liver fat degeneration.
• Free triiodothyronine (FT3), free thyroxine (FT4), and the FT3:FT4 ratio were independently associated with the prevalence of metabolic dysfunction-associated steatotic liver disease (MASLD).
What is the implication, and what should change now?
• In patients with MASLD, serum FT3 and FT4 levels should be monitored as key predictive factors for prognosing liver fat degeneration.
Introduction
Hepatic steatosis is characterized by the accumulation of lipid droplets that are primarily composed of triglycerides in hepatocytes. Within hepatocytes, hepatic steatosis increases the sensitivity to oxidative stress and cytokine-mediated liver damage, rendering affected individuals more susceptible to additional hepatotoxic insults (1). Metabolic dysfunction-associated steatotic liver disease (MASLD), which is associated with an elevated risk for type 2 diabetes mellitus (T2DM), cardiovascular disease (CVD), and hepatocellular carcinoma (HCC), is a primary etiology of hepatic steatosis, affecting over 25% of the global population (2). Moreover, a sizable fraction of patients infected with the chronic hepatitis B (CHB) virus have concurrent hepatic steatosis or even steatohepatitis. Patients with CHB with concurrent hepatic steatosis face elevated risks of progressive liver disease and HCC development (3-5). Thus, evaluating steatosis quantitatively and identifying risk factors of hepatic steatosis among these patient populations may improve clinical management and prognostication.
Liver biopsy remains the gold standard for the assessment of hepatic steatosis. However, its invasive nature limits its more widespread use among a larger population of patients with hepatic steatosis and during disease follow-up (6). Noninvasive ultrasonography is commonly adopted for the initial, rapid hepatic steatosis screening of suspected cases, but conventional ultrasonography provides only qualitative diagnosis and lacks sensitivity for mild liver steatosis (7). Recently, controlled attenuation parameter (CAP) obtained from FibroScan offers quantitative grading of hepatic steatosis severity. However, the repeatability and accuracy of CAP grading require further improvements and can be confounded by several patient-related factors (8), since the A-mode ultrasound technique lacks visualization of liver structural information. Additionally, CAP results can be misguided when different positions or sections of the liver are sampled (9). Given the high prevalence of hepatic steatosis worldwide, there is a critical need to develop a comprehensive quantitative technique that provides both visual structural information of the liver and two-dimensional acoustic attenuation results. Recently, Mindray proposed a novel ultrasound attenuation analysis (USAT) parameter as a noninvasive, quantitative tool for diagnosing and grading hepatic steatosis. USAT quantifies the mean attenuation coefficient in the liver at the region of interest (ROI) under the supervision of two-dimensional gray-scale sonography, during which structures impairing measurement accuracy, such as blood vessels, biliary ducts, and intrahepatic masses, can be automatically avoided (10). In comparison to the tradition CAP method, it can be guided by B-mode imaging during measurement based on translational ultrasound machine, which can guarantee diagnostic accuracy and reduce costs. Therefore, the novel USAT technique provides an accurate and reliable approach to quantifying hepatic steatosis deterioration. The parameterization of hepatic steatosis thus allows for the quantitative and statistical identification of factors that may contribute to hepatic steatosis.
MASLD, one of the most common types of liver diseases in the world, is typically considered to be independent manifestation of metabolic syndrome (11-14). Indeed, numerous studies have consistently shown that the onset of MASLD is closely linked to metabolic disorders, including obesity, insulin resistance, T2DM, and dyslipidemia (15), especially alterations in thyroid function (16). Thyroid hormones play a critical role in modulating lipid metabolism, body weight, and insulin resistance, suggesting their possible relationship with MASLD (17). Similarly, metabolic dysfunctions have been identified as significant contributors to the development of steatosis in patients with CHB with oxidative stress, with insulin resistance, and hyperglycemia being the key factors (18). Thus, metabolic dysfunction is potentially correlated with hepatic steatosis in both patients with MASLD and those with CHB.
Thyroid hormones are critical in preserving the metabolic equilibrium at both the systemic and hepatic levels through various pathways, such as de novo lipogenesis, beta-oxidation, cholesterol metabolism, and carbohydrate metabolism (19). In fact, suboptimal thyroid function [(sub)clinical hypothyroidism or low-normal thyroid function] has been correlated to hepatic steatosis (20). A large-sample meta-analysis reported that hypothyroidism was highly linked to the presence and severity of MASLD (21) and that there was a correlation between subclinical hypothyroidism, low-normal thyroid function, and advanced fibrosis (22). Recently, resmetirom, a liver-directed agonist for the thyroid hormone beta nuclear receptor, has been approved in the United States for the treatment of patients with metabolic dysfunction-associated steatohepatitis (MASH) and hepatic fibrosis. Consequently, augmenting beneficial thyroid hormone signaling in the liver by specific thyromimetics such as resmetirom has emerged as a treatment option for advanced cases of MASLD/MASH (23). However, the identification of patients that would benefit most from this treatment remain to be determined, and the severity of MASLD as well as the thyroid hormone function need to be considered as potential variables (1,24).
There is limited research regarding the direct relationship between thyroid hormones and the degree of hepatic steatosis specifically in patients with MASLD or CHB, mainly due to the difficulty in the quantification of hepatic steatosis via traditional ultrasound techniques. We present this article in accordance with the STROBE reporting checklist (available at https://gs.amegroups.com/article/view/10.21037/gs-2025-62/rc).
Methods
Participants
The study population consisted of consecutive patients who underwent USAT for liver steatosis evaluation for suspected diffuse liver diseases (typically as a result of abnormal liver enzymes or imaging methods, the clinical doctor diagnosed it as hepatic steatosis) between October 1, 2022, to November 5, 2022, at the Fourth Affiliated Hospital of Zhejiang University School of Medicine (Zhejiang, China). MASLD was identified according to the 2018 practice recommendations of the American Association for the Study of Liver Diseases as follows: radiological or histological evidence of hepatic steatosis; absence of additional steatosis-causing factors, no concurrent chronic liver illness, and no considerable alcohol usage (ethanol intake ≥21 standard drinks per week for men and ≥14 standard drinks per week for women) (25). Positivity for hepatitis B surface antigen for more than 6 months was a diagnostic criterion for chronic HBV infection. Meanwhile, the exclusion criteria for patients were as follows: (I) hepatitis viruses other than HBV or other chronic liver diseases, chronic hepatitis virus infection, or long-term drinking cause chronic liver disease; (II) liver operation, such as liver occupation resection and liver transplantation; (III) other severe liver metabolic diseases, such as heart failure and chronic kidney disease; (IV) administration of drugs affecting serum thyroid hormone levels, such as methimazole (MMI), propylthiouracil (PTU), levothyroxine, or amiodarone; and (V) a history of hyperthyroidism or hypothyroidism, or hypothalamus or pituitary dysfunction.
Because this study was a retrospective cohort study based on pre-existing clinical data that were analyzed anonymously, the need for obtaining a written informed consent was waived. The study protocol was approved by the local ethics committee of the Fourth Affiliated Hospital of Zhejiang University School of Medicine (No. K2024121) and conducted in accordance with the principles of the Declaration of Helsinki (as revised in 2013).
Clinical and laboratory data
Clinical data (age, gender, hypertension, T2DM, dyslipidemia medical history, etc.) and anthropometric parameters (weight, height) were recorded. Subsequently, body mass index (BMI) was calculated as follows: BMI = weight (kilograms)/square of height (meters). T2DM is a metabolic disease characterized by a persistent rise in blood glucose levels in the body. Hypertension is defined as blood pressure ≥140/90 mmHg without taking antihypertensive drugs. Dyslipidemia, also known as hyperlipidemia, refers to abnormal lipoprotein profiles in plasma.
After the patients were fasted for at least 8 hours, venous blood was drawn. The following parameters were recorded: the levels of albumin (ALB), total bilirubin (TBil), uric acid (UA), triglyceride (TG), total cholesterol (TC), high-density lipoprotein cholesterol (HDL-C), low-density lipoprotein cholesterol (LDL-C), aspartate aminotransferase (AST), alanine aminotransferase (ALT), and platelet (PLT). The serum levels of triiodothyronine (T3), thyroxine (T4), free triiodothyronine (FT3), free thyroxine (FT4), and thyroid stimulating hormone (TSH) were also obtained. All biochemical parameters were detected with standard automated laboratory methods using commercially available kits following the manufacturers’ protocols. Serum ALT, AST, ALT/AST, ALB, TBil, UA, TC, TG, HDL-C, and LDL-C were determined using biochemical automatic analyzer (LABOSPECT 006, Japan). PLT levels were measured using automatic hematology analyzer (Mindray BC-7500NRCS, China). Thyroid-related hormones, including TSH, T3, FT3, T4, and FT4 were determined using automatic chemiluminescent immunoassay analyzer (Abbott ALINITY, USA).
USAT
In this study, we adopted USAT to accurately grade patients with hepatic steatosis and classify them into four categories based on the cutoffs of USAT determined in our previous study (26) (≤0.62, 0.63–0.65, 0.66–0.71, and ≥0.72 dB/cm/MHz). Using this approach, we sought to examine the correlation between thyroid hormones and the degree of hepatic steatosis, which may support the clinical management of individuals with MASLD in light of the new treatment options.
USAT was conducted with recently developed ultrasound software (Resona R9 with an SC6-1U probe; Mindray, Shenzhen, China) and was performed by an expert radiologist who was unaware of the patient’s clinical diagnosis and laboratory data. USAT examination was performed after more than 8 hours of fasting. In order to obtain the best scanning surface available during the inspection process, patients usually lay with the right and the right upper limbs placed on the head. First, B-mode ultrasound was used to determine the appropriate position in liver segment V/VI at five centimeters below the liver capsule. Second, the ROI was outlined in the USAT mode to avoid the blood vessels and gallbladder. After modifying the measurement box which was a square with a side length of two centimeters, the operator should hit the “UPDATE” button on the instrument console. Thirdly, in order to obtain the attenuation coefficient measurement, the best image was chosen in accordance with the credibility map’s instructions (Figure 1). Five consecutive measurements are taken at the same sampling point, and the results were automatically saved. A median and quartile range/median (of less than 0.30) for five measurements was considered a valid USAT measurement (dB/cm/MHz). Finally, we defined the vertical distance from the skin to the liver envelope as the thickness of abdominal subcutaneous fat on the same section without applying any pressure.

Statistical analysis
Continuous numerical variables are expressed as the mean and standard deviation (SD) or the median and interquartile range (IQR) depending on the nature of their distribution, and categorical variables are described as absolute figures with percentages. The normality of continuous variable distributions was tested with the Shapiro-Wilk test. The t-test or Mann-Whitney U test was used for comparisons of continuous data, whereas the Chi-squared test was used for comparisons of categorical variables. Multivariable logistic regression analysis was performed to identify the association between thyroid and hepatic steatosis. P values of <0.05 were adopted as the threshold for statistical significance. The statistical analysis was performed using SPSS 25.0 (IBM Corp., Armonk, NY, USA) and MedCalc 20.022 (MedCalc Software, Ostend, Belgium).
Results
Patient general characteristics
In this study, a total of 326 participants diagnosed with suspicious hepatic steatosis on USAT were enrolled as the study population. Of these, 195 were excluded (lack of serum thyroid hormone data for 191 participants, lack of complete serum lipid data for 2 participants, and etiologies other than MASLD or CHB for 2 participants), as depicted in Figure 2. Table 1 outlines the general characteristics of the final dataset, which included 131 participants with 86 CHB participants and 45 suspected MASLD participants. Among the participants, there were 98 men and 33 women, with a mean age of 43.2 (SD 15.3) years and a mean BMI of 26.2 (SD 3.7) kg/m2. After classification into MASLD and CHB groups, the characteristics of these groups were compared (Table 1). Participants with CHB were predominantly male (n=70, 81.4%) as were those with MASLD (n=28, 62.2%). Moreover, those with MASLD were likely to have T2DM (MASLD: n=16, 35.6%; CHB: n=9, 10.5%) and hypertension (MASLD: n=21, 46.7%; CHB: n=13, 15.1%). The MASLD group exhibited a significantly higher mean platelet count (227.8×109/L, SD 62.4) compared to the CHB group (187.4×109/L, SD 61.3), as well as lower median T3 levels (MASLD: 1.5 nmol/L, IQR 1.3–1.6 nmol/L; CHB: 1.6 nmol/L, IQR 1.5–1.8 nmol/L). No significant differences were found in age, BMI, distance from skin to liver capsule, albumin, TBil, UA, ALT, AST, ALT/AST, TC, TG, HDL-C, LDL-C, FT3, T4, or FT4 levels between the two groups (P>0.05).
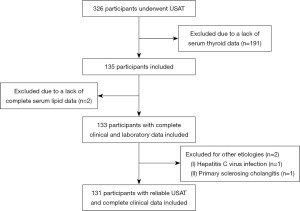
Table 1
| Characteristic | MASLD (n=45) | CHB (n=86) | P |
|---|---|---|---|
| Male | 28 (62.2) | 70 (81.4) | 0.02* |
| Age (years) | 43.2 (15.3) | 43.1 (9.6) | 0.97 |
| BMI (kg/m2) | 26.9 (3.7) | 25.9 (3.0) | 0.15 |
| Distance from the skin to liver capsule (cm) | 1.9 (1.7–2.2) | 1.9 (1.6–2.1) | 0.14 |
| Type 2 diabetes mellitus | 16 (35.6) | 9 (10.5) | 0.001* |
| Hypertension | 21 (46.7) | 13 (15.1) | <0.001* |
| Dyslipidemia | 37 (82.2) | 65 (75.6) | 0.39 |
| USAT (dB/cm/MHz) | 0.7 (0.6–0.8) | 0.7 (0.6–0.7) | 0.009* |
| Steatosis stage using USAT | – | ||
| S0 | 12 (26.7) | 35 (40.7) | |
| S1 | 4 (8.9) | 11 (12.8) | |
| S2 | 5 (11.1) | 22 (25.6) | |
| S3 | 24 (53.3) | 18 (20.9) | |
| ALT (U/L) | 33.0 (19.0–90.0) | 36.5 (25.5–50.3) | 0.72 |
| AST (U/L) | 29.0 (18.5–52.5) | 29.5 (23.0–42.0) | 0.44 |
| ALT/AST | 1.2 (0.9–1.5) | 1.2 (1.0–1.4) | 0.69 |
| PLT count (×109/L) | 227.8 (62.4) | 187.4 (61.3) | 0.001* |
| Albumin (g/L) | 42.2 (40.8–45.7) | 43.7 (41.7–44.7) | 0.32 |
| UA (μmol/L) | 358.0 (307.0–425.5) | 368.5 (319.0–414.0) | 0.77 |
| TBil (μmol/L) | 11.4 (9.3–15.0) | 13.2 (10.1–17.9) | 0.08 |
| TC (mmol/L) | 4.7 (4.1–5.7) | 4.5 (4.1–5.4) | 0.27 |
| TG (mmol/L) | 1.8 (1.3–2.5) | 1.7 (1.3–2.8) | 0.82 |
| HDL-C (mmol/L) | 1.1 (0.9–1.2) | 1.0 (0.9–1.2) | 0.22 |
| LDL-C (mmol/L) | 2.8 (2.1–3.2) | 2.5 (2.1–3.0) | 0.10 |
| T3 (nmol/L) | 1.5 (1.3–1.6) | 1.6 (1.5–1.8) | 0.001* |
| FT3 (pmol/L) | 4.6 (0.8) | 4.8 (0.7) | 0.23 |
| T4 (nmol/L) | 93.5 (84.4–107.7) | 94.2 (82.7–115.3) | 0.96 |
| FT4 (pmol/L) | 12.3 (11.7–13.8) | 12.2 (11.3–13.1) | 0.24 |
| TSH (mIU/L) | 1.7 (1.2–2.9) | 1.7 (1.3–2.3) | 0.89 |
Continuous numerical variables are expressed as the mean (SD) or as the median (IQR) according to their distribution, and categorical variables are described as absolute figures with percentages. *, P<0.05. ALT, alanine aminotransferase; AST, aspartate aminotransferase; BMI, body mass index; CHB, chronic hepatitis B; FT3, free triiodothyronine; FT4, free thyroxine; HDL-C, high-density lipoprotein cholesterol; IQR, interquartile range; LDL-C, low-density lipoprotein cholesterol; MASLD, metabolic dysfunction-associated steatotic liver disease; PLT, platelet; SD, standard deviation; T3, triiodothyronine; T4, thyroxine; TBil, total bilirubin; TC, total cholesterol; TG, triglyceride; TSH, thyroid-stimulating hormone; UA, uric acid; USAT, ultrasound attenuation analysis.
Differences in parameters of hepatic steatosis as determined by USAT
To examine the potential correlation between hepatic steatosis and thyroid hormone, 131 patients with suspicious MASLD or CHB were further divided into two subgroups, including 84 with hepatic steatosis (USAT >0.62 dB/cm/MHz) and 47 without hepatic steatosis (USAT ≤0.62 dB/cm/MHz), as displayed in Table 2. Hepatic steatosis was found in 64.1% of the entire cohort, with the MASLD group accounting for 53.5% of cases. The USAT further indicated that patients at the S0, S1, S2, and S3 stages accounted for 35.9%, 11.5%, 20.6%, and 32.1% of all sample cases, respectively. Interestingly, the MASLD and CHB groups had different distributions of stages. In the MASLD group (N=45), there were 12 (26.7%) patients in the S0 stage, 4 (8.9%) in S1 stage, 5 (11.1%) in S2 stage, and 24 (53.3%) in S3 stage. Meanwhile, in the CHB group, patients with S1 stage accounted for the smallest proportion (12.8%), while those with S0 accounted for the highest (40.7%) proportion, with S2 and S3 constituting 25.6% and 20.9% of the CHB group, respectively. Therefore, the MASLD population was considered more likely to experience severe hepatic steatosis as opposed to the CHB population. On the other hand, stage S0 hepatic steatosis was more commonly observed in patients with CHB. By comparing subgroups with and without hepatic steatosis, we found significant differences in BMI, distance from skin to capsule, USAT values, ALT, and AST (P<0.05). Meanwhile, for parameters including age, TBil, albumin, TC, TG, UA, PLT count, ALT/AST, HDL-C, LDL-C, TSH, T3, FT3, T4, FT4, gender, T2DM, hypertension, and dyslipidemia (P>0.05), no significant difference was found.
Table 2
| Characteristic | Steatosis (n=84) | No steatosis (n=47) | P |
|---|---|---|---|
| Male | 63 (75.0) | 35 (74.5) | 0.64 |
| Age (years) | 43.4 (12.8) | 42.7 (9.7) | 0.75 |
| BMI (kg/m2) | 26.5 (24.7–29.1) | 25.0 (23.6–27.0) | 0.02* |
| Distance from skin to liver capsule (cm) | 1.9 (1.7–2.2) | 1.8 (1.6–2.1) | 0.04* |
| Type 2 diabetes mellitus | 14 (16.7) | 11 (23.4) | 0.35 |
| Hypertension | 24 (28.6) | 10 (21.3) | 0.36 |
| Dyslipidemia | 67 (79.8) | 35 (74.5) | 0.48 |
| USAT (dB/cm/MHz) | 0.7 (0.7–0.8) | 0.6 (0.5–0.6) | <0.001* |
| ALT (U/L) | 39.5 (24.0–83.8) | 29.0 (20.0–43.0) | 0.02* |
| AST (U/L) | 34.0 (23.0–50.5) | 26.0 (21.0–34.0) | 0.02* |
| ALT/AST | 1.3 (1.0–1.6) | 1.1 (0.9–1.3) | 0.09 |
| PLT count (×109/L) | 203.2 (61.6) | 197.8 (69.6) | 0.65 |
| Albumin (g/L) | 43.7 (40.9–45.4) | 43.3 (41.7–44.7) | 0.99 |
| UA (μmol/L) | 372.6 (82.9) | 361.3 (79.6) | 0.45 |
| TBil (μmol/L) | 12.3 (9.5–16.9) | 12.3 (10.0–16.2) | 0.86 |
| TC (mmol/L) | 4.5 (4.0–5.4) | 4.8 (4.1–5.6) | 0.40 |
| TG (mmol/L) | 1.8 (1.4–2.9) | 1.6 (1.2–2.3) | 0.13 |
| HDL-C (mmol/L) | 1.0 (0.9–1.2) | 1.1 (0.9–1.2) | 0.32 |
| LDL-C (mmol/L) | 2.5 (2.1–3.1) | 2.6 (2.2–3.2) | 0.54 |
| T3 (nmol/L) | 1.6 (1.4–1.8) | 1.5 (1.3–1.7) | 0.20 |
| FT3 (pmol/L) | 4.7 (0.7) | 4.7 (0.8) | 0.79 |
| T4 (nmol/L) | 94.9 (85.4–113.5) | 89.1 (81.5–104.8) | 0.19 |
| FT4 (pmol/L) | 12.3 (11.7–13.8) | 11.8 (11.2–13.0) | 0.07 |
| TSH (mIU/L) | 1.7 (1.2–2.4) | 1.6 (1.3–2.4) | 0.66 |
Continuous numerical variables are expressed as the mean (SD) or as the median (IQR) according to their distribution, and categorical variables are expressed as absolute figures with percentages. *, P<0.05. ALT, alanine aminotransferase; AST, aspartate aminotransferase; BMI, body mass index; FT3, free triiodothyronine; FT4, free thyroxine; HDL-C, high-density lipoprotein cholesterol; IQR, interquartile range; LDL-C, low-density lipoprotein cholesterol; PLT, platelet; SD, standard deviation; T3, triiodothyronine; T4, thyroxine; TBil, total bilirubin; TC, total cholesterol; TG, triglyceride; TSH, thyroid-stimulating hormone; UA, uric acid; USAT, ultrasound attenuation analysis.
Prevalence of hepatic steatosis in cases with different TH levels
To clarify the potential relationship between thyroid function and the prevalence of hepatic steatosis, we plotted the proportions of the population at different hepatic steatosis stages versus the interquartile range of those with abnormal thyroid hormone indexes including for TSH, T3, FT3, T4, and FT4. As presented in Figure 3, we found that the incidence of hepatic steatosis was higher among patients with higher T4 and FT4 levels, but this was not statistically significant (P>0.05). A similar tendency was observed for TSH, T3, and FT3 levels.

Correlation of thyroid hormone levels with hepatic steatosis
The levels of T3, T4, FT3, FT4, and TSH were treated as independent variables within different regression models, respectively. Regression models 1, 2, and 3 were constructed regardless of hepatic steatosis. Model 1, which lacked any variable correction, revealed a close relationship between FT4 level and hepatic steatosis [odds ratio (OR) =1.482; P=0.045] (Table 3). Model 2, which included adjustments for gender and age variables, also showed a close correlation between FT4 and hepatic steatosis, but only for the MASLD group (OR =1.561; P=0.03). Model 3, which included adjustments for gender, age, T2DM, hypertension, dyslipidemia, and ALT/AST levels, displayed similar results to those of the other two models (OR =1.539; P=0.04). These results clearly indicated that FT4 level was positively correlated with hepatic steatosis. Moreover, another multiple logistic regression model was performed to assess the correlation between these variables and populations of hepatic steatosis categorized by USAT in MASLD and CHB groups, respectively. In the MASLD group, the multivariate model identified hepatic steatosis as a dependent variable; meanwhile, univariable logistic analysis identified BMI and T3 as independent variables strongly correlated with hepatic steatosis (P<0.15). Moreover, FT3 (OR =0.133; P=0.04) was independently associated with hepatic steatosis in the MASLD group (Table 4). However, no thyroid hormones were associated with hepatic steatosis in patients with CHB.
Table 3
| Variables | Model 1 | Model 2 | Model 3 | |||||
|---|---|---|---|---|---|---|---|---|
| OR (95% CI) | P | OR (95% CI) | P | OR (95% CI) | P | |||
| TSH | 1.154 (0.855–1.558) | 0.35 | 1.196 (0.871–1.641) | 0.27 | 1.200 (0.866–1.662) | 0.27 | ||
| T3 | 1.001 (0.999–1.003) | 0.36 | 1.001 (0.999–1.003) | 0.25 | 1.001 (0.999–1.003) | 0.34 | ||
| FT3 | 0.747 (0.352–1.585) | 0.45 | 0.804 (0.371–1.743) | 0.58 | 0.774 (0.349–1.716) | 0.53 | ||
| T4 | 0.988(0.961–1.014) | 0.36 | 0.985 (0.957–1.014) | 0.31 | 0.985 (0.956–1.016) | 0.34 | ||
| FT4 | 1.482 (1.010–2.174) | 0.045* | 1.561 (1.044–2.334) | 0.03* | 1.539 (1.013–2.338) | 0.04* | ||
| Gender | – | – | 0.919 (0.357–2.364) | 0.86 | 0.894 (0.340–2.354) | 0.82 | ||
| Age | – | – | 1.022 (0.984–1.062) | 0.26 | 1.031 (0.988–1.076) | 0.16 | ||
| Type 2 diabetes mellitus | – | – | – | – | 2.035 (0.719–5.758) | 0.18 | ||
| Hypertension | – | – | – | – | 0.623 (0.224–1.728) | 0.36 | ||
| Dyslipidemia | – | – | – | – | 0.811 (0.325–2.023) | 0.65 | ||
| ALT/AST | – | – | – | – | 2.698 (0.937–7.765) | 0.07 | ||
*, P<0.05. Hepatic steatosis according to USAT: the cutoff of hepatic steatosis was 0.62 dB/cm/MHz. Model 1, lacked any variable correction; Model 2, included adjustments for gender and age variables; Model 3, included adjustments for gender, age, type 2 diabetes mellitus, hypertension, dyslipidemia, and ALT/AST levels. ALT, alanine aminotransferase; AST, aspartate aminotransferase; CI, confidence interval; FT3, free triiodothyronine; FT4, free thyroxine; OR, odds ratio; T3, triiodothyronine; T4, thyroxine; TSH, thyroid stimulating hormone; USAT, ultrasound attenuation analysis.
Table 4
| Variable | Univariate analysis | Multivariate analysis | |||||
|---|---|---|---|---|---|---|---|
| OR | 95% CI | P | OR | 95% CI | P | ||
| TSH | 1.170 | 0.701–1.954 | 0.55 | 1.259 | 0.698–2.270 | 0.44 | |
| T3 | 1.003 | 1.000–1.007 | 0.04* | 1.009 | 1.001–1.016 | 0.02* | |
| FT3 | 1.287 | 0.541–3.064 | 0.57 | 0.133 | 0.020–0.897 | 0.04* | |
| T4 | 1.025 | 0.988–1.063 | 0.18 | 0.958 | 0.879–1.043 | 0.32 | |
| FT4 | 1.306 | 0.810–2.108 | 0.27 | 2.127 | 0.611–7.403 | 0.24 | |
| BMI | 1.153 | 0.950–1.400 | 0.15 | 1.046 | 0.827–1.324 | 0.71 | |
*, P<0.05. BMI, body mass index; CI, confidence interval; FT3, free triiodothyronine; FT4, free thyroxine; MASLD, metabolic dysfunction-associated steatotic liver disease; OR, odds ratio; T3, triiodothyronine; T4, thyroxine; TSH, thyroid-stimulating hormone.
Discussion
As a novel noninvasive ultrasound analysis technique, USAT is capable of diagnosing the severity of hepatic steatosis from various etiologies. Therefore, USAT was applied in our study to ensure the efficacy and stability of hepatic steatosis diagnosis. In this study, we used ultrasound attenuation coefficients from the novel USAT technique to quantify hepatic steatosis parameters and clarified their correlation to thyroid hormones levels in both MASLD and CHB groups. In the MASLD group, there were 53.3% in S3 stage. In the CHB group, there were 40.7% in S0 stage. Consistent with previous research findings, we found that the degree of hepatic steatosis in CHB patients was mainly mild, while the MAFLD population was more likely to have severe hepatic steatosis, indicating that metabolic abnormalities (rather than viral factors) are the core driving force for the progression of steatosis (27,28).
Previous studies had indicated there to be a potential association between thyroid hormones and MASLD or obesity-related parameters, although the findings remain contested (29-31). Hepatic steatosis is also commonly encountered in patients with CHB. However, it remains unclear whether thyroid hormones are correlated with hepatic steatosis in CHB, and thus their potential influence on the related therapeutic response is unknown. In our study, we discovered that hepatic steatosis was common in patients with CHB and that thyroid hormone levels, including those of FT4 and FT3, were associated with hepatic steatosis as assessed by USAT. Firstly, USAT-detected hepatic steatosis was correlated with FT4 level. However, the significance was not obvious, possibly due to the lack of distinct characteristics in the sample population. Through further analysis of the relationship between hepatic steatosis and thyroid hormones in the MASLD and CHB groups, we discovered that FT3 level was independently associated with hepatic steatosis in the MASLD group (P=0.04). In contrast, the same relationship was not observed in the CHB group. These results may help to explain the weaker correlation between hepatic steatosis and thyroid hormone level in CHB as compared to in MASLD despite the fact that hepatic steatosis is widespread in CHB.
Thyroid hormones generally consist of T3, T4, FT3, FT4, and TSH. As the levels of T3 and T4 are affected by T4-binding globulin in serum, thyroid function is usually evaluated by FT3, FT4, and TSH serum levels in clinic. In previous study, an association between hepatic steatosis and FT3 or FT4 was reported, whereas there was some ambiguity regarding the relationship between hepatic steatosis and T3 or T4 (32). Previous studies had also demonstrated a correlation between thyroid hormones and MASLD. For example, in a large cross-sectional study by Liu et al. (33), including 1,773 patients with euthyroid who underwent health check-ups, they found that TSH (OR =1.108; P=0.02) and FT3 (OR =1.258; P<0.001) levels were independently associated with MASLD risk diagnosed by ultrasound; meanwhile, only FT3 level (OR =1.252; P=0.004) was independently associated with the risk of MASLD as estimated by fatty liver index (FLI).
Our study includes a number of limitations. First, we used a relatively small sample size from a single center. On the other hand, we focused on two main types of liver disease causing steatosis and used consistent methodology in both etiologies. Additionally, we chose USAT as the reference for the diagnose of hepatic steatosis, which may improve the accuracy in diagnosing liver steatosis as compared to traditional ultrasound. Second, the relationship between hepatic steatosis and thyroid hormone levels was not confirmed in independent cohorts, and the long-term implications for liver-related complications of steatosis, e.g., progression of fibrosis, require further studies. Thirdly, the relevance of USAT and/or thyroid hormone measurement for defining patient groups that would be more likely to benefit from thyromimetic therapy (e.g., resmetirom) deserves investigation.
Conclusions
Using the newly developed USAT method, we identified a correlation between hepatic steatosis and thyroid hormones, specifically FT3 and FT4. These data emphasize the relevance of thyroid hormone signaling for the development of hepatic steatosis, particularly in patients with MASLD.
Acknowledgments
None.
Footnote
Reporting Checklist: The authors have completed the STROBE reporting checklist. Available at https://gs.amegroups.com/article/view/10.21037/gs-2025-62/rc
Data Sharing Statement: Available at https://gs.amegroups.com/article/view/10.21037/gs-2025-62/dss
Peer Review File: Available at https://gs.amegroups.com/article/view/10.21037/gs-2025-62/prf
Funding: This study was supported by
Conflicts of Interest: All authors have completed the ICMJE uniform disclosure form (available at https://gs.amegroups.com/article/view/10.21037/gs-2025-62/coif). J.P. is from Shenzhen Mindray Bio-medical Electronics Co., Ltd. The other authors have no conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved. The study protocol was approved by the local ethics committee of the Fourth Affiliated Hospital of Zhejiang University School of Medicine (No. K2024121) and conducted in accordance with the principles of the Declaration of Helsinki (as revised in 2013). Individual consent for this retrospective analysis was waived.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- European Association for the Study of the Liver (EASL). European Association for the Study of Obesity (EASO). EASL-EASD-EASO Clinical Practice Guidelines on the management of metabolic dysfunction-associated steatotic liver disease (MASLD). J Hepatol 2024;81:492-542. [Crossref] [PubMed]
- Israelsen M, Francque S, Tsochatzis EA, et al. Steatotic liver disease. Lancet 2024;404:1761-78. [Crossref] [PubMed]
- Lee YB, Ha Y, Chon YE, et al. Association between hepatic steatosis and the development of hepatocellular carcinoma in patients with chronic hepatitis B. Clin Mol Hepatol 2019;25:52-64. [Crossref] [PubMed]
- Chan AW, Wong GL, Chan HY, et al. Concurrent fatty liver increases risk of hepatocellular carcinoma among patients with chronic hepatitis B. J Gastroenterol Hepatol 2017;32:667-76. [Crossref] [PubMed]
- Mak LY, Hui RW, Fung J, et al. Diverse effects of hepatic steatosis on fibrosis progression and functional cure in virologically quiescent chronic hepatitis B. J Hepatol 2020;73:800-6. [Crossref] [PubMed]
- Tamaki N, Ajmera V, Loomba R. Non-invasive methods for imaging hepatic steatosis and their clinical importance in NAFLD. Nat Rev Endocrinol 2022;18:55-66. [Crossref] [PubMed]
- Lee DH. Imaging evaluation of non-alcoholic fatty liver disease: focused on quantification. Clin Mol Hepatol 2017;23:290-301. [Crossref] [PubMed]
- Karlas T, Petroff D, Sasso M, et al. Individual patient data meta-analysis of controlled attenuation parameter (CAP) technology for assessing steatosis. J Hepatol 2017;66:1022-30. [Crossref] [PubMed]
- Wilder J, Patel K. The clinical utility of FibroScan((R)) as a noninvasive diagnostic test for liver disease. Med Devices (Auckl) 2014;7:107-14. [PubMed]
- Wang M, Tang S, Li G, et al. Comparative study of ultrasound attenuation analysis and controlled attenuation parameter in the diagnosis and grading of liver steatosis in non-alcoholic fatty liver disease patients. BMC Gastroenterol 2024;24:81. [Crossref] [PubMed]
- Smits MM, Ioannou GN, Boyko EJ, et al. Non-alcoholic fatty liver disease as an independent manifestation of the metabolic syndrome: results of a US national survey in three ethnic groups. J Gastroenterol Hepatol 2013;28:664-70. [Crossref] [PubMed]
- Cariou B, Byrne CD, Loomba R, et al. Nonalcoholic fatty liver disease as a metabolic disease in humans: A literature review. Diabetes Obes Metab 2021;23:1069-83. [Crossref] [PubMed]
- Weinstein G, Zelber-Sagi S, Preis SR, et al. Association of Nonalcoholic Fatty Liver Disease With Lower Brain Volume in Healthy Middle-aged Adults in the Framingham Study. JAMA Neurol 2018;75:97-104. [Crossref] [PubMed]
- Bellentani S. The epidemiology of non-alcoholic fatty liver disease. Liver Int 2017;37:81-4. [Crossref] [PubMed]
- Ziamanesh F, Mohammadi M, Ebrahimpour S, et al. Unraveling the link between insulin resistance and Non-alcoholic fatty liver disease (or metabolic dysfunction-associated steatotic liver disease): A Narrative Review. J Diabetes Metab Disord 2023;22:1083-94. [Crossref] [PubMed]
- Shi YW, Yang RX, Fan JG. Chronic hepatitis B infection with concomitant hepatic steatosis: Current evidence and opinion. World J Gastroenterol 2021;27:3971-83. [Crossref] [PubMed]
- Ritter MJ, Amano I, Hollenberg AN. Thyroid Hormone Signaling and the Liver. Hepatology 2020;72:742-52. [Crossref] [PubMed]
- Rosato V, Masarone M, Dallio M, et al. NAFLD and Extra-Hepatic Comorbidities: Current Evidence on a Multi-Organ Metabolic Syndrome. Int J Environ Res Public Health 2019;16:3415. [Crossref] [PubMed]
- Tanase DM, Gosav EM, Neculae E, et al. Hypothyroidism-Induced Nonalcoholic Fatty Liver Disease (HIN): Mechanisms and Emerging Therapeutic Options. Int J Mol Sci 2020;21:5927. [Crossref] [PubMed]
- Ratziu V, Scanlan TS, Bruinstroop E. Thyroid hormone receptor-β analogues for the treatment of metabolic dysfunction-associated steatohepatitis (MASH). J Hepatol 2025;82:375-87. [Crossref] [PubMed]
- Mantovani A, Nascimbeni F, Lonardo A, et al. Association Between Primary Hypothyroidism and Nonalcoholic Fatty Liver Disease: A Systematic Review and Meta-Analysis. Thyroid 2018;28:1270-84. [Crossref] [PubMed]
- Kim D, Kim W, Joo SK, et al. Subclinical Hypothyroidism and Low-Normal Thyroid Function Are Associated With Nonalcoholic Steatohepatitis and Fibrosis. Clin Gastroenterol Hepatol 2018;16:123-131.e1. [Crossref] [PubMed]
- Harrison SA, Bedossa P, Guy CD, et al. A Phase 3, Randomized, Controlled Trial of Resmetirom in NASH with Liver Fibrosis. N Engl J Med 2024;390:497-509. [Crossref] [PubMed]
- Noureddin M, Charlton MR, Harrison SA, et al. Expert Panel Recommendations: Practical Clinical Applications for Initiating and Monitoring Resmetirom in Patients With MASH/NASH and Moderate to Noncirrhotic Advanced Fibrosis. Clin Gastroenterol Hepatol 2024;22:2367-77. [Crossref] [PubMed]
- Chalasani N, Younossi Z, Lavine JE, et al. The diagnosis and management of nonalcoholic fatty liver disease: Practice guidance from the American Association for the Study of Liver Diseases. Hepatology 2018;67:328-57. [Crossref] [PubMed]
- Jiang M, Zhang X, Wu X, et al. The diagnostic value of novel ultrasound attenuation analysis in detecting liver steatosis identified by the controlled attenuation parameter: a diagnostic accuracy study. Ann Transl Med 2023;11:38. [Crossref] [PubMed]
- Xu L, Lu W, Li P, et al. A comparison of hepatic steatosis index, controlled attenuation parameter and ultrasound as noninvasive diagnostic tools for steatosis in chronic hepatitis B. Dig Liver Dis 2017;49:910-7. [Crossref] [PubMed]
- Zhu L, Jiang J, Zhai X, et al. Hepatitis B virus infection and risk of non-alcoholic fatty liver disease: A population-based cohort study. Liver Int 2019;39:70-80. [Crossref] [PubMed]
- Nie X, Xu Y, Ma X, et al. Association between Abdominal Fat Distribution and Free Triiodothyronine in a Euthyroid Population. Obes Facts 2020;13:358-66. [Crossref] [PubMed]
- Guo Z, Li M, Han B, et al. Association of non-alcoholic fatty liver disease with thyroid function: A systematic review and meta-analysis. Dig Liver Dis 2018;50:1153-62. [Crossref] [PubMed]
- Wirth EK, Puengel T, Spranger J, et al. Thyroid hormones as a disease modifier and therapeutic target in nonalcoholic steatohepatitis. Expert Rev Endocrinol Metab 2022;17:425-34. [Crossref] [PubMed]
- Zhang Y, Li J, Liu H. Correlation between the thyroid hormone levels and nonalcoholic fatty liver disease in type 2 diabetic patients with normal thyroid function. BMC Endocr Disord 2022;22:144. [Crossref] [PubMed]
- Liu Y, Wang W, Yu X, et al. Thyroid Function and Risk of Non-Alcoholic Fatty Liver Disease in Euthyroid Subjects. Ann Hepatol 2018;17:779-88. [Crossref] [PubMed]
(English Language Editor: J. Gray)


