
Risk factor analysis and clinical experience of treating red breast syndrome in acellular dermal matrix and implant-based breast reconstruction
Highlight box
Key findings
• A history of prior radiotherapy is a significant risk factor for red breast syndrome (RBS) in implant-based breast reconstruction.
What is known and what is new?
• Although several causes have been suggested for RBS, no appropriate treatment has yet been established.
• A 1-week course of antibiotic therapy coupled with a single intravenous steroid injection might be beneficial as an initial treatment approach for RBS.
What is the implication, and what should change now?
• If radiation therapy is planned, it is important to inform and educate patients about RBS. We believe that it may be helpful to try the treatments we suggest rather than just observing.
Introduction
Since the implementation of implant-based breast reconstruction using acellular dermal matrix (ADM), red breast syndrome (RBS) has been the subject of controversy over its existence and etiology. RBS is defined as idiopathic cutaneous erythema overlying the ADM after implant-based breast reconstruction, without other signs or symptoms of infection. There have been a number of reports on the incidence of RBS, ranging from 0% to 27% according to the literature (1-5). The RBS phenomenon was described in an article in 2009 (6). Since then, several etiologies have been suggested for RBS, included lymphatic disruption of mastectomy skin flaps, delayed hypersensitivity reaction to ADM, subclinical infection and residual DNA within ADM (7-11). However, consensus regarding risk factors and treatment for RBS has not been reached yet. Due to the absence of a consensus regarding the underlying cause of RBS, there is a limited body of research available on treatment approaches for RBS. As a result, only a few recommendations or suggestions currently exist.
While some studies have characterized RBS as a condition and a self-limiting disease, some patients do not experience improvement unless ADM is removed or washed out (12,13). Some studies have shown that RBS can effectively manage by administering corticosteroid (14,15). In contrast, other authors have advocated for the bacterial theory of RBS and stressed the importance of antibiotic therapy (16). Since the cause of the disease is ambiguous, a consensus on its appropriate treatment methods has not been established either. Thus, the purpose of this study is to determine risk factors for RBS, providing a clue to identifying the cause of this ambiguous and confusing disease. Another purpose of this study was to introduce our experience of successful treatment of RBS in Korea University Anam Hospital. Here we report six patients with RBS treated successfully with conservative management and two patients with surgical intervention. We present this article in accordance with the STROBE reporting checklist (available at https://gs.amegroups.com/article/view/10.21037/gs-2024-542/rc).
Methods
This retrospective analysis was approved by the Korea University Anam Hospital institutional review board (No. 2020AN0132). The study was conducted in accordance with the Declaration of Helsinki (as revised in 2013) and individual consent for this retrospective analysis was waived due to the retrospective nature. We enrolled 118 patients who had undergone an implant-based breast reconstruction using ADM following nipple-sparing or skin-sparing mastectomy between October 2018 and February 2021. As part of the exclusion criteria, patients with insufficient surgical information or those diagnosed with inflammatory breast cancer were not included in this study. Individuals who underwent breast reconstruction using autologous tissue were also excluded. Medical records including detailed operation notes, follow-up records, and photographs were collected and analyzed.
Operative technique
All surgeries were performed at this institution by two plastic surgeons (E.S.Y. and H.C.L). Generally, patients who underwent skin-sparing mastectomy and those with poor intraoperative mastectomy skin flap perfusion were chosen for two-stage reconstructions. If a patient underwent a nipple-sparing mastectomy and the mastectomy skin flap perfusion was tolerable, reconstruction was performed with permanent silicone implant using a direct-to-implant method. Mastectomy skin flap perfusion was intraoperatively assessed using near-infrared fluorescence camera imaging (Moment K; IANC&S, Seoul, Korea) with intravenous indocyanine green injection after mastectomy.
Two primary methods were employed for the coverage of ADM: pectoralis muscle sling and full-wrapping. For subpectoral reconstruction cases, an 8 cm × 16 cm ADM was utilized and secured as a sling along the lower border of the pectoralis major muscle. For prepectoral reconstruction, the implant was fully covered by ADM and inserted into the pocket. Postoperative antibiotics were used until drain removal.
ADM
All ADM products were ready-to-use type and terminal sterilization was done with gamma-radiation or electron beam radiation. They were derived from decellularized cadaveric dermis. For bilateral reconstruction patients, ADM from different company or different sources were used.
A small piece of ADM and fluid from ADM package were sent for culture and no bacterial growth were found in all cases. We didn’t perform endotoxin screening test of ADM.
ADM was washed 5–10 times with betadine and then 5–10 times with normal saline. And it was soaked in betadine until use.
Study design
RBS was defined as a sterile inflammation of affected mastectomy skin flap, characteristic of erythema over the ADM without fever, leukocytosis, or positive bacterial culture results. When evaluating erythema occurring on the breast, a comprehensive physical examination was conducted, which included assessing for symptoms such as fever and tenderness. Additionally, laboratory tests were employed to measure inflammatory markers. A sono-guided aspiration culture was performed if there was evidence of fluid collection. For patients lacking fever, tenderness, leukocytosis, and positive aspiration culture results, treatment was initiated based on our guidelines specific to RBS (Figure 1). However, in cases where infection was evident, treatment followed protocols established for managing infections. To analyze risk factors of RBS, we conducted statistical analysis by dividing patient’s cohort into an RBS group (n=8) and a non-RBS group (n=138).

Statistical analyses
All statistical analyses were performed using SPSS version 26.0 software (IBM Corp., Redmond, WA, USA). Student’s t-test, Chi-squared test and Fisher’s exact test were used for statistical comparison of both groups. Univariate analysis using a logistic regression model was performed to analyze predictors of RBS. For all analyses, a value of P<0.05 was considered statistically significant.
Results
Patient demographics
A total of 138 implant-based breast reconstructions were performed using ADM. The average follow-up period was 17.33 months (Table 1). Of 118 patients, there were 98 women who had unilateral mastectomy and 20 women who had bilateral mastectomy. Prepectoral reconstructions were performed for 94 breasts and subpectoral reconstructions were performed for 44 breasts. There were 62 breasts with two-stage reconstructions using tissue expander and 76 breasts with direct-to-implant reconstructions. In terms of ADM, Megaderm was used in 67 (48.6%) breasts, CGCryoderm was used in 40 (29.0%) breasts, DermAcell was used in 18 (13.0%) breasts, AlloMend was used in 8 (5.8%) breasts, Alloderm RTU was used in 4 (2.9%) breasts, and Myderm was used in 1 (0.7%) breast (Table 1).
Table 1
| Patient demographics | Total (N=138) | Non-RBS (N=130) | RBS (N=8) | P |
|---|---|---|---|---|
| Age (years) | 45.91±9.07 | 45.92±9.11 | 45.88±9.08 | 0.99 |
| BMI (kg/m2) | 22.50±3.58 | 22.30±3.08 | 25.74±7.92 | 0.26 |
| Hypertension | 14 (10.1) | 13 (92.9) | 1 (7.1) | 0.59 |
| Diabetes mellitus | 9 (6.5) | 8 (88.9) | 1 (11.1) | 0.43 |
| Smoking | 9 (6.5) | 9 (100.0) | 0 (0.0) | >0.99 |
| Mastectomy | ||||
| Unilateral | 98 (83.05) | |||
| Bilateral | 20 (16.95) | |||
| Reconstruction type | 0.73 | |||
| Two-stage | 62 (44.9) | 59 (95.2) | 3 (4.8) | |
| Direct-to-implant | 76 (55.1) | 71 (93.4) | 5 (6.6) | |
| Plane | 0.44 | |||
| Prepectoral | 94 (68.1) | 87 (92.6) | 7 (7.4) | |
| Subpectoral | 44 (31.9) | 43 (97.7) | 1 (2.3) | |
| ADM | ||||
| Megaderm | 67 (48.6) | 62 (92.5) | 5 (7.5) | |
| CGCryoderm | 40 (29.0) | 40 (100.0) | 0 (0.0) | |
| DermACELL | 18 (13.0) | 15 (83.3) | 3 (16.7) | |
| AlloMend | 8 (5.8) | 8 (100.0) | 0 (0.0) | |
| Alloderm RTU | 4 (2.9) | 4 (100.0) | 0 (0.0) | |
| Myderm | 1 (0.7) | 1 (100.0) | 0 (0.0) | |
| Prior history of chemotherapy | 38 (27.5) | 33 (86.8) | 5 (13.2) | 0.04* |
| Prior history of radiotherapy | 7 (5.1) | 4 (57.1) | 3 (42.9) | 0.004* |
Data are presented as mean ± SD or n (%). Other variables on non-RBS, RBS: number of patients (percentage of the total for that variable). *, statistically significant. ADM, acellular dermal matrix; BMI, body mass index; RBS, red breast syndrome; SD, standard deviation; RTU, ready to use.
There were 8 cases of RBS (Table 2). The overall incidence of RBS was 5.8% (n=8) with a median onset time of 36 days. The mean age was 45.88 years for the RBS group, and 45.92 years for the non-RBS group (Table 1). Among 8 cases, 4 cases were from bilateral reconstruction, and only one side was affected in all 4 cases. The mean BMI was 25.74 kg/m2 for the RBS group, and 22.30 kg/m2 for the non-RBS group. Patients in the RBS group had significantly higher rates of prior history of chemotherapy or radiation therapy than patients in the non-RBS group (P=0.04 and P=0.004, respectively). Although not statistically significant, patients in the RBS group had higher average BMI than patients in the non-RBS group (P=0.26). There was no significant difference in age, comorbidity (hypertension, diabetes), smoking status, or perioperative characteristics such as reconstruction type or plane of implant placement between the two groups.
Table 2
| No. | Sex/Age (years) | HTN | DM | BMI (kg/m2) | Smoking | Reconstruction type | Site (RBS) | Onset time (d) | Plane | ADM | Prior history of chemotherapy | Prior history of radiotherapy | Intervention |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 1 | F/59 | Y | Y | 41.26 | N | Two-stage | Right | 677 | Subpectoral | DermACELL | N | N | Conservative |
| 2 | F/48 | N | N | 28.71 | N | DTI | Left | 102 | Prepectoral | MegaDerm | Y | N | Surgical |
| 3 | F/33 | N | N | 30.94 | N | Two-stage | Left | 14 | Prepectoral | MegaDerm | Y | N | Conservative |
| 4 | F/48 | N | N | 24.55 | N | Two-stage | Right | 233 | Prepectoral | MegaDerm | Y | Y | Conservative |
| 5 | F/41 | N | N | 22.46 | N | DTI | Left | 28 | Prepectoral | MegaDerm | Y | N | Conservative |
| 6 | F/37 | N | N | 17.86 | N | DTI | Left | 21 | Prepectoral | MegaDerm | Y | Y | Surgical |
| 7 | F/57 | N | N | 23.56 | N | DTI | Left | 44 | Prepectoral | DermACELL | N | Y | Conservative |
| 8 | F/44 | N | N | 16.60 | N | DTI | Right | 20 | Prepectoral | DermACELL | N | N | Conservative |
ADM, acellular dermal matrix; BMI, body mass index; DM, diabetes mellitus; DTI, direct-to-implant; F, female; HTN, hypertension; N, no; RBS, red breast syndrome; Y, yes.
Risk factor analysis of RBS occurrence
In univariate analysis, prior history of neoadjuvant chemotherapy [odds ratio (OR) 4.899, P=0.04], and prior history of neoadjuvant radiotherapy (OR 18.9, P=0.001) were significant risk factors for RBS. Nonetheless, the risk of developing RBS was not significantly increased by factors such as older age (50 years or older, P=0.70), having obesity [body mass index (BMI) of 25 kg/m2 or higher, P=0.09], having comorbidities like hypertension (P=0.82), having diabetes mellitus (P=0.49), being a smoker (P=0.99), reconstruction stage (P=0.67), pocket location (P=0.25), or using a particular type of ADM (P=0.93) (Table 3).
Table 3
| Risk factors (univariate) | Non-RBS | RBS | OR | 95% CI | P |
|---|---|---|---|---|---|
| Age (years) | 45.92±9.11 | 45.88±9.08 | 1.000 | 0.924–1.082 | 0.99 |
| ≥50 | 41 | 2 | 0.724 | 0.140–3.740 | 0.70 |
| <50 | 89 | 6 | |||
| BMI (kg/m2) | 22.30±3.08 | 25.74±7.92 | 1.173 | 1.024–1.345 | 0.02* |
| ≥25 | 18 | 3 | 3.733 | 0.820–16.992 | 0.09 |
| <25 | 112 | 5 | |||
| Hypertension | 13 (92.9) | 1 (7.1) | 1.286 | 0.146–11.286 | 0.82 |
| Diabetes mellitus | 8 (88.9) | 1 (11.1) | 2.179 | 0.238–19.939 | 0.49 |
| Smoking | 9 (100.0) | 0 (0.0) | 0.99 | ||
| Plane | 0.25 | ||||
| Prepectoral | 87 (92.6) | 7 (7.4) | 3.406 | 0.412–29.024 | |
| Subpectoral | 43 (97.7) | 1 (2.3) | |||
| Stage | 0.67 | ||||
| Direct-to-implant | 71 (93.4) | 5 (6.6) | 1.385 | 0.318–6.038 | |
| Two-stage | 59 (95.2) | 3 (4.8) | |||
| ADM | 0.93 | ||||
| Megaderm | 62 (92.5) | 5 (7.5) | |||
| CGCryoderm | 40 (100.0) | 0 (0.0) | |||
| DermACELL | 15 (83.3) | 3 (16.7) | |||
| AlloMend | 8 (100.0) | 0 (0.0) | |||
| Alloderm RTU | 4 (100.0) | 0 (0.0) | |||
| Myderm | 1 (100.0) | 0 (0.0) | |||
| Prior history of chemotherapy | 33 (86.8) | 5 (13.2) | 4.899 | 1.110–21.626 | 0.04* |
| Prior history of radiotherapy | 4 (57.1) | 3 (42.9) | 18.900 | 3.306–108.052 | 0.001* |
Data are presented as mean ± SD or n (%). *, statistically significant. ADM, acellular dermal matrix; BMI, body mass index; CI, confidence interval; RBS, red breast syndrome; OR, odds ratio; SD, standard deviation; RTU, ready to use.
In multivariate logistic regression analysis, Alloderm RTU and Myderm, which had few cases, were excluded from analysis. As a result, prior history of radiotherapy (OR 22.703, P=0.001) was the only significant risk factor for RBS. Even after accounting for the impact of all other variables, there was no statistically significant evidence to suggest that obesity could significantly increase the risk of RBS (P=0.07) (Table 4).
Table 4
| Risk factors | OR | 95% CI | P |
|---|---|---|---|
| Obesity (BMI ≥25 kg/m2) | 4.789 | 0.866–26.495 | 0.07 |
| Prior history of radiotherapy | 22.703 | 3.493–147.562 | 0.001* |
*, statistically significant. CI, confidence interval; OR, odds ratio; BMI, body mass index.
In the case where RBS was suspected, we administered intravenous corticosteroid with 1 week course of oral antibiotics and followed up the patient 1 week later. If there was no response to conservative treatment, surgical exploration was considered. In the majority of cases (6 out of 8) where RBS was suspected, resolution was achieved through administration of intravenous corticosteroids and 1-week course of oral antibiotics. However, conservative treatment did not yield satisfactory results for the remaining two patients. As a result, surgical intervention, including pocket irrigation, was carried out. Biopsies were done in those two patients, and results were fibrous tissue with synovium like change and fibrocollagenous tissue with chronic inflammation. In one of these two cases, explantation was ultimately required. Next, we will describe our experience of two cases.
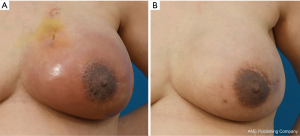
Case 1 (patient 4)
A 48-year-old patient who underwent prepectoral two-staged implant reconstruction with ADM visited the hospital with complaint of erythema above the periareolar area 8 months postoperatively. She has prior history of radiotherapy. Her BMI was 24.55 kg/m2. She underwent treatment with intravenous steroid with 1 week course of oral antibiotics with good response (Figure 2).

Case 2 (patient 2)
A 48-year-old patient who underwent prepectoral implant reconstruction with ADM three months ago visited the hospital with complaint of erythema of reconstructed breast. She has prior history of neoadjuvant chemotherapy. Her BMI was 28.71 kg/m2. In this case, there was no response to conservative treatment. She was hospitalized. She underwent surgical exploration under local anesthesia. In exploration, it was observed that some part of ADM was not well taken with some serous fluid collection. Thus, partial debridement of ADM and pocket irrigation was performed. After surgical intervention, her symptoms were resolved (Figure 3).
Discussion
As mentioned earlier, RBS has been the subject of controversy over its existence and etiology. It is difficult to distinguish RBS from cellulitis due to its very ambiguous characteristics. Since the description of RBS, many studies have been conducted to investigate its pathophysiology and causes.
There have been several perspectives on the etiology of RBS. Ganske et al. have proposed that the cause of RBS might be an immune reaction called delayed-type hypersensitivity triggered by the ADM product (14). Through a skin biopsy of affected areas, they suggested that certain components present in ADM, such as haptens and allergens, could be responsible for allergic response. In contrast, Danino et al. took a different approach to understanding RBS compared to previous studies (16). They suggested a bacterial hypothesis, supported by their analysis using scanning electron microscopy, which revealed the presence of biofilm in ADM samples from RBS cases. Their study raised the possibility of RBS being an infectious condition. Nahabedian has suggested that RBS is more closely linked to lymphatic obstruction and lymphedema, than being solely attributed to the type of ADM used or other factors (1). On the other hand, Nguyen et al. argued for the endotoxin hypothesis in RBS (17). Their research discovered detectable levels of endotoxin in ADM associated with RBS, indicating the need for endotoxin screening to prevent RBS. Despite ongoing research and investigations, a consensus regarding the exact cause of RBS has not reached yet, resulting in a lack of clarity in defining this disease.
The incidence of RBS has been reported to vary from 0 to 27% in the literature, and in our study it occurred in 5.8%, with previous radiotherapy as a significant risk factor. Eichler et al. compared acellular bovine matrix with decellularized human skin and reported RBS as 4.8% and 14.1% (5). In recent studies using sterile ready-to-use type ADM, the rate of RBS was variable as 2.7%, 0.7% and 26% (2,3,10). When excluding patients with prior radiotherapy, the rate of RBS was 3.8% in our study. Although it is not a high rate, we think it is important to explain RBS to patients especially when radiotherapy is expected.
Differentiating between RBS and cellulitis is important. However, it can be quite challenging due to the subtle nature of their distinction. When the ADM is situated only in the lower part of the breast with the prosthesis positioned beneath the pectoralis major muscle in a subpectoral manner, one can easily differentiate RBS from cellulitis based on whether the erythema extends beyond the boundary of the ADM. However, if the prosthesis is placed in a prepectoral manner, which is predominantly done in our hospital currently, the ADM exists throughout the breast, making it more difficult to distinguish between RBS and cellulitis based on the extent of redness. Consequently, in certain cases, the final diagnosis could only be established once the treatment was completed. Basically, we initiated treatment based on our initial impression, prioritizing patient’s symptoms and conducting a physical examination.
As a result of our analysis, prior history of radiotherapy was suggested as a significant risk factor for RBS. It is widely known that radiation therapy can negatively affect the body’s microvascular and lymphatic systems (18,19). In this way, if there is a prior history of radiotherapy, blood and lymphatic circulation might be compromised. The patients might be vulnerable to bacterial infection. In severe cases, it can lead to a local inflammatory reaction manifested by erythema over the ADM. It has been claimed that RBS is attributable to a noninfectious etiology because antibiotics are not effective. We believe that the reason why antibiotics are not effective for RBS is due to compromised blood and lymphatic flow to the mastectomy skin flap and underlying ADM.
Although RBS is often recognized as a self-limiting disease, it is our opinion that it is not adequate to simply follow-up without intervention because it is difficult to completely rule out the possibility of subclinical infection. For some cases of RBS, surgical intervention is required. As mentioned earlier, although some authors perceive RBS as a self-limiting condition that can resolve spontaneously, there are patients who do not improve unless the ADM is removed or rinsed out (12,13). Some studies have reported that RBS can effectively manage through administration of corticosteroids (14,15). On the other hand, there are authors who support the bacterial theory of RBS and emphasize the crucial role of antibiotic therapy (16).
We have diagnosed and treated several cases of RBS since breast reconstructions using ADM have been performed in Korea University Anam Hospital. We hypothesize that RBS has complex elements of compromised circulation of mastectomy skin flap, subclinical infection, and immune response. In most of our cases, intravenous steroid injection and 1 week course of oral antibiotic treatment were sufficient for resolution of RBS. Surgical intervention was needed if there was no response.
In treatment of RBS, antibiotics not only could treat subclinical infections, but also could serve as a safeguard to buffer devastating consequences when clinical infections are misdiagnosed with RBS. In the case where RBS is suspected, we administered intravenous (IV) corticosteroid with oral antibiotics and followed up the patient 1 week later. If there was no response to conservative treatment, surgical exploration was considered (Figure 3).
However, several limitations should be considered when interpreting results of this study. First, since this was a retrospective study, there might be a selection bias. It was also limited in that it could only reveal associations rather than a causal relationship. Second, being a single center study, different results might be obtained in different institutions or regions. Third, we assumed that a compromised circulation of mastectomy skin flap might be associated with RBS. However, quantitative measurement of the thickness of skin flap or circulation was not performed. Fourth, we used various types of ADM, and did not perform endotoxin screening tests. If RBS is affected by ADM, these variables may have an effect. Lastly, due to its ambiguity and low prevalence, the sample size was relatively small, which inevitably led to low statistical power. If a larger sample size is utilized with data collected from multiple hospitals, the study could yield different outcomes with enhanced statistical power compared to findings of this study.
Conclusions
According to our study, the incidence of RBS was approximately 5.8%. In the context of implant-based breast reconstruction, a history of prior radiotherapy was identified as significant risk factor for RBS. Consequently, it is imperative for surgeons to thoroughly inform and educate patients about risks associated with RBS when planning reconstruction, particularly for individuals presenting these risk factors. Despite the absence of a consensus regarding the treatment of RBS, based on our experience, a 1-week course of antibiotic therapy coupled with a single intravenous steroid injection might be beneficial as an initial treatment approach for RBS.
Acknowledgments
None.
Footnote
Reporting Checklist: The authors have completed the STROBE reporting checklist. Available at https://gs.amegroups.com/article/view/10.21037/gs-2024-542/rc
Data Sharing Statement: Available at https://gs.amegroups.com/article/view/10.21037/gs-2024-542/dss
Peer Review File: Available at https://gs.amegroups.com/article/view/10.21037/gs-2024-542/prf
Funding: None.
Conflicts of Interest: All authors have completed the ICMJE uniform disclosure form (available at https://gs.amegroups.com/article/view/10.21037/gs-2024-542/coif). The authors have no conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved. The study was conducted in accordance with the Declaration of Helsinki (as revised in 2013). The study was approved by the Korea University Anam Hospital institutional review board (No. 2020AN0132) and individual consent for this retrospective analysis was waived due to the retrospective nature.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Nahabedian MY. Prosthetic Breast Reconstruction and Red Breast Syndrome: Demystification and a Review of the Literature. Plast Reconstr Surg Glob Open 2019;7:e2108. [Crossref] [PubMed]
- Nahabedian MY, Cocilovo C. Two-Stage Prosthetic Breast Reconstruction: A Comparison Between Prepectoral and Partial Subpectoral Techniques. Plast Reconstr Surg 2017;140:22S-30S. [Crossref] [PubMed]
- Lewis P, Jewell J, Mattison G, et al. Reducing postoperative infections and red breast syndrome in patients with acellular dermal matrix-based breast reconstruction: the relative roles of product sterility and lower body mass index. Ann Plast Surg 2015;74:S30-2. [Crossref] [PubMed]
- Govshievich A, Somogyi RB, Brown MH. Conservative mastectomies and immediate reconstruction with the use of ADMs. Gland Surg 2015;4:453-62. [Crossref] [PubMed]
- Eichler C, Vogt N, Brunnert K, et al. A Head-to-head Comparison between SurgiMend and Epiflex in 127 Breast Reconstructions. Plast Reconstr Surg Glob Open 2015;3:e439. [Crossref] [PubMed]
- Eichler C, Efremova J, Brunnert K, et al. A Head to Head Comparison Between SurgiMend® - Fetal Bovine Acellular Dermal Matrix and Tutomesh® - A Bovine Pericardium Collagen Membrane in Breast Reconstruction in 45 Cases. In Vivo 2017;31:677-82. [Crossref] [PubMed]
- Momeni A, Kanchwala SK. Improved pocket control in immediate microsurgical breast reconstruction with simultaneous implant placement through the use of mesh. Microsurgery 2018;38:450-7. [Crossref] [PubMed]
- Ortiz JA. Clinical Outcomes in Breast Reconstruction Patients Using a Sterile Acellular Dermal Matrix Allograft. Aesthetic Plast Surg 2017;41:542-50. [Crossref] [PubMed]
- Venturi ML, Mesbahi AN, Boehmler JH 4th, et al. Evaluating sterile human acellular dermal matrix in immediate expander-based breast reconstruction: a multicenter, prospective, cohort study. Plast Reconstr Surg 2013;131:9e-18e. Erratum in: Plast Reconstr Surg 2013;131:669. [Crossref] [PubMed]
- Pittman TA, Fan KL, Knapp A, et al. Comparison of Different Acellular Dermal Matrices in Breast Reconstruction: The 50/50 Study. Plast Reconstr Surg 2017;139:521-8. [Crossref] [PubMed]
- Nahabedian MY. AlloDerm performance in the setting of prosthetic breast surgery, infection, and irradiation. Plast Reconstr Surg 2009;124:1743-53. [Crossref] [PubMed]
- Wu PS, Winocour S, Jacobson SR. Red breast syndrome: a review of available literature. Aesthetic Plast Surg 2015;39:227-30. [Crossref] [PubMed]
- Cicilioni OJ Jr, Foles VB, Sieger B, et al. Mycobacterium fortuitum Infection following Reconstructive Breast Surgery: Differentiation from Classically Described Red Breast Syndrome. Plast Reconstr Surg Glob Open 2013;1:e50. [Crossref] [PubMed]
- Ganske I, Hoyler M, Fox SE, et al. Delayed hypersensitivity reaction to acellular dermal matrix in breast reconstruction: the red breast syndrome? Ann Plast Surg 2014;73:S139-43. [Crossref] [PubMed]
- Wu C, Cipriano J, Osgood G Jr, et al. Human acellular dermal matrix (AlloDerm®) dimensional changes and stretching in tissue expander/implant breast reconstruction. J Plast Reconstr Aesthet Surg 2013;66:1376-81. [Crossref] [PubMed]
- Danino MA, El Khatib AM, Doucet O, et al. Preliminary Results Supporting the Bacterial Hypothesis in Red Breast Syndrome following Postmastectomy Acellular Dermal Matrix- and Implant-Based Reconstructions. Plast Reconstr Surg 2019;144:988e-92e. [Crossref] [PubMed]
- Nguyen TC, Brown AM, Kulber DA, et al. The Role of Endotoxin in Sterile Inflammation After Implanted Acellular Dermal Matrix: Red Breast Syndrome Explained? Aesthet Surg J 2020;40:392-9. [Crossref] [PubMed]
- Avraham T, Yan A, Zampell JC, et al. Radiation therapy causes loss of dermal lymphatic vessels and interferes with lymphatic function by TGF-beta1-mediated tissue fibrosis. Am J Physiol Cell Physiol 2010;299:C589-605. [Crossref] [PubMed]
- Li YQ, Chen P, Haimovitz-Friedman A, et al. Endothelial apoptosis initiates acute blood-brain barrier disruption after ionizing radiation. Cancer Res 2003;63:5950-6.


