
Prognostic analysis of N3 locally advanced breast cancer according to the 8th edition of AJCC clinical stage: a propensity-matched SEER analysis
Highlight box
Key findings
• The study found significant differences in survival outcomes among the N3 subgroups (N3a, N3b, and N3c) both before and after propensity score matching (PSM).
• Before PSM, the N3b group had the most favorable prognosis, followed by N3a, with N3c exhibiting the poorest outcomes.
• After PSM, the N3b group continued to show the best prognosis, though the differences in breast cancer-specific survival (BCSS) and overall survival (OS) were less pronounced.
What is known and what is new?
• The 8th edition of the American Joint Committee on Cancer (AJCC) clinical staging system, which includes both anatomical and prognostic staging, is well-established for predicting prognosis in breast cancer.
• N3 locally advanced breast cancer (LABC) has been classified into three subgroups (N3a, N3b, and N3c), with differences in clinical outcomes observed, but their prognostic significance has been debated.
What is the implication, and what should change now?
• This manuscript provides a detailed application of the 8th edition AJCC staging system to N3 LABC, demonstrating that N3b patients have the most favorable prognosis, even after adjusting for baseline differences through PSM.
• This study highlights the potential for more tailored treatment strategies based on the N3 subgroup classification, particularly for N3b patients who may have a more favorable prognosis.
• Clinicians should consider incorporating the 8th edition AJCC staging criteria into clinical decision-making to better guide treatment and prognosis predictions for N3 LABC.
Introduction
Since the American Joint Committee on Cancer (AJCC) staging system was first introduced in 1977, it has been continuously updated, with eight versions released. The 8th edition of the AJCC staging system has been in official use since 2018 (1). This edition combines estrogen receptor (ER), progesterone receptor (PR), human epidermal growth factor receptor 2 (HER2), histological grade (G), and multi-gene detection tools into the traditional anatomical staging for the first time (2). It creates clinical and pathological prognostic stage groups. Several experts have recognized the prognostic effectiveness of this new staging system. Plichta et al. (3) confirmed that the 8th edition of the AJCC Cancer staging system has advanced clinical applications.
Over the past 30 years, with modifications to the tumor, node, and metastasis (TNM) staging system for breast cancer, the understanding and definition of locally advanced breast cancer (LABC) have also evolved. The TNM staging, revised by the AJCC prior to 1988, defined ipsilateral supraclavicular lymph node metastasis as M1 (stage IV). However, experts such as Professor Brito (4) confirmed that breast cancer limited to supraclavicular lymph node metastasis without other site metastasis has a survival outcome similar to stage IIIB. Therefore, in the TNM staging after 2002, ipsilateral supraclavicular lymph node metastasis was classified as N3, making patients with breast cancer with supraclavicular lymph node metastasis, but no metastasis to other parts, potentially curable. The status of regional lymph nodes affects the staging, prognosis, and treatment plans of patients. However, since N3 LABC is collectively referred to as stage IIIc in traditional anatomical TNM staging, the prognosis of different N3 breast cancers has not been compared in detail, nor has the diagnosis and treatment of different N3 LABCs received more specific guidance.
Currently, the 8th edition of the AJCC clinical staging system, combined with biological characteristics, has introduced prognostic staging for the first time. However, detailed staging of N3 LABC has not included the N3 lymph node status, limiting the system’s ability to predict clinical outcomes more accurately. The prognostic value of combining biological characteristics with prognostic staging for different N3 cancers still needs confirmation. The Surveillance, Epidemiology, and End Results (SEER) database, known for its high quality and wide coverage, offers an opportunity to conduct such validation. Therefore, we conducted a validation study based on the SEER database to evaluate the prognosis of different N3 LABCs, demonstrating the application of the 8th edition of the AJCC staging system. The study aims to assess its rationality and feasibility, providing a reference for clinical practice and research. We present this article in accordance with the STROBE reporting checklist (available at https://gs.amegroups.com/article/view/10.21037/gs-24-437/rc).
Methods
Data source
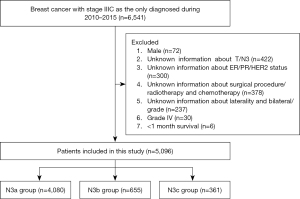
This study included data on the first female patients diagnosed with primary in situ or invasive breast cancer from the National Cancer Institute’s SEER Program 18 Registered Research Database. The study was conducted in accordance with the Declaration of Helsinki (as revised in 2013). The case list in this study was generated using SEER*Stat software (version 8.4.1). Study case inclusion criteria: female; diagnosed between 2010 and 2015; unilateral breast cancer; confirmed invasive carcinoma by pathological examination; stage IIIc; breast cancer diagnosed as the only primary cancer. According to the exclusion criteria, we excluded patients who did not meet the criteria, such as those lacking any of the following information: tissue grade, subtype, ER/PR/HER2 status; patients with multiple tumors; patients with bilateral breast cancer; and patients with distant metastasis. Finally, 5,096 patients who met the criteria were included in the analysis (Figure 1, patient screening flowchart).
Variable evaluation and definition
Patient data at the time of diagnosis, including age, race, marital status, histological grade, histological type, laterality, primary tumor stage, lymph node stage, distant metastasis, ER status, PR status, HER2 status, chemotherapy, radiotherapy, surgical methods, and follow-up status, were obtained from the database. Age was categorized as <60 and ≥60 years. In the SEER database, N3 is defined as the lymph node metastasis status at the first diagnosis of cancer. Patients were divided into three groups based on their N3 status: N3a, N3b, and N3c. The pathological types of tumors were divided into three categories: invasive ductal carcinoma (IDC), invasive lobular carcinoma (ILC), and other types. Histological grades were categorized into three groups: grade 1 (high differentiation), grade 2 (moderate differentiation), and grade 3 (low differentiation). According to the expression status of ER, PR, and HER2, breast cancer was classified into eight subtypes: ER+/PR+/HER2−, ER+/PR−/HER2−, ER−/PR−/HER2−, ER−/PR+/HER2−, ER+/PR+/HER2+, ER−/PR+/HER2+, ER+/PR−/HER2+, and ER−/PR−/HER2+. In this study, breast cancer-specific survival (BCSS) and overall survival (OS) were the primary outcomes.
Statistical analysis
Clinicopathological parameters were converted into categorical variables for statistical analysis. The χ2 test or Fisher’s exact test was used for group comparisons. The Kaplan-Meier method was used for survival analysis, and survival curves were drawn. The log-rank test was used for survival rate comparisons. A univariate Cox proportional hazards model was used to analyze prognostic factors for BCSS and OS, and hazard ratios (HRs) with 95% confidence intervals (CIs) were estimated. Parameters with statistical or clinical significance in the univariate analysis were included in the multivariate Cox model for independent prognostic factor analysis. Propensity score matching (PSM) was performed using the “TriMatch” package in R, based on clinical and pathological characteristics, excluding the four biological indicators of prognostic stage. A logistic regression model was used to calculate propensity scores, with a matching ratio of 1:7:2 and a caliper value of 0.3. The matched samples were reanalyzed, with the significance level set at α=0.05 (two-tailed). All statistical analyses and visualizations were conducted using SPSS (version 27.0) and R (version 4.4.1).
Results
Statistical analyses results of N3 LABC before PSM
A total of 5,096 patients with newly diagnosed N3 locally advanced invasive breast cancer were included in this study. The clinical and pathological characteristics of the three N3 subgroups (N3a, N3b, and N3c) are summarized in Table 1. Among these patients were classified 4,080 as N3a (80.06%), 655 as N3b (12.85%), and 361 as N3c (7.09%). Significant differences were observed among the three groups in terms of age (P<0.001), race (P<0.001), marital status (P=0.003), laterality (P=0.005), histological grade (P<0.001), histological subtype (P<0.001), tumor stage (P<0.001), ER status (P<0.001), PR status (P<0.001), HER2 status (P<0.001), breast surgery (P<0.001), chemotherapy (P<0.001), and radiotherapy (P=0.07). The N3a group comprised the largest proportion of patients, followed by the N3b group, with the N3c group having the smallest proportion (Table 1).
Table 1
| Variables | N3a (n=4,080) | N3b (n=655) | N3c (n=361) | P value |
|---|---|---|---|---|
| Age (years) | <0.001 | |||
| <60 | 2,199 (53.90) | 463 (70.69) | 227 (62.88) | |
| ≥60 | 1,881 (46.10) | 192 (29.31) | 134 (37.12) | |
| Race | <0.001 | |||
| White | 3,198 (78.38) | 474 (72.37) | 267 (73.96) | |
| Black | 490 (12.01) | 109 (16.64) | 66 (18.28) | |
| Other | 392 (9.61) | 72 (10.99) | 28 (7.76) | |
| Marital status | 0.003 | |||
| Married | 3,172 (77.74) | 509 (77.71) | 272 (75.35) | |
| Unmarried | 707 (17.33) | 118 (18.02) | 84 (23.27) | |
| Unknown | 201 (4.93) | 28 (4.27) | 5 (1.38) | |
| Laterality | 0.005 | |||
| Left | 2,070 (50.74) | 342 (52.21) | 208 (57.62) | |
| Right | 2,010 (49.26) | 313 (47.79) | 153 (42.38) | |
| Histological grade | <0.001 | |||
| Grade I | 309 (7.57) | 21 (3.21) | 7 (1.94) | |
| Grade II | 1,632 (40.00) | 200 (30.53) | 98 (27.15) | |
| Grade III | 2,139 (52.43) | 434 (66.26) | 256 (70.91) | |
| Histological subtype | <0.001 | |||
| Invasive ductal carcinoma | 2,761 (67.67) | 542 (82.75) | 295 (81.72) | |
| Invasive lobular carcinoma | 728 (17.84) | 23 (3.51) | 12 (3.32) | |
| Other | 591 (14.49) | 90 (13.74) | 54 (14.96) | |
| Tumor stage | <0.001 | |||
| T0–1 | 618 (15.15) | 79 (12.06) | 43 (11.91) | |
| T2 | 1,951 (47.82) | 250 (38.17) | 126 (34.90) | |
| T3 | 1,071 (26.25) | 169 (25.80) | 66 (18.28) | |
| T4 | 440 (10.78) | 157 (23.97) | 126 (34.90) | |
| ER status | <0.001 | |||
| Positive | 3,147 (77.13) | 400 (61.07) | 197 (54.57) | |
| Negative | 933 (22.87) | 255 (38.93) | 164 (45.43) | |
| PR status | <0.001 | |||
| Positive | 2,625 (64.34) | 293 (44.73) | 138 (38.23) | |
| Negative | 1,455 (35.66) | 362 (55.27) | 223 (61.77) | |
| HER2 status | <0.001 | |||
| Positive | 871 (21.35) | 177 (27.02) | 132 (36.57) | |
| Negative | 3,209 (78.65) | 478 (72.98) | 229 (63.43) | |
| Breast surgery | <0.001 | |||
| Modified radical mastectomy | 3,285 (80.51) | 473 (72.22) | 228 (63.16) | |
| Breast-conserving surgery | 745 (18.26) | 121 (18.47) | 69 (19.11) | |
| No | 50 (1.23) | 61 (9.31) | 64 (17.73) | |
| Chemotherapy | <0.001 | |||
| Yes | 3,291 (80.66) | 596 (90.99) | 329 (91.14) | |
| No | 789 (19.34) | 59 (9.01) | 32 (8.86) | |
| Radiotherapy | 0.07 | |||
| Yes | 2,747 (67.33) | 469 (71.60) | 238 (65.93) | |
| No | 1,333 (32.67) | 186 (28.40) | 123 (34.07) |
Data are presented as n (%). PSM, propensity score matching; ER, estrogen receptor; PR, progesterone receptor; HER2, human epidermal growth factor receptor 2.
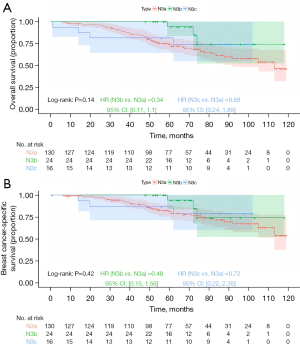
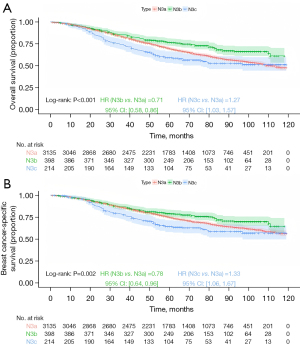
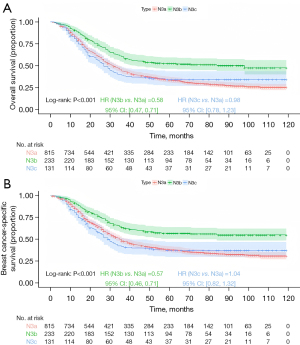
Kaplan-Meier survival analysis stratified by anatomical staging revealed significant differences in prognosis among the groups. The N3b group had the most favorable prognosis, followed by the N3a group, with the N3c group having the worst prognosis (Figure 2; OS, P<0.001; BCSS, P<0.001). In Kaplan-Meier analysis stratified by prognostic staging, for stage IIIa, the N3b group demonstrated the best prognosis, followed by the N3a group, and the N3c group had the worst outcomes, though the differences were not statistically significant (Figure 3; OS, P=0.14; BCSS, P=0.42). For stage IIIb, the N3b group had the most favorable prognosis, followed by N3a, with N3c showing the poorest outcomes (Figure 4; OS, P<0.001; BCSS, P=0.002). Similarly, for stage IIIc, the N3b group had the best prognosis, followed by the N3a group, with the N3c group again showing the worst outcomes (Figure 5; OS, P<0.001; BCSS, P<0.001).

Univariate Cox regression analysis identified that the survival risk of Histological Grade III patients is significantly higher than that of Grade I and Grade II (P<0.001). As the tumor stage progresses (from T2 to T4), the survival risk of patients significantly increases, especially for T4 patients, whose survival risk is significantly higher (P<0.001). The survival risk of patients in the N3b group is significantly lower than that in the N3a group, while the survival risk of patients in the N3c group is significantly higher than that in the N3a group (P<0.001). The survival risk of ER-negative and PR-negative patients is significantly higher than that of ER-positive and PR-positive patients (P<0.001). The survival risk of HER2-negative patients is significantly higher than that of HER2-positive patients (P<0.001). Parameters with statistical or clinical significance in the univariate analysis were included in the multivariate Cox model for independent prognostic factor analysis (Table 2).
Table 2
| Variables | OS | BCSS | |||||
|---|---|---|---|---|---|---|---|
| HR | 95% CI | P value | HR | 95% CI | P value | ||
| Age (years) (vs. <60) | |||||||
| ≥60 | 1.554 | 1.431–1.688 | <0.001 | 1.284 | 1.172–1.406 | <0.001 | |
| Race (vs. White) | |||||||
| Black | 1.504 | 1.345–1.681 | <0.001 | 1.567 | 1.388–1.769 | <0.001 | |
| Other | 0.805 | 0.690–0.939 | 0.006 | 0.851 | 0.720–1.005 | 0.06 | |
| Marital status (vs. married) | |||||||
| Unmarried | 1.181 | 1.063–1.312 | 0.002 | 1.249 | 1.115–1.399 | <0.001 | |
| Unknown | 1.038 | 0.853–1.265 | 0.70 | 1.026 | 0.824–1.277 | 0.82 | |
| Laterality (vs. left) | |||||||
| Right | 0.981 | 0.903–1.065 | 0.65 | 0.955 | 0.872–1.046 | 0.32 | |
| Histological grade (vs. grade I) | |||||||
| Grade II | 1.195 | 0.981–1.455 | 0.08 | 1.300 | 1.032–1.638 | 0.03 | |
| Grade III | 1.795 | 1.483–2.173 | <0.001 | 2.144 | 1.714–2.682 | <0.001 | |
| Histological subtype (vs. invasive ductal carcinoma) | |||||||
| Invasive lobular carcinoma | 0.920 | 0.818–1.034 | 0.16 | 0.900 | 0.791–1.024 | 0.11 | |
| Other | 0.864 | 0.764–0.978 | 0.02 | 0.828 | 0.722–0.950 | 0.007 | |
| Tumor stage (vs. T0–1) | |||||||
| T2 | 1.203 | 1.046–1.384 | 0.009 | 1.179 | 1.011–1.376 | 0.04 | |
| T3 | 1.653 | 1.428–1.913 | <0.001 | 1.684 | 1.434–1.977 | <0.001 | |
| T4 | 2.726 | 2.337–3.181 | <0.001 | 2.776 | 2.344–3.287 | <0.001 | |
| Lymph stage (vs. N3a) | |||||||
| N3b | 0.764 | 0.666–0.876 | <0.001 | 0.817 | 0.706–0.947 | 0.007 | |
| N3c | 1.299 | 1.116–1.512 | <0.001 | 1.387 | 1.178–1.633 | <0.001 | |
| ER status (vs. positive) | |||||||
| Negative | 2.000 | 1.833–2.182 | <0.001 | 2.219 | 2.020–2.438 | <0.001 | |
| PR status (vs. positive) | |||||||
| Negative | 1.907 | 1.756–2.072 | <0.001 | 2.064 | 1.885–2.261 | <0.001 | |
| HER2 status (vs. positive) | |||||||
| Negative | 1.404 | 1.263–1.561 | <0.001 | 1.365 | 1.217–1.532 | <0.001 | |
| Breast surgery (vs. modified radical mastectomy) | |||||||
| Breast-conserving surgery | 0.742 | 0.661–0.833 | <0.001 | 0.787 | 0.695–0.892 | <0.001 | |
| No | 2.210 | 1.827–2.674 | <0.001 | 2.305 | 1.876–2.831 | <0.001 | |
| Chemotherapy (vs. yes) | |||||||
| No | 2.205 | 2.006–2.423 | <0.001 | 1.751 | 1.568–1.955 | <0.001 | |
| Radiotherapy (vs. yes) | |||||||
| No | 1.687 | 1.550–1.836 | <0.001 | 1.628 | 1.483–1.787 | <0.001 | |
OS, overall survival; BCSS, breast cancer-specific survival; PSM, propensity score matching; HR, hazard ratio; CI, confidence interval; ER, estrogen receptor; PR, progesterone receptor; HER2, human epidermal growth factor receptor 2.
Multivariate Cox regression analysis further identified that Patients with Grade II and Grade III have significantly higher survival risks compared to Grade I (P<0.05). Patients in the T3 and T4 groups have significantly increased survival risks compared to those in the T0-1 group (P<0.001), with the risk being most pronounced for T4 patients. Patients in the N3b group have significantly lower survival risks compared to those in the N3a group (P<0.001), while there is no significant difference in survival risks between N3c and N3a groups (P>0.05). ER-negative and PR-negative patients have significantly higher survival risks compared to ER-positive and PR-positive patients (P<0.001). HER2-negative patients have significantly higher survival risks compared to HER2-positive patients (P<0.001). Histological grade (Grade II, and Grade III), tumor stage (T3 and T4), hormone receptor status (ER-negative and PR-negative), and HER2 status (HER2-negative) are identified as independent risk factors for poor outcomes in patients with N3 LABC. In contrast, the N3b stage represents a lower risk compared to N3a, indicating a more favorable prognosis for these patients (Table 3).
Table 3
| Variables | OS | BCSS | |||||
|---|---|---|---|---|---|---|---|
| HR | 95% CI | P | HR | 95% CI | P | ||
| Age (years) (vs. <60) | |||||||
| ≥60 | 1.432 | 1.309–1.565 | <0.001 | 1.244 | 1.128–1.372 | <0.001 | |
| Race (vs. White) | |||||||
| Black | 1.328 | 1.184–1.490 | <0.01 | 1.327 | 1.171–1.505 | <0.01 | |
| Other | 0.811 | 0.694–0.948 | 0.009 | 0.842 | 0.712–0.996 | 0.045 | |
| Marital status (vs. married) | |||||||
| Unmarried | 1.162 | 1.042–1.295 | 0.007 | 1.183 | 1.052–1.331 | 0.005 | |
| Unknown | 0.971 | 0.796–1.184 | 0.77 | 0.968 | 0.777–1.206 | 0.77 | |
| Histological grade (vs. grade I) | |||||||
| Grade II | 1.249 | 1.021–1.527 | 0.03 | 1.356 | 1.072–1.715 | 0.01 | |
| Grade III | 1.662 | 1.352–2.043 | <0.001 | 1.918 | 1.509–2.439 | <0.001 | |
| Histological subtype (vs. invasive ductal carcinoma) | |||||||
| Invasive lobular carcinoma | 1.045 | 0.915–1.195 | 0.51 | 1.142 | 0.985–1.323 | 0.08 | |
| Other | 0.922 | 0.813–1.046 | 0.21 | 0.908 | 0.790–1.045 | 0.18 | |
| Tumor stage (vs. T0–1) | |||||||
| T2 | 1.144 | 0.994–1.317 | 0.06 | 1.116 | 0.955–1.304 | 0.17 | |
| T3 | 1.595 | 1.371–1.857 | <0.001 | 1.647 | 1.394–1.945 | <0.001 | |
| T4 | 2.450 | 2.084–2.880 | <0.001 | 2.519 | 2.109–3.008 | <0.001 | |
| Lymph stage (vs. N3a) | |||||||
| N3b | 0.601 | 0.521–0.694 | <0.001 | 0.600 | 0.514–0.700 | <0.001 | |
| N3c | 0.982 | 0.835–1.155 | 0.83 | 0.963 | 0.810–1.146 | 0.67 | |
| ER status (vs. positive) | |||||||
| Negative | 1.524 | 1.346–1.726 | <0.001 | 1.612 | 1.407–1.846 | <0.001 | |
| PR status (vs. positive) | |||||||
| Negative | 1.586 | 1.414–1.778 | <0.001 | 1.601 | 1.410–1.818 | <0.001 | |
| HER2 status (vs. positive) | |||||||
| Negative | 1.739 | 1.556–1.943 | <0.001 | 1.777 | 1.575–2.005 | <0.001 | |
| Breast surgery (vs. modified radical mastectomy) | |||||||
| Breast-conserving surgery | 0.847 | 0.750–0.956 | 0.007 | 0.907 | 0.795–1.034 | 0.14 | |
| No | 1.489 | 1.213–1.828 | <0.001 | 1.522 | 1.220–1.900 | <0.001 | |
| Chemotherapy (vs. yes) | |||||||
| No | 1.774 | 1.591–1.978 | <0.001 | 1.470 | 1.297–1.665 | <0.001 | |
| Radiotherapy (vs. yes) | |||||||
| No | 1.282 | 1.166–1.409 | <0.001 | 1.323 | 1.220–1.468 | <0.001 | |
OS, overall survival; BCSS, breast cancer-specific survival; PSM, propensity score matching; HR, hazard ratio; CI, confidence interval; ER, estrogen receptor; PR, progesterone receptor; HER2, human epidermal growth factor receptor 2.
Statistical analyses results of N3 LABC after PSM
Given the potential significant impact of baseline characteristic imbalances on survival outcomes, the method proposed by Zhao et al. (5) was adopted to maximize the sample size of N3b cases while retaining as many samples as possible. A 1:7:2 PSM analysis was conducted to address baseline variations in age, race, marital status, pathological subtype, surgical treatment, chemotherapy, laterality, and radiotherapy. Due to insufficiently defined baseline characteristics, a total of 3,880 N3a patients, 537 N3b patients, and 74 N3c patients were excluded from the analysis. Ultimately, 200 N3a patients (33.06%), 118 N3b patients (19.50%), and 287 N3c patients (47.44%) were included for matching. After PSM, baseline characteristics were largely balanced, with no significant differences observed across most variables, except for histological grade (P=0.04), tumor stage (P<0.001), ER status (P<0.001), and PR status (P<0.001) (Table 4). This indicates that PSM effectively minimized bias caused by other variables. Among the matched cohorts, the N3c group had the highest proportion of patients, followed by the N3a group, with the N3b group having the smallest proportion.
Table 4
| Variables | N3a (n=200) | N3b (n=118) | N3c (n=287) | P value |
|---|---|---|---|---|
| Age (years) | 0.98 | |||
| <60 | 120 (60.00) | 72 (61.02) | 172 (59.93) | |
| ≥60 | 80 (40.0) | 46 (38.98) | 115 (40.07) | |
| Race | 0.22 | |||
| White | 124 (62.00) | 76 (64.41) | 198 (68.99) | |
| Black | 47 (23.50) | 31 (26.27) | 65 (22.65) | |
| Other | 29 (14.50) | 11 (9.32) | 24 (8.36) | |
| Marital status | 0.40 | |||
| Married | 127 (63.50) | 80 (67.80) | 204 (71.08) | |
| Unmarried | 66 (33.00) | 34 (28.81) | 78 (27.18) | |
| Unknown | 7 (3.50) | 4 (3.39) | 5 (1.74) | |
| Laterality | 0.43 | |||
| Left | 100 (50.00) | 66 (55.93) | 159 (55.40) | |
| Right | 100 (50.00) | 52 (44.07) | 128 (44.60) | |
| Histological grade | 0.04 | |||
| Grade I | 11 (5.50) | 4 (3.39) | 5 (1.74) | |
| Grade II | 71 (35.50) | 37 (31.36) | 79 (27.53) | |
| Grade III | 118 (59.00) | 77 (65.25) | 203 (70.73) | |
| Histological subtype | 0.40 | |||
| Invasive ductal carcinoma | 146 (73.00) | 90 (76.27) | 229 (79.79) | |
| Invasive lobular carcinoma | 10 (5.00) | 5 (4.24) | 7 (2.44) | |
| Other | 44 (22.00) | 23 (19.49) | 51 (17.77) | |
| Tumor stage | <0.001 | |||
| T0–1 | 30 (15.00) | 21 (17.80) | 38 (13.24) | |
| T2 | 87 (43.50) | 44 (37.29) | 94 (32.75) | |
| T3 | 51 (25.50) | 28 (23.73) | 50 (17.42) | |
| T4 | 32 (16.00) | 25 (21.18) | 105 (36.59) | |
| ER status | <0.001 | |||
| Positive | 148 (74.00) | 87 (73.73) | 158 (55.05) | |
| Negative | 52 (26.00) | 31 (26.27) | 129 (44.95) | |
| PR status | <0.001 | |||
| Positive | 106 (53.00) | 67 (56.78) | 112 (39.02) | |
| Negative | 94 (47.00) | 51 (43.22) | 175 (60.98) | |
| HER2 status | 0.24 | |||
| Positive | 52 (26.00) | 36 (30.51) | 95 (33.10) | |
| Negative | 148 (74.00) | 82 (69.49) | 192 (66.90) | |
| Breast surgery | 0.32 | |||
| Modified radical mastectomy | 121 (60.50) | 60 (50.85) | 166 (57.84) | |
| Breast-conserving surgery | 50 (25.00) | 31 (26.27) | 66 (23.00) | |
| No | 29 (14.50) | 27 (22.88) | 55 (19.16) | |
| Chemotherapy | 0.052 | |||
| Yes | 168 (84.00) | 99 (83.90) | 260 (90.59) | |
| No | 32 (16.00) | 19 (16.10) | 27 (9.41) | |
| Radiotherapy | 0.07 | |||
| Yes | 113 (56.50) | 59 (50.00) | 174 (60.63) | |
| No | 87 (43.50) | 59 (50.00) | 113 (39.37) |
Data are presented as n (%). PSM, propensity score matching; ER, estrogen receptor; PR, progesterone receptor; HER2, human epidermal growth factor receptor 2.
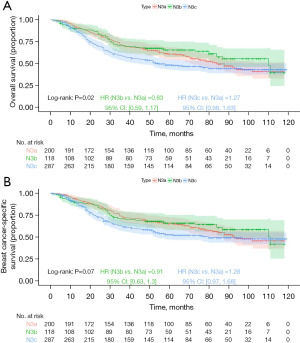
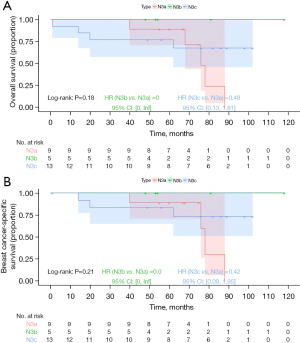
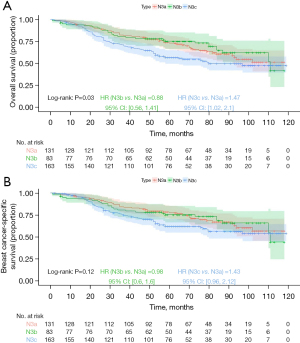
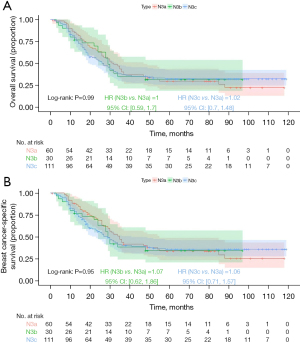
Kaplan-Meier analysis demonstrated that the N3b group had the best prognosis, followed by the N3a group, with the N3c group exhibiting the worst outcomes (Figure 6; OS, P=0.02; BCSS, P=0.07), but the differences in BCSS were not statistically significant. For stage IIIa prognostic staging, Kaplan-Meier analysis indicated that the N3b group had the best prognosis, followed by the N3a group, with the N3c group showing the worst prognosis; however, the differences were not statistically significant (Figure 7; OS, P=0.18; BCSS, P=0.21). For stage IIIb prognostic staging, the N3b group again exhibited the most favorable prognosis, followed by the N3a group, with the N3c group showing the poorest outcomes (Figure 8; OS, P=0.03; BCSS, P=0.12), the differences in BCSS were also not statistically significant. Interestingly, for stage IIIc prognostic staging, the N3a group showed the best prognosis, followed by the N3b group, with the N3c group demonstrating the worst outcomes; however, the differences were not statistically significant (Figure 9; OS, P=0.99; BCSS, P=0.95).
Univariate Cox regression analysis identified that patients with Grade III have significantly higher survival risks compared to Grade I and Grade II (P<0.05). As the tumor stage progresses, especially in T3 and T4 stages, the survival risk of patients significantly increases, with the highest risk observed in T4 patients (P<0.001). N3b patients have a lower survival risk compared to the N3a group (P>0.05), while N3c patients have a higher survival risk than the N3a group, but the difference does not reach statistical significance (P>0.05). ER-negative patients have significantly higher survival risks compared to ER-positive patients (P<0.001). PR-negative patients have significantly higher survival risks compared to PR-positive patients (P<0.001). HER2-negative patients have significantly higher survival risks compared to HER2-positive patients (P<0.01). Parameters with statistical or clinical significance in the univariate analysis were included in the multivariate Cox model for independent prognostic factor analysis (Table 5).
Table 5
| Variables | OS | BCSS | |||||
|---|---|---|---|---|---|---|---|
| HR | 95% CI | P value | HR | 95% CI | P value | ||
| Age (years) (vs. <60) | |||||||
| ≥60 | 1.743 | 1.389–2.188 | <0.001 | 1.456 | 1.140–1.860 | 0.003 | |
| Race (vs. White) | |||||||
| Black | 1.458 | 1.128–1.884 | 0.004 | 1.512 | 1.151–1.986 | 0.003 | |
| Other | 1.063 | 0.726–1.555 | 0.76 | 1.069 | 0.710–1.608 | 0.75 | |
| Marital status (vs. married) | |||||||
| Unmarried | 1.177 | 0.923–1.502 | 0.19 | 1.233 | 0.952–1.596 | 0.11 | |
| Unknown | 0.542 | 0.223–1.317 | 0.18 | 0.376 | 0.120–1.177 | 0.09 | |
| Laterality (vs. left) | |||||||
| Right | 0.957 | 0.762–1.202 | 0.71 | 0.915 | 0.717–1.168 | 0.47 | |
| Histological grade (vs. grade I) | |||||||
| Grade II | 2.173 | 0.881–5.359 | 0.09 | 2.122 | 0.773–5.827 | 0.14 | |
| Grade III | 3.086 | 1.271–7.496 | 0.01 | 3.503 | 1.301–9.433 | 0.01 | |
| Histological subtype (vs. invasive ductal carcinoma) | |||||||
| Invasive lobular carcinoma | 0.682 | 0.362–1.285 | 0.24 | 0.786 | 0.417–1.484 | 0.46 | |
| Other | 0.684 | 0.502–0.933 | 0.02 | 0.671 | 0.480–0.938 | 0.02 | |
| Tumor stage (vs. T0–1) | |||||||
| T2 | 1.485 | 0.967–2.280 | 0.07 | 1.534 | 0.966–2.437 | 0.07 | |
| T3 | 1.920 | 1.225–3.010 | 0.004 | 2.008 | 1.238–3.257 | 0.005 | |
| T4 | 3.801 | 2.494–5.791 | <0.001 | 3.833 | 2.430–6.047 | <0.001 | |
| Lymph stage (vs. N3a) | |||||||
| N3b | 0.828 | 0.586–1.169 | 0.28 | 0.906 | 0.631–1.302 | 0.59 | |
| N3c | 1.266 | 0.982–1.631 | 0.07 | 1.274 | 0.969–1.674 | 0.08 | |
| ER status (vs. positive) | |||||||
| Negative | 2.049 | 1.629–2.577 | <0.001 | 2.345 | 1.837–2.993 | <0.001 | |
| PR status (vs. positive) | |||||||
| Negative | 2.053 | 1.624–2.597 | <0.001 | 2.263 | 1.754–2.919 | <0.001 | |
| HER2 status (vs. positive) | |||||||
| Negative | 1.561 | 1.194–2.040 | 0.001 | 1.532 | 1.152–2.038 | 0.003 | |
| Breast surgery (vs. modified radical mastectomy) | |||||||
| Breast-conserving surgery | 0.895 | 0.671–1.195 | 0.45 | 0.886 | 0.650–1.209 | 0.45 | |
| No | 2.526 | 1.919–3.326 | <0.001 | 2.606 | 1.946–3.491 | <0.001 | |
| Chemotherapy (vs. yes) | |||||||
| No | 1.672 | 1.239–2.255 | <0.001 | 1.432 | 1.022–2.007 | 0.04 | |
| Radiotherapy (vs. yes) | |||||||
| No | 1.358 | 1.082–1.704 | 0.008 | 1.391 | 1.091–1.773 | 0.008 | |
OS, overall survival; BCSS, breast cancer-specific survival; PSM, propensity score matching; HR, hazard ratio; CI, confidence interval; ER, estrogen receptor; PR, progesterone receptor; HER2, human epidermal growth factor receptor 2.
Multivariate Cox regression analysis further identified that the survival risk for Grade III patients is higher than that of the Grade I group, but it does not reach statistical significance (P>0.05). Patients in stages T3 and T4 have a significantly higher survival risk than those in stages T0-1, with the highest risk observed in stage T4 (P<0.001). There is no significant difference in survival risk between N3b and N3c groups compared to the N3a group (P>0.05). ER-negative patients have a significantly higher survival risk compared to ER-positive patients (P<0.05). PR-negative patients have a significantly higher survival risk compared to PR-positive patients (P<0.001). HER2-negative patients have a significantly higher survival risk compared to HER2-positive patients (P<0.001). Tumor stage (T3 and T4), hormone receptor status (ER-negative and PR-negative), and HER2 status (HER2-negative) are identified as independent risk factors for poor outcomes in patients with N3 LABC. The survival risk in the N3b group is slightly lower than that in the N3a group, but the difference does not reach statistical significance (Table 6).
Table 6
| Variables | OS | BCSS | |||||
|---|---|---|---|---|---|---|---|
| HR | 95% CI | P | HR | 95% CI | P | ||
| Age (years) (vs. <60) | |||||||
| ≥60 | 1.890 | 1.468–2.434 | <0.001 | 1.614 | 1.229–2.118 | <0.001 | |
| Race (vs. White) | |||||||
| Black | 1.440 | 1.086–1.911 | 0.01 | 1.393 | 1.030–1.884 | 0.03 | |
| Other | 1.133 | 0.758–1.695 | 0.54 | 1.105 | 0.718–1.701 | 0.65 | |
| Marital status (vs. married) | |||||||
| Unmarried | 1.256 | 0.966–1.633 | 0.09 | 1.283 | 0.972–1.694 | 0.08 | |
| Unknown | 0.626 | 0.253–1.551 | 0.31 | 0.403 | 0.126–1.285 | 0.12 | |
| Histological grade (vs. grade I) | |||||||
| Grade II | 2.074 | 0.828–5.195 | 0.12 | 1.920 | 0.688–5.355 | 0.21 | |
| Grade III | 2.256 | 0.903–5.638 | 0.08 | 2.418 | 0.870–6.719 | 0.09 | |
| Histological subtype (vs. invasive ductal carcinoma) | |||||||
| Invasive lobular carcinoma | 1.135 | 0.566–2.276 | 0.72 | 1.545 | 0.759–3.143 | 0.23 | |
| Other | 0.861 | 0.621–1.194 | 0.37 | 0.872 | 0.612–1.241 | 0.45 | |
| Tumor stage (vs. T0–1) | |||||||
| T2 | 1.458 | 0.944–2.250 | 0.09 | 1.505 | 0.942–2.405 | 0.09 | |
| T3 | 2.085 | 1.302–3.339 | 0.002 | 2.172 | 1.308–3.607 | 0.003 | |
| T4 | 3.196 | 2.032–5.026 | <0.001 | 3.371 | 2.068–5.495 | <0.001 | |
| Lymph stage (vs. N3a) | |||||||
| N3b | 0.810 | 0.570–1.152 | 0.24 | 0.905 | 0.625–1.310 | 0.60 | |
| N3c | 1.077 | 0.817–1.421 | 0.60 | 1.053 | 0.781–1.419 | 0.74 | |
| ER status (vs. positive) | |||||||
| Negative | 1.437 | 1.053–1.960 | 0.02 | 1.609 | 1.154–2.245 | 0.005 | |
| PR status (vs. positive) | |||||||
| Negative | 1.930 | 1.408–2.647 | <0.001 | 1.876 | 1.329–2.646 | <0.001 | |
| HER2 status (vs. positive) | |||||||
| Negative | 1.811 | 1.366–2.401 | <0.001 | 1.812 | 1.342–2.448 | <0.001 | |
| Breast surgery (vs. modified radical mastectomy) | |||||||
| Breast-conserving surgery | 1.071 | 0.769–1.491 | 0.69 | 1.094 | 0.765–1.565 | 0.62 | |
| No | 1.869 | 1.362–2.563 | <0.001 | 1.916 | 1.370–2.678 | <0.001 | |
| Chemotherapy (vs. yes) | |||||||
| No | 1.723 | 1.223–2.427 | 0.002 | 1.595 | 1.090–2.335 | 0.02 | |
| Radiotherapy (vs. yes) | |||||||
| No | 1.089 | 0.834–1.422 | 0.53 | 1.123 | 0.843–1.496 | 0.43 | |
OS, overall survival; BCSS, breast cancer-specific survival; PSM, propensity score matching; HR, hazard ratio; CI, confidence interval; ER, estrogen receptor; PR, progesterone receptor; HER2, human epidermal growth factor receptor 2.
Discussion
Breast cancer is the most common malignant tumor and the leading cause of cancer-related deaths among women. It has been reported that the incidence of breast cancer has been continuously increasing, with an annual rise of 1% from 2012 to 2021 (6). The prognostic staging is a reliable tool for its diagnosis and treatment, serving as a solid foundation by categorizing patients’ test results. Beyond the anatomical staging, this process integrates biological markers—such as ER, PR, HER2, histological grade (G), and multi-gene detection tools. These tumor biomarkers are instrumental in predicting prognosis and play a vital role in guiding personalized treatment strategies (7). Some studies have shown that prognostic staging advances the comprehensive evaluation of breast cancer (8,9). The 8th edition of the Prognostic Stage Group is the most comprehensive staging tool for breast cancer. In this study, histological grading, N-staging, T-staging, ER, PR, and HER2 status were significantly associated with OS and BCSS of breast cancer in multifactorial analysis. It was confirmed that in N3 LABC, the 8th edition prognostic staging system further incorporates biological markers, making it more comprehensive and reasonable than traditional anatomical staging.
LABC refers to breast cancer with extensive infiltration of lesions in the breast or more severe regional lymph node involvement without distant metastases being clinically detected. With the modification of the TNM staging system for breast cancer, LABC includes N3 lymph node status, which is divided into N3a, N3b, and N3c (10). After more than 10 years of clinical practice and validation (4), it was found that patients with only ipsilateral supraclavicular lymph nodes without distant metastases had similar OS rates to patients with stage IIIB, and the ipsilateral supraclavicular lymph nodes were redefined as N3. However, due to the relatively broad range of N3 and the prognostic limitations that still exist between N3 subtypes, several studies have examined the prognosis of N3. Park et al. (11) found that the N3b DFS and OS were both longer than those of N3a and N3c. Additionally, a study indicates that bilateral breast cancer generally has more lymph node metastases than unilateral breast cancer (12). Therefore, in this study, we analyzed the prognosis of patients with different N3 subtypes of unilateral breast cancer and their correlation with OS and BCSS. We also examined the differences between traditional anatomical staging and prognostic staging.
Internal mammary lymph nodes (IMLNs) in breast cancer are located in the parasternal intercostal space, with an average of four nodes, accounting for 25% of the breast’s lymphatic fluid return. Previous study has shown (13) that IMLN metastases may be present in approximately 28–52% of patients with positive axillary lymph nodes in breast cancer. In this study, we comprehensively analyzed the correlation between N3 LABC intervals and OS and BCSS by controlling for several clinicopathological variables. The results showed that OS and BCSS of breast cancer were correlated with different N3 subtypes, with the prognosis of N3b patients being better than that of the N3a group, and the N3c group having the worst prognosis. This suggests that different N3 subtypes are closely related to the prognosis of breast cancer patients, and that different pathological features of breast cancer are associated with N3 status and have varying prognostic implications. Patients with different N3 intervals were validated according to different prognostic stages.
This study has significant implications for clinical practice. First, assessment based on different prognostic staging plays an important role in the era of personalized medicine. Previous study (14) has shown that the prognosis and risk of metastasis for breast cancers with different ER, PR, HER2+, and histological grades are significantly different, greatly affecting the survival and treatment of breast cancer. In this study, the significant association of histological grade, N-stage, T-stage, and the statuses of ER, PR, and HER2 with OS and BCSS highlights the critical role of integrating these biological markers into prognostic staging systems. The N3b group had the best prognosis, followed by the N3a group, while the N3c group had the worst prognosis. N3b had the best prognosis regardless of traditional anatomical or prognostic staging, while the prognosis in N3a and N3c crossed over or even inverted after a certain time point, regardless of staging. It cannot be ruled out that other confounders influenced their prognosis. Finally, the results of multivariate Cox analysis showed that patients with N3b might have better survival regardless of anatomical or prognostic staging outcomes, suggesting that these patients have a better prognosis than those with N3a or N3c. Joo et al. (15) stratified N3 using prophylactic staging and confirmed significant differences in survival rates between the groups. With the increasing optimization of imaging tools, precision radiotherapy techniques, and minimally invasive surgical techniques, IMLN metastases are more easily detected and treated, improving the prognosis of patients whose IMLN were previously poorly managed. This may contribute to the better prognosis observed in patients with N3b compared to N3a. Additionally, internal breast lymph node status may play a role, as Qiu et al. (16) demonstrated that the sentinel lymph node metastatic status of axillary lymph node-positive patients can affect survival time and influence therapeutic decisions. Therefore, future research should focus on further refining the N3 staging system by incorporating new biomarkers, such as IMLN status. This could improve the precision of prognostic assessments and guide more targeted and effective treatment approaches, ultimately improving survival outcomes for patients with N3 LABC.
There are some limitations in this study. First, it exclusively included female patients with unilateral LABC diagnosed as N3, which may introduce potential selection bias. Consequently, the findings may not be generalizable to other subtypes of N3 breast cancer, necessitating further data analysis to validate these conclusions in broader patient populations. Second, as a retrospective study, it is inherently prone to selection bias due to incomplete data. Although PSM was applied, the potential influence of residual confounders cannot be entirely ruled out. Additionally, the SEER database lacks critical information, such as updated classifications for N3a staging and detailed data on endocrine and targeted therapies, which may have varying degrees of impact on patient prognosis.
Conclusions
In the study of N3 LABC, histological grade, N-stage, T-stage, and the statuses of ER, PR, and HER2 are significantly linked to OS and BCSS. These findings emphasize the importance of integrating these biological markers into prognostic staging to enhance survival prognosis and treatment strategies for N3 LABC patients. Among the N3 sub-stages, N3b demonstrates the most favorable prognosis, while prognoses for N3a and N3c may converge or reverse over time. It is essential to consider other confounding factors that might affect prognosis. Therefore, future research should investigate further subdivisions of N3 staging, including additional biomarkers such as IMLN status, to refine prognostic accuracy and inform treatment approaches for LABC patients classified as N3.
Acknowledgments
We would like to thank the SEER program for providing open access to the database.
Footnote
Reporting Checklist: The authors have completed the STROBE reporting checklist. Available at https://gs.amegroups.com/article/view/10.21037/gs-24-437/rc
Peer Review File: Available at https://gs.amegroups.com/article/view/10.21037/gs-24-437/prf
Funding: This research was supported by grant from
Conflicts of Interest: All authors have completed the ICMJE uniform disclosure form (available at https://gs.amegroups.com/article/view/10.21037/gs-24-437/coif). The authors have no conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved. The study was conducted in accordance with the Declaration of Helsinki (as revised in 2013).
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Brierley JD, Gospodarowicz MK, Wittekind C, et al. editors. TNM Classification of Malignant Tumours. 8th ed. Oxford, UK: Wiley Blackwell; 2017.
- Amin MB, Greene FL, Edge SB, et al. The Eighth Edition AJCC Cancer Staging Manual: Continuing to build a bridge from a population-based to a more "personalized" approach to cancer staging. CA Cancer J Clin 2017;67:93-9.
- Plichta JK, Ren Y, Thomas SM, et al. Implications for Breast Cancer Restaging Based on the 8th Edition AJCC Staging Manual. Ann Surg 2020;271:169-76.
- Brito RA, Valero V, Buzdar AU, et al. Long-term results of combined-modality therapy for locally advanced breast cancer with ipsilateral supraclavicular metastases: The University of Texas M.D. Anderson Cancer Center experience. J Clin Oncol 2001;19:628-33. [Crossref] [PubMed]
- Zhao QY, Luo JC, Su Y, et al. Propensity score matching with R: conventional methods and new features. Ann Transl Med 2021;9:812. [Crossref] [PubMed]
- Giaquinto AN, Sung H, Newman LA, et al. Breast cancer statistics 2024. CA Cancer J Clin 2024;74:477-95. [Crossref] [PubMed]
- Poorolajal J, Nafissi N, Akbari ME, et al. Breast Cancer Survival Analysis Based on Immunohistochemistry Subtypes (ER/PR/HER2): a Retrospective Cohort Study. Arch Iran Med 2016;19:680-6.
- Giuliano AE, Connolly JL, Edge SB, et al. Breast Cancer-Major changes in the American Joint Committee on Cancer eighth edition cancer staging manual. CA Cancer J Clin 2017;67:290-303.
- Wong RX, Wong FY, Lim J, et al. Validation of the AJCC 8th prognostic system for breast cancer in an Asian healthcare setting. Breast 2018;40:38-44. [Crossref] [PubMed]
- Giuliano AE, Edge SB, Hortobagyi GN. Eighth Edition of the AJCC Cancer Staging Manual: Breast Cancer. Ann Surg Oncol 2018;25:1783-5. [Crossref] [PubMed]
- Park HJ, Shin KH, Cho KH, et al. Outcomes of positron emission tomography-staged clinical N3 breast cancer treated with neoadjuvant chemotherapy, surgery, and radiotherapy. Int J Radiat Oncol Biol Phys 2011;81:e689-95. [Crossref] [PubMed]
- Mruthyunjayappa S, Zhang K, Zhang L, et al. Synchronous and metachronous bilateral breast cancer: clinicopathologic characteristics and prognostic outcomes. Hum Pathol 2019;92:1-9. [Crossref] [PubMed]
- Huang O, Wang L, Shen K, et al. Breast cancer subpopulation with high risk of internal mammary lymph nodes metastasis: analysis of 2,269 Chinese breast cancer patients treated with extended radical mastectomy. Breast Cancer Res Treat 2008;107:379-87. [Crossref] [PubMed]
- Purdie CA, Quinlan P, Jordan LB, et al. Progesterone receptor expression is an independent prognostic variable in early breast cancer: a population-based study. Br J Cancer 2014;110:565-72. [Crossref] [PubMed]
- Joo JH, Kim SS, Son BH, et al. Evaluation of the Prognostic Stage in the 8th Edition of the American Joint Committee on Cancer in Patients with Breast Cancer and Internal Mammary Lymph Node Metastasis. Anticancer Res 2018;38:5357-61.
- Qiu PF, Zhao RR, Wang W, et al. Internal Mammary Sentinel Lymph Node Biopsy in Clinically Axillary Lymph Node-Positive Breast Cancer: Diagnosis and Implications for Patient Management. Ann Surg Oncol 2020;27:375-83. [Crossref] [PubMed]


