
Development and validation of a nomogram based on preoperative factors for predicting clinically relevant postoperative pancreatic fistula following pancreaticoduodenectomy
Highlight box
Key findings
• The nomogram based on preoperative computed tomography (CT) parameters and laboratory factors, which indicate the degree of fat infiltration in the pancreas, predicts clinically relevant postoperative pancreatic fistula (CR-POPF) accurately. These parameters include thickness of the pancreas (TP), main pancreatic duct (MPD) size, pancreatic index (PI), triglyceride (TG), and neutrophils.
What is known and what is new?
• Several POPF risk factors have been identified, including preoperative biliary drainage (PBD), intraoperative assessed TP, MPD size, pancreatic stump texture, and postoperative drain amylase values. Various predictive models aiming to improve the accuracy of POPF risk assessment based on those factors have been proposed.
• Predictors that are often assessed intraoperatively (e.g., MPD, TP) can be measured preoperatively using CT parameters, reducing bias due to subjectivity and improving accuracy. Additionally, factors related to pancreatic fat infiltration, including TP, PI, and TG are independently associated with CR-POPF.
What is the implication, and what should change now?
• Regular preoperative radiologic assessment of MPD, TP, and PI, along with early detection of abnormal lipid metabolism markers, is helpful for accurate POPF risk assessment in the preoperative setting. This promotes a more proactive approach to surveillance and intervention to mitigate fistula development.
Introduction
Pancreaticoduodenectomy (PD) is a major surgical approach for treating diseases of the periampullary region. Despite significant progress in technology and advancements in perioperative management strategies, the morbidity rate following PD remains elevated, ranging from 30% to 60% (1,2). Postoperative pancreatic fistula (POPF) is one of the major drivers of severe postoperative morbidity, with an incidence ranging from 10% to 40% (3,4).
POPF can be divided into two major groups according to the consensus of 2016 International Study Group of Pancreatic Surgery (ISGPS): biochemical leak (BL) and clinically relevant postoperative pancreatic fistula (CR-POPF) (5). CR-POPF is a major driver of life-threatening postoperative complications, especially when accompanied by intra-abdominal infections or delayed massive bleeding (6). Previous studies have identified several risk factors for POPF, including preoperative biliary drainage (PBD), thickness of the pancreas (TP), main pancreatic duct (MPD) size, pancreatic stump texture, and drain amylase levels (7-9). Based on these risk factors, numerous scoring systems have been proposed to improve CR-POPF risk stratification (10-12). The most extensively validated predictive models, the Fistula Risk Score (FRS) and the Alternative Fistula Risk Score (a-FRS), rely on intraoperative details such as pancreatic duct diameter, gland texture, and blood loss. However, these parameters are not always accessible preoperatively (13,14), and their reliance on surgeons’ subjective judgment introduces potential bias, potentially compromising the accuracy and reliability of the predictions.
Recently, increasing evidence has emerged that certain computed tomography (CT) findings, including TP, MPD size, and pancreatic index (PI)—calculated by dividing pancreatic CT density by splenic CT density—are independent risk factors for CR-POPF (15-18). However, few studies using nomograms have assessed the risk of CR-POPF based solely on preoperative radiologic and clinical variables.
Therefore, this study aims to develop and validate a novel nomogram for predicting the risk of CR-POPF by incorporating preoperative clinical and radiological data, including CT-derived measurements that reflect pancreatic tissue characteristics. By integrating basic patient characteristics with histologic information extracted from preoperative CT scans, this model seeks to provide a more comprehensive and accurate risk prediction tool for patients undergoing PD. We present this article in accordance with the TRIPOD reporting checklist (available at https://gs.amegroups.com/article/view/10.21037/gs-24-249/rc).
Methods
Patients
Data from all 262 consecutive patients who underwent PD between February 2021 and December 2022 at the Department of Pancreatic and Metabolic Surgery, Nanjing Drum Tower Hospital, the Affiliated Hospital of Medical School, Nanjing University were retrospectively collected. The study was approved by the Health Research Ethics Board of Nanjing Drum Tower Hospital, the Affiliated Hospital of Medical School, Nanjing University (No. 2024-795-01). The process of our study adhered to the Declaration of Helsinki (as revised in 2013). Every patient signed written informed consent for this retrospective study and for the use of their clinical data. The inclusion criteria were as follows: (I) patients meeting the indications for conventional PD or pylorus-preserving PD (PPPD); (II) absence of distant metastasis at the time of diagnosis; (III) no history of chemotherapy or radiotherapy; and (IV) age greater than 18 years old. The exclusion criteria were: (I) patients who had undergone simultaneous hepatic/colon resection; (II) those requiring emergency surgery for trauma; (III) total pancreatectomy; and (IV) lack of medical records. All preoperative demographics, laboratory tests, and preoperative assessments from CT images were obtained. To evaluate the applicability of our novel predictive nomogram, patients were randomly assigned to a training cohort (n=209) and a validation cohort (n=53).
Surgical techniques and perioperative management
All PDs were performed using a standardized technique with modified Child’s reconstruction by the same experienced surgical team. The pancreas was transected at the level of the superior mesenteric vein in all patients. An interrupted double-layer, duct-to-mucosa pancreaticojejunostomy (PJ) anastomosis was performed manually using an end-to-side technique. Depending on the diameter of the MPD, a non-absorbable internal pancreatic duct stent was routinely placed. Hepaticojejunostomy (HJ) was completed manually with an end-to-side, monolayer continuous suture anastomosis on the same segment of the jejunum. Prior to closing the surgical incision, two closed-suction peritoneal drainage tubes were routinely inserted at the superior and inferior positions of the PJ.
All enrolled patients received standard perioperative management. PBD, performed via endoscopic nasobiliary drainage (ENBD) or percutaneous transhepatic cholangial drainage (PTCD), was indicated in patients with hyperbilirubinemia (total bilirubin ≥250 µmol/L), preoperative cholangitis, or compromised nutritional status requiring support (19,20). Prophylactic antibiotics, specifically third-generation cephalosporin (ceftriaxone), were routinely administered intravenously for 3 days [the day of surgery and postoperative days (POD) 1 and 2] (21). Drain amylase levels, along with bacterial smears and cultures for both aerobic and anaerobic microorganisms, were routinely assessed from each peritoneal drain on POD 1, 3, 5, and 7. The peritoneal drainage tubes were removed on POD 7 after enhanced abdominal computed tomography (CT) confirmed the absence of CR-POPF or fluid accumulation. For patients with evidence of CR-POPF or fluid accumulation on CT, the drains were kept in place until the CR-POPF resolved. Additional surgical drainage was performed under ultrasonographic guidance, and broad-spectrum antibiotics were administered as needed.
Clinical data collection and outcome
Preoperative clinical data were collected to investigate predictors of CR-POPF, including age, gender, body mass index (BMI), comorbidities (e.g., high blood pressure, diabetes mellitus, and coronary artery disease), preoperative jaundice, PBD, abdominal surgery history, preoperative laboratory results, preoperative CT indicators (e.g., the size of MPD, PI, TP) and the occurrence of CR-POPF.
POPF was defined according to the 2016 ISGPS: amylase-rich fluid (amylase levels exceeding three times the upper limit of normal) gathered from the peripancreatic drains after POD 3. The severity of POPF was classified as follows: BL: no clinical impact; Grade B: requiring a deviation from the expected postoperative management pathway; Grade C: a Grade B POPF resulting in organ failure or clinical instability necessitating reoperation. Grade B/C POPF was defined as CR-POPF.
CT measurement
All patients underwent cross-sectional imaging with an abdominal CT scan within 30 days prior to surgery. Images were transferred to the picture archiving and communication system (PACS) for analysis. CT images were evaluated in the non-contrast phase by two blinded radiologists. CT scans were reviewed to assess the size of the MPD, PI, and TP. PI was assessed using the formula PI = Pancreatic density/Spleen density, as per the method widely accepted for diagnosing fatty liver (22). Briefly, pancreatic CT density was assessed by attenuation, which was measured in Hounsfield units (HU). We selected pancreatic parenchyma sufficiently large to place three round regions of interest (ROIs), each with an area of 1.0 cm2, at the estimated resection line of the confluence of the superior mesenteric and portal veins. Spleen density was also measured with three circular ROIs of 1.0 cm2 at the level of the splenic hilum. Vasculature, pancreatic lesions, pancreatic ducts, and peripheral margins were avoided in the assessment. The average density of both organs was calculated over the measurements. The size of the MPD was measured at the level of the superior mesenteric and portal vein confluence, and TP was assessed at the same level from the anterior to posterior pancreatic margins.
Data presentation and statistical analysis
All statistical analyses were done using R version 4.1.2. Categorical variables were analyzed using the Chi-squared test or Fisher’s exact test, as appropriate, and are expressed as absolute numbers and percentages. Wilcoxon Mann-Whitney test or t-test was used depending on whether the data were distributed normally to compare the distribution of continuous variables, described as mean ± standard deviation (SD) or median [interquartile range (IQR)]. A two-tailed P<0.05 was considered statistically significant.
The nomogram model was constructed following a prospectively defined plan. Model building and validation involved several key steps. Firstly, the dataset was split into a training cohort (70–80%) and a validation cohort (20–30%) using the caret package in R, employing a 10-fold cross-validation method with stratified sampling to ensure a balanced distribution of categorical variables. Secondly, potential predictors were selected based on clinical relevance, prior literature, and expert judgment. Variables with more than 10% missing data were excluded, while the remaining missing values were imputed using the Mice package in R. Thirdly, to identify significant predictors, adaptive least absolute shrinkage and selection operator (LASSO) regression was applied using the Glmnet package in R. Lasso performs variable selection by adding a penalty term to the regression model, shrinking less important coefficients towards zero and removing them from the model. Finally, the selected variables were used in a binary logistic regression model to construct the nomogram, estimating odds ratios (ORs) and 95% confidence intervals (CIs) for each predictor.
Different approaches were utilized to evaluate the nomogram model’s performance. To assess discrimination, the area under the receiver operating characteristic (ROC) curve (AUC) was calculated using the pROC package in R, with AUC values >0.7 considered indicative of a good nomogram, and values >0.8 suggesting excellent performance. Calibration was assessed using calibration plots generated with the rms package in R, comparing the predicted probability of CR-POPF with the observed probability, and the Hosmer-Lemeshow test was applied to assess model fit. Additionally, decision curve analysis (DCA) was performed (rmda package in R) to evaluate the clinical benefit of the nomogram. Sensitivity and specificity were calculated at various thresholds to assess the model’s ability to correctly classify patients with and without CR-POPF.
Results
Demographic and clinical characteristics
The baseline characteristics of all 262 consecutive patients are shown in Table 1. During the study period, 209 patients were included to construct the nomogram, defined as the training cohort, while another 53 patients constituted the validation cohort. The study included 161 (61.45%) male and 101 (38.55%) female participants, with a median age of 63.50 years (range, 55–70 years). A total of 102 (38.93%) patients presented with preoperative jaundice, and 76 (29.01%) underwent PBD. Overall, CR-POPF was diagnosed in 96 (36.2%) patients based on the ISGPF diagnostic criteria. There were no significant differences in preoperative baseline characteristics.
Table 1
| Variables | Total (n=262) | Training cohort (n=209) | Validation cohort (n=53) | P value |
|---|---|---|---|---|
| Age (years) | 63.5 (55.00–70.00) | 64.00 (57.00–70.00) | 59.00 (51.00–67.00) | 0.01 |
| Gender (male) | 161 (61.45) | 135 (64.59) | 26 (49.06) | 0.04 |
| BMI (kg/m2) | 23.41±3.46 | 23.17±3.62 | 23.68±3.45 | 0.67 |
| HBP | 87 (33.21) | 75 (35.89) | 12 (22.64) | 0.07 |
| DM | 47 (17.94) | 43 (20.57) | 4 (7.55) | 0.03 |
| Coronary artery disease | 11 (4.20) | 10 (4.78) | 1 (1.89) | 0.70 |
| Drinking | 46 (17.56) | 40 (19.14) | 6 (11.32) | 0.18 |
| Jaundice | 102 (38.93) | 85 (40.67) | 17 (32.08) | 0.25 |
| Preoperative surgery history | 76 (29.01) | 57 (27.27) | 19 (35.85) | 0.22 |
| PBD | 76 (29.01) | 61 (29.19) | 15 (28.30) | 0.90 |
| ALT (U/L) | 45.40 (17.80–102.60) | 44.9 (18.80–92.70) | 47.00 (17.40–109.90) | 0.73 |
| AST (U/L) | 30.20 (17.90–63.30) | 30.50 (18.70–63.30) | 29.20 (16.00–61.30) | 0.49 |
| TB (μmol/L) | 15.75 (9.10–58.10) | 16.00 (9.00–60.00) | 13.50 (9.20–56.10) | 0.41 |
| DB (μmol/L) | 6.40 (2.20–44.10) | 7.4 (2.30–47.10) | 4.90 (2.00–40.00) | 0.26 |
| AKP (U/L) | 136.50 (69.30–286.90) | 141.30 (70.80–294.90) | 110.60 (62.40–221.10) | 0.22 |
| γ-GGT (U/L) | 123.95 (22.80–387.80) | 121.50 (23.60–375.70) | 132.30 (18.30–418.10) | 0.45 |
| TG (mmol/L) | 1.39 (1.00–2.03) | 1.39 (1.02–2.03) | 1.34 (0.92–1.87) | 0.36 |
| TC (mmol/L) | 4.25 (3.55–5.14) | 4.27 (3.57–5.12) | 4.01 (3.54–5.17) | 0.56 |
| HDL (mmol/L) | 0.85 (0.59–1.12) | 0.83 (0.56–1.11) | 0.92 (0.67–1.14) | 0.18 |
| LDL (mmol/L) | 2.34 (1.82–3.04) | 2.38 (1.83–3.02) | 2.23 (1.72–3.14) | 0.49 |
| Apo A (g/L) | 0.82±0.26 | 0.81±0.27 | 0.83±0.22 | 0.60 |
| Apo B (g/L) | 0.88±0.36 | 0.90±0.37 | 0.80±0.34 | 0.08 |
| Albumin (g/L) | 38.74±3.10 | 38.77±3.10 | 38.60±3.13 | 0.72 |
| CRP (mg/L) | 4.5 (3.10–6.80) | 4.50 (3.10–6.40) | 4.60 (3.20–9.10) | 0.39 |
| WBC (109/L) | 5.60 (4.40–6.60) | 5.70 (4.40–6.60) | 5.60 (4.40–6.60) | 0.72 |
| Neutrophils (109/L) | 3.4 (2.60–4.40) | 3.40 (2.60–4.40) | 3.50 (2.50–4.00) | 0.40 |
| Lymphocyte (109/L) | 1.40 (1.10–1.80) | 1.40 (1.10–1.80) | 1.50 (1.20–1.70) | 0.86 |
| Hb (g/L) | 122.54±0.17.88 | 122.46±17.77 | 122.87±18.50 | 0.88 |
| The size of MPD (mm) | 3.71 (2.40–5.05) | 3.71 (2.35–5.20) | 3.51 (2.54–4.60) | 0.42 |
| PI | 0.66 (0.51–0.79) | 0.65 (0.49–0.78) | 0.68 (0.58–0.80) | 0.04 |
| TP (mm) | 16.74 (13.83–19.35) | 16.48 (13.76–19.13) | 16.93 (15.30–19.65) | 0.46 |
Data are presented as n (%) or mean ± standard deviation or median (interquartile range). BMI, body mass index; HBP, high blood pressure; DM, diabetes mellitus; PBD, preoperative biliary drainage; ALT, alanine aminotransferase; AST, aspartate aminotransferase; TB, total bilirubin; DB, direct bilirubin; AKP, alkaline phosphate; γ-GGT, γ-glutamyl transferase; TG, triglyceride; TC, total cholesterol; HDL, high-density lipoprotein; LDL, low-density lipoprotein; Apo A, apolipoprotein A; Apo B, apolipoprotein B; CRP, C-reactive protein; WBC, white blood cell; Hb, hemoglobin; MPD, main pancreatic duct; PI, pancreatic index; TP, thickness of the pancreas.
Risk factors for CR-POPF
Univariate logistic regression analysis was performed to identify independent risk factors for CR-POPF, revealing that preoperative jaundice (P=0.046), TG (P=0.005), neutrophils (P=0.008), PI (P<0.001), TP (P=0.02), and the size of MPD (P<0.001) were significantly associated with CR-POPF. In contrast, the occurrence of CR-POPF was not influenced by alcohol consumption (P=0.11). Furthermore, multivariate logistic regression analysis demonstrated that TG (P=0.03), neutrophils (P=0.03), PI (P<0.001), TP (P=0.02), and the size of MPD (P<0.001) were independent risk factors for CR-POPF (Table 2).
Table 2
| Variables | Univariate | Multivariate | ||||
|---|---|---|---|---|---|---|
| OR (95% CI) | P value | β coefficient | OR (95% CI) | P value | ||
| TG | 1.48 (1.13–1.94) | 0.005 | 0.3286 | 1.389 (1.03–1.87) | 0.03 | |
| Drinking | 0.55 (0.17–1.13) | 0.11 | ||||
| PI | 0.07 (0.02–0.27) | <0.001 | −3.8867 | 0.021 (0.00–0.11) | <0.001 | |
| The size of MPD | 0.79 (0.70–0.91) | <0.001 | −0.2461 | 0.707 (0.60–0.83) | <0.001 | |
| Jaundice | 1.69 (1.01–2.82) | 0.046 | ||||
| TP | 1.07 (1.01–1.14) | 0.02 | 0.0814 | 1.085 (1.02–1.16) | 0.02 | |
| Neutrophils | 1.25 (1.06–1.47) | 0.008 | 0.1995 | 1.221 (1.02–1.46) | 0.03 | |
CR-POPF, clinically relevant postoperative pancreatic fistula; OR, odds ratio; CI, confidence interval; TG, triglyceride; PI, pancreatic index; MPD, main pancreatic duct; TP, thickness of the pancreas.
Establishment of predictive nomogram for CR-POPF
Based on the results of multivariate logistic regression analysis, we integrated five predictive factors—TG, neutrophils, PI, TP, and MPD—to develop the predictive nomogram. The nomogram for predicting CR-POPF was constructed using these five independent risk factors identified by multivariate regression analysis (Figure 1). As shown in Table 2, the size of MPD made the greatest contribution to predicting CR-POPF. Other factors that independently contributed to CR-POPF risk included neutrophils, TG, PI, and TP. Additionally, as PI and the size of MPD increased, the risk of CR-POPF was reduced.

Internal validation and calibration of the nomogram
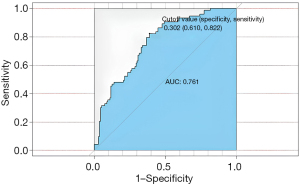
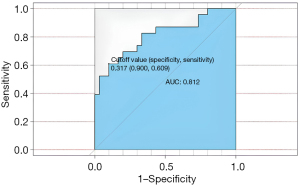
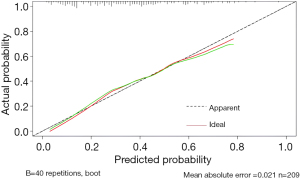
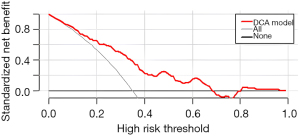
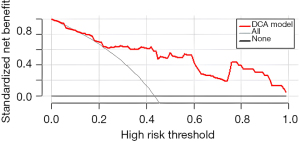
First, the nomogram was implemented on a training cohort of 209 patients. The internal validation of the model showed an AUC of 0.761 (Figure 2). Next, data from the validation cohort (n=53) were utilized to cross-validate the nomogram model, achieving a C-index of 0.812, which exceeded that of the training cohort (Figure 3). The calibration curve indicated that the predictions from the nomogram model were in strong agreement with the observed data (Figures 4,5). To determine the clinical utility of the prediction nomogram, DCA was utilized. In both the training and validation cohorts, the decision curves indicated that the nomogram model for predicting CR-POPF was more beneficial than treating all patients or none as infected (Figures 6,7).

Discussion
This current study aimed to establish a nomogram based on preoperative data, including CT parameters, to predict CR-POPF. We retrospectively collected preoperative data from 262 patients who underwent PD and developed a novel predictive nomogram for assessing the risk of CR-POPF prior to surgery. The nomogram demonstrated good discrimination and calibration. It incorporated the following five factors: (I) the size of MPD; (II) TP; (III) triglyceride (TG); (IV) pancreatic index (PI); and (V) neutrophils. All predictors were objectively measurable preoperatively, without relying on intraoperative or postoperative parameters.
The overall incidence of CR-POPF in our study was 36.2%. The high incidence rate may be attributed to the patient cohort, as 199 (75.95%) of the included patients were non-pancreatic cancer cases, while 63 (24.05%) were diagnosed with pancreatic ductal adenocarcinoma. This study identified MPD size as the most significant independent contributor to CR-POPF risk. Consistent with previous studies, a small MPD was recognized as a strong independent risk factor for CR-POPF, with a relatively high odds ratio (23,24). In this study, each 1 mm increase in MPD diameter was associated with a 29.3% reduction in the odds of CR-POPF (Table 2). The increased risk associated with a smaller MPD may be due to the greater technical difficulty encountered during the reconstruction of the PJ (25,26). In contrast to prior models, where MPD diameter was assessed intraoperatively, this study measured MPD size using preoperative CT images. This approach reduces bias introduced by subjective intraoperative assessment, enhancing the accuracy and reliability of the predictive model.
In addition to MPD, pancreatic parenchymal thickness was significantly associated with the risk of CR-POPF, consistent with findings from previous studies. Sugimoto et al. (15) demonstrated that a thick pancreatic parenchyma and fatty infiltration are risk factors for CR-POPF, in addition to a small MPD. A thicker pancreatic stump can indirectly reflect the thickness and volume of the pancreatic stump, which serve as indicators of exocrine pancreatic function. Chen et al.’s (27) systematic review and meta-analysis further highlighted the role of pancreatic parenchymal thickness and related biomarkers in predicting postoperative pancreatic complications, while Parray et al. (28) discussed how high-risk pancreatic features, such as parenchymal thickness, contribute to the increased risk of CR-POPF and proposed evidence-based strategies to mitigate this risk. Several studies indicated that increased pancreatic parenchymal thickness and remnant pancreatic volume could lead to elevated pancreatic secretions rich in proteolytic enzymes, thereby inducing CR-POPF (29,30). At the same time, thicker pancreatic tissue increases the technical difficulty of PJ stitches, further elevating the risk of CR-POPF.
There is a clear association between the preoperative PI and fat infiltration in the pancreas (16,31,32). Kim et al. (31) analyzed the relationship between PI, calculated as the ratio of pancreatic to splenic attenuation values, and pathological fat infiltration of the pancreas. Since this study focuses on assessing the risk of CR-POPF prior to PD, we utilized PI instead of pathological data. Interestingly, our study demonstrated that a higher PI, indicative of increased fat infiltration in the pancreas, was independently associated with CR-POPF. Studies on pancreatic steatosis have identified fatty pancreas as a significant risk factor for CR-POPF development in the postoperative period (31-34). Additionally, a previous case-control study confirmed that a fatty pancreas is a risk factor for postoperative pancreatic fistula (35).
To our best knowledge, the current study is the first to identify TG as an independent risk factor for CR-POPF after PD. As previously published, higher serum TG levels are significantly correlated with a fatty pancreas (36-39). A systematic review and meta-analysis conducted by Singh et al. (36), including nearly 12,000 individuals, found that markers of lipid metabolism, including circulating TGs and HDL-C, correlated with the percentage of pancreatic fat. Among the lipid metabolism parameters, TGs and HDL-C demonstrated moderate correlations with pancreatic fat, with coefficients of 0.38 and −0.33, respectively. Fatty pancreas, which is well known to affect pancreatic consistency, has been reported to be associated with CR-POPF (17). We hypothesize that the distribution of adipose tissue in the pancreas influences its softness and fragility by disrupting the normal microstructure, thereby increasing the difficulty of creating secure PJ stitches.
In addition, our study identified preoperative neutrophils, the most abundant type of white blood cells, as a significant risk factor for CR-POPF. This finding may be explained by the fact that elevated neutrophil levels correlate with underlying inflammation. Tumors originating from the periampullary region may interfere with the Oddi sphincter, causing gastrointestinal tract contents to flow back into the biliary system and pancreas, which in turn causes a state of preoperative inflammation (40).
There are certain limitations in this study. As with other retrospective studies, the possibility of bias related to historical data and selection cannot be entirely excluded. Moreover, while the nomogram incorporates parameters associated with fatty infiltration, such as PI and TG, the absence of histological confirmation limits a more nuanced understanding of the relationship between pancreatic fat infiltration and CR-POPF. Additionally, recent studies have questioned the significance of fatty infiltration, particularly in the absence of fibrosis (41). Furthermore, the relatively small sample size of the validation cohort may impact the generalizability of our conclusions. Future studies with larger, independent cohorts are needed to validate the nomogram’s robustness and clinical utility, while incorporating pathological confirmation and advanced imaging techniques to elucidate underlying mechanisms and explore risk stratification into distinct categories, thereby enhancing its applicability in diverse clinical settings.
Conclusions
The current study for predicting CR-POPF after PD has been well developed and validated. Our findings demonstrated that preoperative parameters, including TG, neutrophils count, the size of MPD, PI, and TP, were independent predictive factors in the nomogram for predicting CR-POPF. This applicable nomogram could be used to identify individuals at risk of CR-POPF preoperatively, facilitating physicians’ decision-making.
Acknowledgments
We would like to extend our special thanks to members of the multidisciplinary biliopancreatic cancer team of Nanjing Drum Tower Hospital, the Affiliated Hospital of Medical School, Nanjing University for their guidance.
Footnote
Reporting Checklist: The authors have completed the TRIPOD reporting checklist. Available at https://gs.amegroups.com/article/view/10.21037/gs-24-249/rc
Data Sharing Statement: Available at https://gs.amegroups.com/article/view/10.21037/gs-24-249/dss
Peer Review File: Available at https://gs.amegroups.com/article/view/10.21037/gs-24-249/prf
Funding: None.
Conflicts of Interest: All authors have completed the ICMJE uniform disclosure form (available at https://gs.amegroups.com/article/view/10.21037/gs-24-249/coif). The authors have no conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved. The process of our study adhered to the Declaration of Helsinki (as revised in 2013). The study was approved by the Health Research Ethics Board of Nanjing Drum Tower Hospital, the Affiliated Hospital of Medical School, Nanjing University (No. 2024-795-01). Every patient signed written informed consent for this retrospective study and for the use of their clinical data.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- D'Angelica MI, Ellis RJ, Liu JB, et al. Piperacillin-Tazobactam Compared With Cefoxitin as Antimicrobial Prophylaxis for Pancreatoduodenectomy: A Randomized Clinical Trial. JAMA 2023;329:1579-88. [Crossref] [PubMed]
- Bassi C, Marchegiani G, Giuliani T, et al. Pancreatoduodenectomy at the Verona Pancreas Institute: the Evolution of Indications, Surgical Techniques, and Outcomes: A Retrospective Analysis of 3000 Consecutive Cases. Ann Surg 2022;276:1029-38. [Crossref] [PubMed]
- Chen H, Wang W, Ying X, et al. Predictive factors for postoperative pancreatitis after pancreaticoduodenectomy: A single-center retrospective analysis of 1465 patients. Pancreatology 2020;20:211-6. [Crossref] [PubMed]
- Beane JD, Borrebach JD, Zureikat AH, et al. Optimal Pancreatic Surgery: Are We Making Progress in North America?. Ann Surg 2021;274:e355-e363. [Crossref] [PubMed]
- Bassi C, Marchegiani G, Dervenis C, et al. The 2016 update of the International Study Group (ISGPS) definition and grading of postoperative pancreatic fistula: 11 Years After. Surgery 2017;161:584-91. [Crossref] [PubMed]
- Lu JW, Ding HF, Wu XN, et al. Intra-abdominal hemorrhage following 739 consecutive pancreaticoduodenectomy: Risk factors and treatments. J Gastroenterol Hepatol 2019;34:1100-7. [Crossref] [PubMed]
- Ecker BL, McMillan MT, Allegrini V, et al. Risk Factors and Mitigation Strategies for Pancreatic Fistula After Distal Pancreatectomy: Analysis of 2026 Resections From the International, Multi-institutional Distal Pancreatectomy Study Group. Ann Surg 2019;269:143-9. [Crossref] [PubMed]
- PARANOIA Study Group. Perioperative interventions to reduce pancreatic fistula following pancreatoduodenectomy: meta-analysis. Br J Surg 2022;109:812-21. [Crossref] [PubMed]
- Bannone E, Marchegiani G, Vollmer C, et al. Postoperative Serum Hyperamylasemia Adds Sequential Value to the Fistula Risk Score in Predicting Pancreatic Fistula after Pancreatoduodenectomy. Ann Surg 2023;278:e293-301. [Crossref] [PubMed]
- Callery MP, Pratt WB, Kent TS, et al. A prospectively validated clinical risk score accurately predicts pancreatic fistula after pancreatoduodenectomy. J Am Coll Surg 2013;216:1-14. [Crossref] [PubMed]
- Mungroop TH, van Rijssen LB, van Klaveren D, et al. Alternative Fistula Risk Score for Pancreatoduodenectomy (a-FRS): Design and International External Validation. Ann Surg 2019;269:937-43. [Crossref] [PubMed]
- Mungroop TH, Klompmaker S, Wellner UF, et al. Updated Alternative Fistula Risk Score (ua-FRS) to Include Minimally Invasive Pancreatoduodenectomy: Pan-European Validation. Ann Surg 2021;273:334-40. [Crossref] [PubMed]
- Shinde RS, Acharya R, Chaudhari VA, et al. External validation and comparison of the original, alternative and updated-alternative fistula risk scores for the prediction of postoperative pancreatic fistula after pancreatoduodenectomy. Pancreatology 2020;20:751-6. [Crossref] [PubMed]
- PARANOIA Study Group. External validation of postoperative pancreatic fistula prediction scores in pancreatoduodenectomy: a systematic review and meta-analysis. HPB (Oxford) 2022;24:287-98. [Crossref] [PubMed]
- Sugimoto M, Takahashi S, Kojima M, et al. In Patients with a Soft Pancreas, a Thick Parenchyma, a Small Duct, and Fatty Infiltration Are Significant Risks for Pancreatic Fistula After Pancreaticoduodenectomy. J Gastrointest Surg 2017;21:846-54. [Crossref] [PubMed]
- Fukuda Y, Yamada D, Eguchi H, et al. A novel preoperative predictor of pancreatic fistula using computed tomography after distal pancreatectomy with staple closure. Surg Today 2017;47:1180-7. [Crossref] [PubMed]
- Tanaka K, Yamada S, Sonohara F, et al. Pancreatic Fat and Body Composition Measurements by Computed Tomography are Associated with Pancreatic Fistula After Pancreatectomy. Ann Surg Oncol 2021;28:530-8. [Crossref] [PubMed]
- Shi HY, Lu ZP, Li MN, et al. Dual-Energy CT Iodine Concentration to Evaluate Postoperative Pancreatic Fistula after Pancreatoduodenectomy. Radiology 2022;304:65-72. [Crossref] [PubMed]
- Fang Y, Gurusamy KS, Wang Q, et al. Pre-operative biliary drainage for obstructive jaundice. Cochrane Database Syst Rev 2012;2012:CD005444. [Crossref] [PubMed]
- Iacono C, Ruzzenente A, Campagnaro T, et al. Role of preoperative biliary drainage in jaundiced patients who are candidates for pancreatoduodenectomy or hepatic resection: highlights and drawbacks. Ann Surg 2013;257:191-204. [Crossref] [PubMed]
- Bratzler DW, Dellinger EP, Olsen KM, et al. Clinical practice guidelines for antimicrobial prophylaxis in surgery. Surg Infect (Larchmt) 2013;14:73-156. [Crossref] [PubMed]
- Park SH, Kim PN, Kim KW, et al. Macrovesicular hepatic steatosis in living liver donors: use of CT for quantitative and qualitative assessment. Radiology 2006;239:105-12. [Crossref] [PubMed]
- Roberts KJ, Hodson J, Mehrzad H, et al. A preoperative predictive score of pancreatic fistula following pancreatoduodenectomy. HPB (Oxford) 2014;16:620-8. [Crossref] [PubMed]
- Schuh F, Mihaljevic AL, Probst P, et al. A Simple Classification of Pancreatic Duct Size and Texture Predicts Postoperative Pancreatic Fistula: A classification of the International Study Group of Pancreatic Surgery. Ann Surg 2023;277:e597-608. [Crossref] [PubMed]
- Ansorge C, Strömmer L, Andrén-Sandberg Å, et al. Structured intraoperative assessment of pancreatic gland characteristics in predicting complications after pancreaticoduodenectomy. Br J Surg 2012;99:1076-82. [Crossref] [PubMed]
- Wu JM, Lin YJ, Wu CH, et al. Novel Non-duct-to-Mucosa Pancreaticojejunostomy Reconstruction After Pancreaticoduodenectomy: Focus on the Occurrence of Post-pancreatectomy Hemorrhage and Intra-abdominal Abscess. Ann Surg Oncol 2023;30:5063-70. [Crossref] [PubMed]
- Chen G, Yi H, Zhang J. Diagnostic value of C-reactive protein and procalcitonin for postoperative pancreatic fistula following pancreatoduodenectomy: a systematic review and meta-analysis. Gland Surg 2021;10:3252-63. [Crossref] [PubMed]
- Parray AM, Chaudhari VA, Shrikhande SV, et al. "Mitigation strategies for post-operative pancreatic fistula after pancreaticoduodenectomy in high-risk pancreas: an evidence-based algorithmic approach"-a narrative review. Chin Clin Oncol 2022;11:6. [Crossref] [PubMed]
- Okano K, Murakami Y, Nakagawa N, et al. Remnant pancreatic parenchymal volume predicts postoperative pancreatic exocrine insufficiency after pancreatectomy. Surgery 2016;159:885-92. [Crossref] [PubMed]
- Hartman V, Op de Beeck B, Chapelle T, et al. Prediction of exocrine and endocrine insufficiency after pancreaticoduodenectomy using volumetry. Acta Chir Belg 2020;120:257-64. [Crossref] [PubMed]
- Kim SY, Kim H, Cho JY, et al. Quantitative assessment of pancreatic fat by using unenhanced CT: pathologic correlation and clinical implications. Radiology 2014;271:104-12. [Crossref] [PubMed]
- Fukuda Y, Yamada D, Eguchi H, et al. CT Density in the Pancreas is a Promising Imaging Predictor for Pancreatic Ductal Adenocarcinoma. Ann Surg Oncol 2017;24:2762-9. [Crossref] [PubMed]
- Pecorelli N, Carrara G, De Cobelli F, et al. Effect of sarcopenia and visceral obesity on mortality and pancreatic fistula following pancreatic cancer surgery. Br J Surg 2016;103:434-42. [Crossref] [PubMed]
- Coppola A, La Vaccara V, Angeletti S, et al. Postoperative procalcitonin is a biomarker for excluding the onset of clinically relevant pancreatic fistula after pancreaticoduodenectomy. J Gastrointest Oncol 2023;14:1077-86. [Crossref] [PubMed]
- Mathur A, Pitt HA, Marine M, et al. Fatty pancreas: a factor in postoperative pancreatic fistula. Ann Surg 2007;246:1058-64. [Crossref] [PubMed]
- Singh RG, Yoon HD, Poppitt SD, et al. Ectopic fat accumulation in the pancreas and its biomarkers: A systematic review and meta-analysis. Diabetes Metab Res Rev 2017;33: [Crossref] [PubMed]
- Lesmana CR, Pakasi LS, Inggriani S, et al. Prevalence of Non-Alcoholic Fatty Pancreas Disease (NAFPD) and its risk factors among adult medical check-up patients in a private hospital: a large cross sectional study. BMC Gastroenterol 2015;15:174. [Crossref] [PubMed]
- Wu WC, Wang CY. Association between non-alcoholic fatty pancreatic disease (NAFPD) and the metabolic syndrome: case-control retrospective study. Cardiovasc Diabetol 2013;12:77. [Crossref] [PubMed]
- Wang CY, Ou HY, Chen MF, et al. Enigmatic ectopic fat: prevalence of nonalcoholic fatty pancreas disease and its associated factors in a Chinese population. J Am Heart Assoc 2014;3:e000297. [Crossref] [PubMed]
- Yang Y, Fu X, Zhu S, et al. Vater's ampullary carcinoma increases the risk of clinically relevant postoperative pancreatic fistula after pancreaticoduodenectomy: A retrospective and propensity score-matched analysis. BMC Gastroenterol 2022;22:51. [Crossref] [PubMed]
- Perri G, Marchegiani G, Partelli S, et al. Either High or Low Risk: The Acinar Score at the Resection Margin Dichotomizes the Risk Spectrum of Pancreas-specific Complications After Pancreatoduodenectomy. Ann Surg 2023;278:e1242-9. [Crossref] [PubMed]


