
A systematic review and meta-analysis comparing the use of elagolix therapy alone or in combination with add-back therapy to treat women with uterine fibroid associated heavy menstrual bleeding
Highlight box
Key findings
• Elagolix alone or in combination with add-back therapy can be considered a treatment for individuals with uterine fibroids and heavy menstrual bleeding (HMB).
What is known and what is new?
• Elagolix is a small molecule, second-generation, nonpeptide gonadotropin-releasing hormone (GnRH) antagonist taken orally. Randomized controlled trials (RCTs) have evaluated the efficacy and safety of elagolix used alone or combined with add-back therapy in women with HMB caused by uterine fibroids.
• Elagolix alone or in combination with add-back therapy can be used for patients with uterine fibroids and HMB.
What are the implications, and what should change now?
• Our study demonstrated the benefits of elagolix for patients with uterine fibroids and HMB.
Introduction
Uterine fibroids (leiomyomas) are common noncancerous tumors of the uterus that originate in the uterine musculature compartment, with a prevalence of approximately 11 million women in the United States (1,2). The lifetime morbidity rates of White and Black women can reach approximately 70% and 80%, respectively (3,4). The main risk factors for uterine fibroids include age, race, premenopausal status, time of fetus delivery, and higher body mass index (BMI) (5). By the age of 50 years old, up to 50% of affected women have symptoms that include heavy menstrual bleeding (HMB) or pelvic pain. HMB, as the primary symptom, can lead to anemia women with uterine fibroids report that these symptoms interfere with daily activities (6-10). Other symptoms, such as dysmenorrhea, urinary and gastrointestinal reactions, may also affect the psychology, social well-being, and quality of life of patients, with 25–50% of requiring treatment (11-15). According to published literature, uterine fibroids have resulted in a heavy economic burden on both women and society, with the cost of treating uterine fibroids in the United States being $590–$344 million dollars every year (16-19).
There are several options for women who experience persistent symptoms due to uterine fibroids, including a hysterectomy, uterine myomectomy, uterine artery embolization, nonsteroidal anti-inflammatory drugs, and hormonal contraceptives. There are noninvasive alternatives to hysterectomies in women with fibroids and HMB, such as laser myolysis, radiofrequency ablation, and magnetic resonance-guided focused ultrasound surgery (20-23). However, owing to the increasing demand for uterine-sparing noninvasive therapies, patients’ acceptance of surgical treatments may be limited (11,18,24). Recently, peptide gonadotropin-releasing hormone (GnRH) analogs have been widely used to treat endometriosis and fibroids, and have assisted treatment for reproductive issues. Owing to their peptide structure, most GnRH agonists require frequent injections or implantation of long-acting formulations (25-27). Therefore, the long-term, safe, and effective use of oral formulations for the treatment of women suffering from uterine fibroids would be a useful alternative to existing therapies.
Elagolix is an orally administered, small molecule second-generation nonpeptide GnRH antagonist that can quickly and reversibly inhibit female gonadotropins and ovarian sex hormones (28-30). Recently, several randomized controlled trials (RCTs) have evaluated the efficacy and safety of elagolix alone or in combination with add-back therapy in women with HMB due to uterine fibroids. However, there are no systematic comprehensive assessments of the existing evidence that incorporate all relevant RCT data published to date. Therefore, the main purpose of this review is to report the efficacy and safety of treatment with elagolix alone or with add-back therapy for women with uterine fibroids who are suffering from HMB, through a systematic review and meta-analysis. We present this article in accordance with the PRISMA reporting checklist (31) (available at https://gs.amegroups.com/article/view/10.21037/gs-24-386/rc).
Methods
In this review, we considered RCTs and only extracted data that included elagolix use alone or with add-back therapy or from the placebo groups of the included studies. This meta-analysis was registered on the PROSPERO website (CRD42021260687).
Search strategy
A systematic literature search was carried out exclusive of language restrictions in the Cochrane Library, PubMed, Embase and ClinicalTrials.gov databases starting from the date of database establishment and concluding on June 15, 2021. The following keywords were searched: “elagolix”, “elagolix sodium”, “orilissa”, “uterine fibroids”, “leiomyomas”, “tumors fibroid”, “RCTs”, and “randomized controlled trial”. In addition. The database search strategy is described in Table S1. Further manual searches of the references in selected studies and existing literature were conducted to identify potential studies that were not captured by the electronic database search.
Eligibility and exclusion criteria
Relevant literature that met the following criteria was included: (I) premenopausal women aged 18 to 50 years old; (II) having a normal menstrual cycle interval of 24–35 days; (III) having HMB results measured using the alkaline hematin method (32) (>80 mL per cycle during 2–3 screening cycles); (IV) a diagnosis of uterine fibroids; (V) having one or more fibroids larger than 2 cm in diameter, or having a total volume of multiple uterine fibroids between 200 and 2,500 cm3 (by pelvic ultrasound recording, as evaluated by a central reader); (VI) having an adequate endometrial biopsy that did not exhibit clinically significant endometrial pathology; and (VII) RCTs.
The exclusion criteria were as follows: (I) having a menstrual cycle exceeding 38 days; (II) having focal intracavitary lesions; (III) a diagnoses of hereditary coagulopathy or those with a severe or long-term bleeding history; and (IV) reviews, animal trials, or non-RCTs.
Study selection
Two professional researchers (Xianying Wang and J.L.) independently screened the retrieved documents by title and abstract. Disagreements that arose during the screening process were resolved through discussions with a third researcher. We read the full texts that met the initial requirements to determine whether they should be included in this study. Any doubts in the selection of eligible studies was resolved by discussion with a third researcher.
Data extraction and quality assessment
Two researchers (Xianying Wang and J.L.) used standardized data collection forms to independently extract data from eligible studies. Cross-checking was applied to ensure the accuracy of the extracted data when extracting, and any differences were resolved through discussion with a third author. The extracted data included baseline characteristics, outcomes, and literature quality. The following data were extracted: trial name, year of publication, number of participants in the trial, national clinical trial (NCT) number, average age, BMI, menstrual bleeding, baseline uterine fibroid symptoms, quality of life (UFS-QoL) score, baseline bone mineral density z score, and hemoglobin level.
The study outcomes included the number of women whose menstrual blood loss (MBL) met the criteria (MBL <80 mL and MBL reduced by more than 50% in the last month), amenorrhea (no bleeding or spotting), UFS-QoL score (change from baseline to the 6th month), the number of women whose hemoglobin levels increased by more than 2 g/dL from baseline to the final month, any adverse events (AEs), and the percent change in bone mineral density from baseline to the 6th month. We used the Cochrane risk of bias to assess the quality of the RCTs (33).
Statistical analysis
Continuous data were expressed as ranges, and discontinuous data were expressed as frequencies or percentages. For dichotomous outcomes, the correlation report is the risk ratio (RR), and the continuous outcomes are related to the mean difference (MD); both of these include 95% confidence intervals (CIs). The heterogeneity of each study was evaluated by Cochran’s Q and I2. When the P value is less than 0.10 or I2 is greater than 50%, the study is highly heterogeneous. Owing to the limited number of trials included, we did not apply Egger’s or Begg’s tests. Publication bias was assessed by applying the Luis Furuya-Kanamori asymmetry (LFK) index. Review Manager 5.4 (RevMan5.4) and Stata16.0 software were used to analyze the data of this systematic analysis.
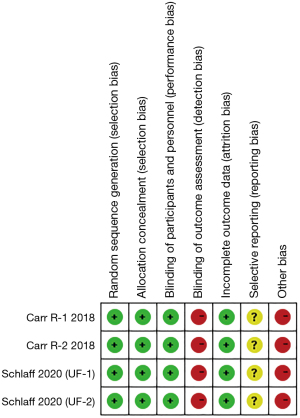
The Cochrane criteria were utilized to assess the quality of the chosen trials, and RevMan5.4 software was employed to generate risk-of-bias graphs. The domains evaluated included random sequence generation, allocation concealment, blinding of participants and personnel, blinding of outcome assessment, incomplete outcome data, selective reporting, and other potential biases, which were categorized as posing low, high, or unclear risks.
Results
Paper selection results
The literature search and research selection tasks were carried out by two authors (Xianying Wang and J.L.). After strict implementation of the selection and exclusion criteria, a total of 105 studies were retrieved. Seventy-five articles remained after the duplicate studies were removed, and 4 studies remained after the titles and abstracts were read and deemed appropriate for review. Finally, two studies with four RCTs were included in this meta-analysis (34,35). The study selection process is detailed in Figure 1.
General characteristics of the studies and patients
The characteristics and clinical information of the trials and patients is summarized in Table 1. The two included studies comprised a two-group parallel test (34), a two-group test with the same daily dose (35). Additionally, two were double-blind studies (34,35). There were 1,217 participants in the two studies, including 341 in the elagolix group, 537 in the elagolix add-back group, and 339 in the placebo group.
Table 1
| Trial name | NCT | Country | Treatment duration (months) | Study arm | Patients | Age, years | Race | BMI, kg/m2 | Menstrual blood loss (mL) | Hemoglobin level, g/dL | UFS-QOL score | Bone mineral density score | ||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Black | White | Others | Symptom severity score | Health-related quality-of-life total score | Lumbar spine | Total hip | Femoral neck | |||||||||||
| Schlaff 2020 (UF-1) (34) | NCT02654054 | United States | 6 | Elagolix 300 mg/BID | 104 | 42.6±5.2 | 69 | 27 | 7 | 33.4±7.7 | 248.9±169.6 | 10.6±1.5 | 60.4±22.5 | 42.5±23.3 | 1.1±1.2 | 0.8±0.9 | 0.6±0.9 | |
| Elagolix 300 mg (BID) + E2 1/0.5 mg NETA(QD) | 206 | 42.6±5.3 | 141 | 59 | 6 | 33.3±6.8 | 238±150.1 | 11.1±1.5 | 57.3±22.2 | 44.1±23.5 | 1.0±1.0 | 0.8±0.9 | 0.6±0.9 | |||||
| Placebo | 102 | 41.6±5.7 | 70 | 30 | 2 | 33.8±7.7 | 255.3±174 | 11±1.4 | 61.7±19.2 | 40.7±20.3 | 0.9±1.0 | 0.7±0.9 | 0.5±0.8 | |||||
| Schlaff 2020 (UF-2) (34) | NCT02691494 | United States, Canada | 6 | Elagolix 300 mg/BID | 95 | 42.2±5.4 | 66 | 27 | 2 | 34.5±7.9 | 224.9±146.2 | 11±1.6 | 63.7±20.4 | 42.2±24.0 | 0.9±1.2 | 0.8±1.0 | 0.6±0.9 | |
| Elagolix 300 mg (BID)+ E2 1/0.5 mg NETA(QD) | 189 | 42.5±5.3 | 124 | 59 | 5 | 33.2±6.9 | 228.5±148.8 | 11.1±1.5 | 60.9±21.6 | 43.3±24.2 | 1.1±1.2 | 0.8±0.9 | 0.6±0.9 | |||||
| Placebo | 94 | 42.5±5.4 | 63 | 30 | 1 | 33.8±7.2 | 254.3±178.5 | 11±1.6 | 60.5±23.4 | 43.0±22.8 | 1.1±1.1 | 0.7±1.0 | 0.6±0.9 | |||||
| Carr R-1 2018 (35) | NCT01817530 | United States, Puerto Rico, Canada, Chile, United Kingdom | 6 | Elagolix 300 mg/BID | 65 | 43 | 41 | 21 | 3 | 30±5.4 | 265±146 | 10.5±1.5 | 63.1±2.5 | 39.9±3.1 | 0.73±1.16 | 0.32±0.73 | 0.27±0.74 | |
| Elagolix 300 mg (BID) + E2 1/0.5 mg NETA(QD) | 65 | 44 | 50 | 14 | 1 | 30±5.1 | 296±253 | 10.4±1.7 | 64.8±2.5 | 35.9±3.1 | 0.8±1.2 | 0.28±1.08 | 0.32±1.16 | |||||
| Placebo | 65 | 44 | 44 | 18 | 3 | 30±4.7 | 238±189 | 11.1±1.6 | 62.1±2.5 | 39.4±3.1 | 0.72±1.25 | 0.56±0.98 | 0.47±0.97 | |||||
| Carr R-2 2018 (35) | United States, Puerto Rico, Canada, Chile, United Kingdom | 6 | Elagolix 600 mg/QD | 77 | 42 | 51 | 22 | 4 | 30±5.2 | 208±141 | 10.7±1.9 | 66.3±2.4 | 34.3±2.6 | 0.87±1.18 | 0.4±0.96 | 0.37±0.99 | ||
| Elagolix 600 mg (QD) + E2 1/0.5 mg NETA(QD) | 77 | 43 | 55 | 18 | 3 | 31±5.7 | 247±187 | 11±1.8 | 62.1±2.4 | 37.5±2.6 | 1.16±1.22 | 0.62±0.97 | 0.5±1.04 | |||||
| Placebo | 78 | 43 | 61 | 17 | 0 | 30.7±5.1 | 222±132 | 10.9±1.6 | 65.9±2.4 | 37.2±2.6 | 1.09±1.21 | 0.52±0.95 | 0.44±1.01 | |||||
Data are presented as numbers or mean ± standard deviation. NCT, national clinical trial; UFS-QOL, uterine fibroid symptom quality of life; BID, bis in die; NETA(QD), norethisterone acetate (quaque die); BMI, body mass index.
Risk of bias in the included studies
The risk bias of the individual studies is shown in Figure 2. Selection bias was assessed as low in all of the included trials. Performance bias was low in two studies; one was generated through the technology system (34), whereas the other was generated by the interactive response system (35). In accordance with the ClinicalTrials.gov protocols, and the results in the literature, attrition bias and reporting bias was deemed low in all two studies. As they were supported by the manufacturer, the studies conducted by Schlaff et al. and Carr et al. (34,35) were deemed high in terms of the other bias risk.
Efficacy outcomes
Main endpoint: MBL
This endpoint was measured by the number of women who met the MBL criteria mentioned above. Two studies (34,35), including four independent pilot projects (involving 1,217 participants), reported this outcome. The results are displayed in Table 2 and Figures 3-5. The LFK indices are shown in Table 3.
Table 2
| Outcomes | Comparisons | Participants | Statistical method | RR (95% CI) | Heterogeneity | Test for overall effect |
|---|---|---|---|---|---|---|
| Menstrual blood loss | Elagolix vs. placebo | 680 | RR (M-H, random, 95% CI) | 4.92 (2.68, 9.05) | P=0.0002, I2=85% | P<0.00001 |
| Elagolix add-back vs. placebo | 876 | RR (M-H, random, 95% CI) | 4.43 (2.31, 8.50) | P<0.0001, I2=86% | P<0.00001 | |
| Elagolix vs. elagolix add-back | 878 | RR (M-H, fixed, 95% CI) | 1.12 (1.04, 1.19) | P=0.15, I2=44% | P=0.002 | |
| Amenorrhea | Elagolix vs. placebo | 853 | RR (M-H, fixed, 95% CI) | 11.83 (6.59, 21.23) | P=0.93, I2=0% | P<0.00001 |
| Elagolix add-back vs. placebo | 260 | RR (M-H, fixed, 95% CI) | 36.13 (9.04, 144.35) | P=0.94, I2=0% | P<0.00001 | |
| Hemoglobin level | Elagolix vs. placebo | 393 | RR (M-H, fixed, 95% CI) | 2.50 (1.91, 3.26) | P=0.41, I2=0% | P<0.00001 |
| Elagolix add-back vs. placebo | 430 | RR (M-H, fixed, 95% CI) | 2.27 (1.72, 2.99) | P=0.53, I2=0% | P<0.00001 | |
| Elagolix vs. elagolix add-back | 433 | RR (M-H, fixed, 95% CI) | 1.09 (0.93, 1.28) | P=0.19, I2=37% | P=0.26 | |
| UFS-QoL (symptom severity) | Elagolix add-back vs. placebo | 876 | MD (M-H, random, 95% CI) | −21.64 (−30.96, −12.32) | P<0.00001, I2=89% | P<0.00001 |
| UFS-QoL (health-related) | Elagolix add-back vs. placebo | 876 | MD (M-H, random, 95% CI) | 23.82 (13.86, 33.78) | P<0.00001, I2=89% | P<0.00001 |
| Bone mineral density (Lumbar spine) | Elagolix vs. placebo | 574 | MD (M-H, random, 95% CI) | −3.38 (−3.90, −2.87) | P<0.00001, I2=98% | P<0.00001 |
| Elagolix add-back vs. placebo | 740 | MD (M-H, random, 95% CI) | −0.70 (−0.85, −0.55) | P<0.0001, I2=87% | P<0.00001 | |
| Elagolix vs. elagolix add-back | 729 | MD (M-H, random, 95% CI) | −2.60 (−2.98, −2.23) | P<0.00001, I2=97% | P<0.00001 | |
| Bone mineral density (total hip) | Elagolix vs. placebo | 575 | MD (M-H, random, 95% CI) | −2.11 (−2.43, −1.78) | P<0.00001, I2=97% | P<0.00001 |
| Elagolix add-back vs. placebo | 740 | MD (M-H, random, 95% CI) | −0.37 (−0.73, −0.02) | P<0.00001, I2=98% | P=0.04 | |
| Elagolix vs. elagolix add-back | 729 | MD (M-H, random, 95% CI) | −1.73 (−1.94, −1.52) | P<0.00001, I2=94% | P<0.00001 | |
| Bone mineral density (femoral neck) | Elagolix vs. placebo | 574 | MD (M-H, random, 95% CI) | −2.01 (−2.77, −1.26) | P<0.00001, I2=98% | P<0.00001 |
| Elagolix add-back vs. placebo | 740 | MD (M-H, random, 95% CI) | −0.48 (−0.68, −0.27) | P=0.0003, I2=84% | P<0.00001 | |
| Elagolix vs. elagolix add-back | 729 | MD (M-H, random, 95% CI) | −1.49 (−2.07, −0.92) | P<0.00001, I2=98% | P<0.00001 | |
| Any AEs | Elagolix vs. placebo | 680 | RR (M-H, fixed, 95% CI) | 1.23 (1.13, 1.34) | P=0.59, I2=0% | P<0.00001 |
| Elagolix add-back vs. placebo | 876 | RR (M-H, fixed, 95% CI) | 1.06 (0.97, 1.17) | P=0.36, I2=7% | P=0.18 | |
| Elagolix vs. elagolix add-back | 878 | RR (M-H, random, 95% CI) | 1.16 (1.01, 1.33) | P=0.01, I2=72% | P=0.04 | |
| Any serious AEs | Elagolix vs. placebo | 680 | RR (M-H, fixed, 95% CI) | 1.15 (0.55, 2.38) | P=0.14, I2=45% | P=0.71 |
| Elagolix add-back vs. placebo | 876 | RR (M-H, random, 95% CI) | 0.84 (0.17, 4.02) | P=0.04, I2=63% | P=0.83 | |
| Elagolix vs. elagolix add-back | 878 | RR (M-H, fixed, 95% CI) | 1.48 (0.72, 3.03) | P=0.86, I2=0% | P=0.28 | |
| Hot flush | Elagolix vs. placebo | 680 | RR (M-H, fixed, 95% CI) | 9.14 (5.84, 14.29) | P=0.81, I2=0% | P<0.00001 |
| Elagolix add-back vs. placebo | 876 | RR (M-H, fixed, 95% CI) | 3.02 (1.87, 4.86) | P=0.72, I2=0% | P<0.00001 | |
| Elagolix vs. elagolix add-back | 878 | RR (M-H, fixed, 95% CI) | 2.97 (2.40, 3.67) | P=0.30, I2=18% | P<0.00001 |
RR, risk ratio; MD, mean difference; M-H, Mantel-Haenszel; CI, confidence interval; UFS-QoL, uterine fibroid symptom quality of life; AEs, adverse events


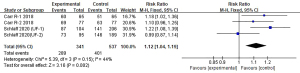
Table 3
| Outcomes | Comparisons | LFK index |
|---|---|---|
| Menstrual blood loss | Elagolix vs. placebo | 1.65 (minor asymmetry) |
| Elagolix add-back vs. placebo | −1.72 (minor asymmetry) | |
| Elagolix vs. elagolix add-back | 0.8 (no asymmetry) | |
| Amenorrhea | Elagolix vs. placebo | −2.26 (major asymmetry) |
| Elagolix add-back vs. placebo | 1.29 (minor asymmetry) | |
| Hemoglobin level | Elagolix vs. placebo | 1.90 (minor asymmetry) |
| Elagolix add-back vs. placebo | 2.26 (major asymmetry) | |
| Elagolix vs. elagolix add-back | −0.08 (no asymmetry) | |
| UFS-QoL (symptom severity) | Elagolix add-back vs. placebo | 0.62 (no asymmetry) |
| UFS-QoL (health-related) | Elagolix add-back vs. placebo | −1.63 (minor asymmetry) |
| Bone mineral density (Lumbar spine) | Elagolix vs. placebo | −3.85 (major asymmetry) |
| Elagolix add-back vs. placebo | −5.89 (major asymmetry) | |
| Elagolix vs. elagolix add-back | −3.13 (major asymmetry) | |
| Bone mineral density (total hip) | Elagolix vs. placebo | −3.59 (major asymmetry) |
| Elagolix add-back vs. placebo | −5.67 (major asymmetry) | |
| Elagolix vs. elagolix add-back | 6.93 (major asymmetry) | |
| Bone mineral density (femoral neck) | Elagolix vs. placebo | −2.10 (major asymmetry) |
| Elagolix add-back vs. placebo | −3.3 (major asymmetry) | |
| Elagolix vs. elagolix add-back | −2.11 (major asymmetry) | |
| Any AEs | Elagolix vs. placebo | 1.07 (minor asymmetry) |
| Elagolix add-back vs. placebo | 0.85 (no asymmetry) | |
| Elagolix vs. elagolix add-back | 1.62 (minor asymmetry) | |
| Any serious AEs | Elagolix vs. placebo | 2.48 (major asymmetry) |
| Elagolix add-back vs. placebo | 2.5 (major asymmetry) | |
| Elagolix vs. elagolix add-back | 2.91 (major asymmetry) | |
| Hot flush | Elagolix vs. placebo | −1.14 (minor asymmetry) |
| Elagolix add-back vs. placebo | 1.74 (minor asymmetry) | |
| Elagolix vs. elagolix add-back | −0.45 (no asymmetry) |
LFK, Luis Furuya-Kanamori; AEs, adverse events; UFS-QoL, uterine fibroid symptom quality of life.
Compared with those in the placebo group, more women in the elagolix group reached the criteria, and the difference was statistically significant [RR 4.92, 95% CI: 2.68, 9.05; P<0.00001]. Additionally, there was high heterogeneity among these studies (I2=85%, P=0.0002). The detailed results are shown in Figure 3.
Moreover, there was a significant difference between the elagolix add-back group (RR 4.43, 95% CI: 2.31, 8.50, P<0.00001) and the placebo groups in terms of this study endpoint. The heterogeneity (I2=86%, P<0.0001) was high (Figure 4).
Marginally, more women in the add-back group met the criteria than the women who used elagolix alone (RR 1.12, 95% CI: 1.04, 1.19, P=0.002). The heterogeneity was moderate (I2=44%, P=0.15). Figure 5 displays the details.
Amenorrhea (no bleeding or spotting)
Two studies (34,35) including a total of 977 participants reported on this endpoint, which was defined as the number of women without bleeding or spotting by observing the hemoglobin data. The detailed information is shown in Table 2 and Figure S1. The LFK indices are shown in Table 3.
A greater number of women had amenorrhea in the elagolix treatment group (RR 11.83, 95% CI: 6.59, 21.23, P<0.00001) than in the placebo group (Figure S1A). We found no heterogeneity among these trials (I2=0%, P=0.93).
Compared with those in the placebo group, more women in the elagolix add-back group experienced amenorrhea (RR 36.13, 95% CI: 9.04, 144.35, P<0.00001) compared to the placebo group (Figure S1B). No obvious heterogeneity was found among these studies (I2=0%, P=0.94).
Hemoglobin level
Information about this outcome was available in two studies (34,35), involving a total of 628 participants. The women whose hemoglobin level increased >2 g/dL satisfied these criteria.
More women met these criteria in the elagolix group (RR 2.50, 95% CI: 1.91, 3.26; P<0.00001) than in the placebo group (Table 2, Figure S2A). No evidence of heterogeneity was found (I2=0%, P=0.41).
Compared with placebo, the number of women who satisfied these criteria using elagolix in combination with add-back therapy, was greater (RR 2.27, 95% CI: 1.72, 2.99, P<0.00001). There was no heterogeneity among the studies (I2=0%, P=0.53) (Table 2, Figure S2B).
There were no differences between the elagolix used alone and elagolix with add-back therapy in terms of this endpoint (RR 1.09, 95% CI: 0.93, 1.28, P=0.26), and the heterogeneity among the studies was mild (I2=37%, P=0.19) (Table 2, Figure S2C).
Uterine fibroid symptom quality of life (UFS-QoL)
The UFS-QoL outcome includes two aspects, symptom severity and health-related quality of life. Evaluation is in the form of a scoring system. Data on the UFS-QoL are available for 876 participants (Table 2).
In terms of symptom severity, the scores of the elagolix with add-back therapy group (MD −21.64, 95% CI: −30.96, −12.32; P<0.00001) were significantly lower than those of the placebo group. High heterogeneity existed among the included studies (I2=89%, P<0.00001) (Table 2, Figure S3A).
With respect to health-related quality of life, women treated with elagolix and add-back therapy (MD 23.82, 95% CI: 13.86, 33.78; P<0.00001) had higher scores than those treated with the placebo. There was high heterogeneity among the included trials (I2=89%, P<0.00001) (Table 2, Figure S3B).
Safety
Bone mineral density
Bone mineral density change was described in three locations, the lumbar spine, the total hip, and the femoral neck. Two articles (34,35) with four trials reported on this endpoint.
For lumbar spine bone density, the percentages of density change in the elagolix (MD −3.38, 95% CI: −3.90, −2.87, P<0.00001) (Figure S4A) and elagolix add-back (MD −0.70, 95% CI: −0.85, −0.55, P<0.00001) groups (Figure S4B) were lower than those in the placebo group. Compared with the elagolix alone group, the elagolix add-back therapy group (MD −2.60, 95% CI: −2.98, −2.23; P<0.00001) (Figure S4C) had greater lumbar spine bone density change. There was high heterogeneity among these three comparisons (Table 2).
In terms of the total hip, the pooled analysis revealed that elagolix reduced bone density more than the placebo (MD −2.11, 95% CI: −2.43, −1.78, P<0.00001) (Figure S5A); however, the elagolix add-back group was similar to the placebo group (MD −0.37, 95% CI: −0.73, −0.02, P=0.04) (Figure S5B). Compared to the elagolix add-back therapy, elagolix alone (MD −1.73, 95% CI: −1.94, −1.52, P<0.00001) (Figure S5C) significantly reduced total hip bone density. The heterogeneity among the trials that reported on this outcome was very high (Table 2).
When evaluating the percent change in femoral neck bone density, the meta-analysis revealed that both the elagolix used alone (MD −2.01, 95% CI: −2.77, −1.26, P<0.00001) and the elagolix add-back therapy (MD −0.48, 95% CI: −0.68, −0.27, P<0.00001) group have greater reduction of bone density than the placebo group, and the review revealed that there was high heterogeneity among the two comparisons (Figures S6A,S6B). Compared with that of the elagolix add-back group, the bone density of the elagolix used alone group (MD −1.49, 95% CI: −2.07, −0.92, P<0.00001) (Figure S6C) was lower. There was evidence of heterogeneity amongst the included trials (Table 2).
AEs
Any AEs or hot flushes were included in our meta-analysis. Compared to the placebo group, the elagolix group was associated with a greater risks of any AEs and hot flushes (RR 1.23, 95% CI: 1.13, 1.34, P<0.00001; RR 9.14, 95% CI: 5.84, 14.29, P<00001). There was no heterogeneity among these comparisons [(I2=0%, P=0.59), (I2 =0%, P=0.81)] (Table 2, Figure S7A,S7B).
Additionally, the risk of any AE associated with elagolix alone was similar to the risk associated with elagolix with add-back therapy (RR 1.16, 95% CI: 1.01, 1.33, P=0.04), but the risk associated with hot flushes was greater when elagolix was used alone (RR 2.97, 95% CI: 2.40, 3.67, P<0.00001) than when elagolix was used with add-back therapy. There was high heterogeneity in terms of any AEs (I2=72%, P=0.01) (Figure S7C) but no heterogeneity was found in terms of hot flush events (I2=18%, P=0.30) (Figure S8A).
There was no difference in any AEs between elagolix add-back therapy and the placebo (RR 1.06, 95% CI: 0.97, 1.17; P=0.18) and the heterogeneity was low (I2=7%, P=0.36) (Figure S8B). In terms of hot flushes, those in the elagolix add-back group experienced more than those in the placebo group (RR 3.02, 95% CI: 1.87, 4.86; P<0.0001), and there was no heterogeneity among the trials (I2=0%, P=0.72) (Figure S8C).
No differences or obvious heterogeneity was found in the comparison between elagolix, elagolix add-back therapy, and placebo in terms of serious AEs (Figure S9A-S9C).
Discussion
This was the first meta-analysis to evaluate the efficacy and safety of elagolix alone or combination with add-back therapy in the treatment of HMB caused by uterine fibroids. This represents the inaugural meta-analysis to assess the effectiveness and safety of elagolix, both as a monotherapy and in combination with add-back therapy, for the treatment of HMB resulting from uterine fibroids. The results indicated that elagolix used alone or with add-back therapy was superior to the placebo in terms of MBL, causing amenorrhea, and increasing hemoglobin levels. There was no difference between the elagolix and elagolix add-back groups in terms of MBL and hemoglobin levels. In terms of the UFS-QoL, elagolix add-back therapy was superior to the placebo. The elagolix alone or with add-back groups was inferior to the placebo in terms of less AEs but the three groups were similar regarding incidents of serious AEs. Compared to patients in the placebo group, patients in the elagolix group had lower level of bone mineral density. Treatment with elagolix add-back group was more effective at preventing the loss of bone mineral density than elagolix therapy alone.
Elagolix is a highly effective, nonpeptide GnRH receptor antagonist (36,37) that can competitively bind to the GnRH receptor in the pituitary, and by blocking endogenous GnRH signaling, it inhibits luteinizing hormone (LH) and follicle-stimulating hormone (FSH), thereby reducing the production of estradiol and progesterone (28,29,38). Its nonpeptide structure means that because it does not undergo gastrointestinal proteolysis, it can be administered orally, and inhibit ovarian function in a dose-dependent manner. However, by reducing the concentration of estradiol, the drug results in several adverse reactions. Elagolix alone or in combination with add-back therapy results in no clinically significant endometrial abnormalities. Additionally, these patients also have low incidences of serious AEs, which are not associated with drug-induced liver injuries. Hot flushes were a common AE reported in the elagolix group, and fewer patients in the elagolix add-back group experienced hot flushes than those in the elagolix alone group. Elagolix add-back therapy was obviously more advantageous in terms of preventing bone mineral density reduction, which is related to an increase in estradiol. The hypoestrogenic effect of elagolix on bone mineral density is reversible (39). To alleviate the reduction of bone mineral density, elagolix should be administered at the lowest effective dose with a limited duration. Even in use with estradiol and norethindrone acetate, it is necessary to pay attention to the dosage and course of treatment (40). Before taking elagolix, hormonal contraceptives should be discontinued for at least 1 week, in order to not influence the treatment effect (36).
The treatment options for uterine fibroids include medications and surgery. Surgical management can include a hysterectomy, myomectomy, endometrial ablation, or uterine artery embolization, procedure price ranges from $6,000 to $15,000 (41). Surgical treatment also involves several complications, including intraoperative bleeding, postoperative fever, surgical site infection, and gastrointestinal injury (37). Compared to surgical options, treatment with pharmaceuticals have several advantages: (I) they cost between $6,000 and $9,000 (41); (II) it is non-invasive or can be less physically traumatic; and (III) most importantly, they encourage faster return to normal life, without hospitalization. According to the literature (42,43), patients who are not considered responders may have a clinically significant response to elagolix with add-back therapy. Additionally, Muneyyirci-Delale et al. (44) reported that elagolix with add-back therapy was effective in reducing HMB in women with uterine fibroids and concurrent adenomyosis. However, the duration of dosing and the low estrogen effects that lead to hot flushes and bone loss are the main disadvantages of pharmaceutical treatments.
The results indicate that elagolix is a comparatively advantageous treatment, and this study provides clinically relevant information to guide treatment decisions. Despite the high quality of the included studies, several limitations in this meta-analysis should be noted and considered. First, the relatively small number of included studies and the total number of patients, coupled with the risk of bias in the included trials presents limited data on some results, such as the volume of fibroids after oral medication and the UFS-QoL of the elagolix alone group. Second, different clinical studies have dissimilar patient populations, so even if statistical adjustments are made in the analysis, clinical heterogeneity may also exist; a correctly defined patient population is one of the key factors related to success. Third, owing to the limited amount of data and the five comparisons consisting of only three studies, subgroup analysis was challenging. Additionally, elagolix is only approved in only the US and Canada in combination with add-back therapy (E2/NETA) and elagolix used alone is not a formally approved treatment. Finally, all included studies were sponsored by pharmaceutical companies. The confounding factors brought about by these limitations may introduce a bias in this meta-analysis, and addressing potential confounding factors or variations in study populations would provide a more balanced interpretation of the results.
Together, the aforementioned aspects reinforce the need for more large, well-designed trials on the safety and efficiency of oral elagolix in patients suffering from HMB with uterine fibroids.
Conclusions
In conclusion, elagolix used alone or in combination with an add-back therapy is effective and safe in patients with HMB caused by uterine fibroids. It could be considered as a reliable treatment option for uterine fibroids patients with HMB Additionally, more high-quality randomized controlled studies are needed to verify or modify the results of this work.
Acknowledgments
None.
Footnote
Reporting Checklist: The authors have completed the PRISMA reporting checklist. Available at https://gs.amegroups.com/article/view/10.21037/gs-24-386/rc
Peer Review File: Available at https://gs.amegroups.com/article/view/10.21037/gs-24-386/prf
Funding: This research was supported by
Conflicts of Interest: All authors have completed the ICMJE uniform disclosure form (available at https://gs.amegroups.com/article/view/10.21037/gs-24-386/coif). The authors have no conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Holdsworth-Carson SJ, Zhao D, Cann L, et al. Differences in the cellular composition of small versus large uterine fibroids. Reproduction 2016;152:467-80. [Crossref] [PubMed]
- Al-Hendy A, Bradley L, Owens CD, et al. Predictors of response for elagolix with add-back therapy in women with heavy menstrual bleeding associated with uterine fibroids. Am J Obstet Gynecol 2021;224:72.e1-72.e50. [Crossref] [PubMed]
- Stewart EA, Cookson CL, Gandolfo RA, et al. Epidemiology of uterine fibroids: a systematic review. BJOG 2017;124:1501-12. [Crossref] [PubMed]
- Stewart EA, Nicholson WK, Bradley L, et al. The burden of uterine fibroids for African-American women: results of a national survey. J Womens Health (Larchmt) 2013;22:807-16. [Crossref] [PubMed]
- Pavone D, Clemenza S, Sorbi F, et al. Epidemiology and Risk Factors of Uterine Fibroids. Best Pract Res Clin Obstet Gynaecol 2018;46:3-11. [Crossref] [PubMed]
- Stewart EA, Laughlin-Tommaso SK, Catherino WH, et al. Uterine fibroids. Nat Rev Dis Primers 2016;2:16043. [Crossref] [PubMed]
- Moravek MB, Bulun SE. Endocrinology of uterine fibroids: steroid hormones, stem cells, and genetic contribution. Curr Opin Obstet Gynecol 2015;27:276-83. [Crossref] [PubMed]
- Baird DD, Dunson DB, Hill MC, et al. High cumulative incidence of uterine leiomyoma in black and white women: ultrasound evidence. Am J Obstet Gynecol 2003;188:100-7. [Crossref] [PubMed]
- Zimmermann A, Bernuit D, Gerlinger C, et al. Prevalence, symptoms and management of uterine fifibroids: An international internet-based survey of 21,746 women. BMC Womens Health 2012;12:6. [Crossref] [PubMed]
- Napolitano M, Dolce A, Celenza G, et al. Iron-dependent erythropoiesis in women with excessive menstrual blood losses and women with normal menses. Ann Hematol 2014;93:557-63. [Crossref] [PubMed]
- Borah BJ, Nicholson WK, Bradley L, et al. The impact of uterine leiomyomas: a national survey of affected women. Am J Obstet Gynecol 2013;209:319.e1-319.e20. [Crossref] [PubMed]
- Al-Hendy A, Myers ER, Stewart E. Uterine Fibroids: Burden and Unmet Medical Need. Semin Reprod Med 2017;35:473-80. [Crossref] [PubMed]
- Fuldeore MJ, Soliman AM. Patient-reported prevalence and symptomatic burden of uterine fibroids among women in the United States: findings from a cross-sectional survey analysis. Int J Womens Health 2017;9:403-11. [Crossref] [PubMed]
- Soliman AM, Margolis MK, Castelli-Haley J, et al. Impact of uterine fibroid symptoms on health-related quality of life of US women: evidence from a cross-sectional survey. Curr Med Res Opin 2017;33:1971-8. [Crossref] [PubMed]
- Ghant MS, Sengoba KS, Recht H, et al. Beyond the physical: a qualitative assessment of the burden of symptomatic uterine fibroids on women's emotional and psychosocial health. J Psychosom Res 2015;78:499-503. [Crossref] [PubMed]
- Cardozo ER, Clark AD, Banks NK, et al. The estimated annual cost of uterine leiomyomata in the United States. Am J Obstet Gynecol 2012;206:211.e1-9. [Crossref] [PubMed]
- Fuldeore M, Yang H, Soliman AM, et al. Healthcare utilization and costs among women diagnosed with uterine fibroids: a longitudinal evaluation for 5 years pre- and post-diagnosis. Curr Med Res Opin 2015;31:1719-31. [Crossref] [PubMed]
- Soliman AM, Anand SB, Coyne KS, et al. Examining the Relationship Between Symptomatic Burden and Self-reported Productivity Losses Among Patients With Uterine Fibroids in the United States. J Occup Environ Med 2017;59:974-81. [Crossref] [PubMed]
- Ali M. A R S, Al Hendy A. Elagolix in the treatment of heavy menstrual bleeding associated with uterine fibroids in premenopausal women. Expert Rev Clin Pharmacol 2021;14:427-37. [Crossref] [PubMed]
- Vitale SG, Saponara S, Sicilia G, et al. Hysteroscopic diode laser myolysis: from a case series to literature review of incisionless myolysis techniques for managing heavy menstrual bleeding in premenopausal women. Arch Gynecol Obstet 2024;309:949-59. [Crossref] [PubMed]
- Vitale SG, Moore O, Riemma G, et al. Hysteroscopic laser ablation of symptomatic uterine fibroids: insights from a prospective study. Climacteric 2023;26:497-502. [Crossref] [PubMed]
- Lee BB, Yu SP. Radiofrequency Ablation of Uterine Fibroids: a Review. Curr Obstet Gynecol Rep 2016;5:318-24. [Crossref] [PubMed]
- Peregrino PFM, de Lorenzo Messina M, Dos Santos Simões R, et al. Review of magnetic resonance-guided focused ultrasound in the treatment of uterine fibroids. Clinics (Sao Paulo) 2017;72:637-41. [Crossref] [PubMed]
- Donnez J, Dolmans MM. Uterine fibroid management: from the present to the future. Hum Reprod Update 2016;22:665-86. [Crossref] [PubMed]
- Engel JB, Schally AV. Drug Insight: clinical use of agonists and antagonists of luteinizing-hormone-releasing hormone. Nat Clin Pract Endocrinol Metab 2007;3:157-67. [Crossref] [PubMed]
- Zhang Y, Wei W, Chang E, et al. The short- and mid-term efficacy and safety of elagolix in the management of pain associated with endometriosis: A systematic review and meta-analysis. J Gynecol Obstet Hum Reprod 2024;53:102829. [Crossref] [PubMed]
- Brown E, Kroll R, Li H, et al. Low-Dose Elagolix for the Treatment of Heavy Menstrual Bleeding in Patients With Uterine Leiomyomas: A Randomized Controlled Trial. Obstet Gynecol 2023;142:1068-76. [Crossref] [PubMed]
- Struthers RS, Nicholls AJ, Grundy J, et al. Suppression of gonadotropins and estradiol in premenopausal women by oral administration of the nonpeptide gonadotropin-releasing hormone antagonist elagolix. J Clin Endocrinol Metab 2009;94:545-51. [Crossref] [PubMed]
- Ng J, Chwalisz K, Carter DC, et al. Dose-Dependent Suppression of Gonadotropins and Ovarian Hormones by Elagolix in Healthy Premenopausal Women. J Clin Endocrinol Metab 2017;102:1683-91. [Crossref] [PubMed]
- Taylor HS, Giudice LC, Lessey BA, et al. Treatment of Endometriosis-Associated Pain with Elagolix, an Oral GnRH Antagonist. N Engl J Med 2017;377:28-40. [Crossref] [PubMed]
- Moher D, Liberati A, Tetzlaff J, et al. Preferred reporting items for systematic reviews and meta-analyses: the PRISMA statement. Int J Surg 2010;8:336-41. [Crossref] [PubMed]
- Hallberg L, Nilsson L. Determination of menstrual blood loss. Scand J Clin Lab Invest 1964;16:244-8.
- Higgins JPT, Thomas J, Chandler J, et al. Cochrane Handbook for Systematic Reviews of Interventions version 6.0 (updated July 2019). Cochrane; 2019. Available online: www.training.cochrane.org/handbook
- Schlaff WD, Ackerman RT, Al-Hendy A, et al. Elagolix for Heavy Menstrual Bleeding in Women with Uterine Fibroids. N Engl J Med 2020;382:328-40. [Crossref] [PubMed]
- Carr BR, Stewart EA, Archer DF, et al. Elagolix Alone or With Add-Back Therapy in Women With Heavy Menstrual Bleeding and Uterine Leiomyomas: A Randomized Controlled Trial. Obstet Gynecol 2018;132:1252-64. [Crossref] [PubMed]
- AbbVie Inc. Prescribing information for Orilissa TM (elagolix) tablets, for oral use. 2018. Available online: http://www.accessdata.fda.gov/. Accessed 15 Aug 2018.
- Miller CE, Kim JH, Kroll R, et al. Efficacy, tolerability, and bone density outcomes of elagolix with add-back therapy for endometriosis-associated pain: twelve months of an ongoing randomized phase 3 trial. Am J Obstet Gynecol 2024;231:630.e1-630.e13. [Crossref] [PubMed]
- Snabes MC, Ng J, Li H, et al. Phase 2, double-blind, randomized, placebo-controlled study of the safety and efficacy of elagolix in women with polycystic ovary syndrome. F S Rep 2023;4:206-12. [Crossref] [PubMed]
- Surrey E, Taylor HS, Giudice L, et al. Long-Term Outcomes of Elagolix in Women With Endometriosis: Results From Two Extension Studies. Obstet Gynecol 2018;132:147-60. [Crossref] [PubMed]
- Beck D, Winzenborg I, Liu M, et al. Population Pharmacokinetics of Elagolix in Combination with Low-Dose Estradiol/Norethindrone Acetate in Women with Uterine Fibroids. Clin Pharmacokinet 2022;61:577-87. [Crossref] [PubMed]
- Carls GS, Lee DW, Ozminkowski RJ, et al. What are the total costs of surgical treatment for uterine fibroids? J Womens Health (Larchmt) 2008;17:1119-32. [Crossref] [PubMed]
- Hodges KR, Davis BR, Swaim LS. Prevention and management of hysterectomy complications. Clin Obstet Gynecol 2014;57:43-57. [Crossref] [PubMed]
- Stewart EA, Archer DF, Owens CD, et al. Reduction of Heavy Menstrual Bleeding in Women Not Designated as Responders to Elagolix Plus Add Back Therapy for Uterine Fibroids. J Womens Health (Larchmt) 2022;31:698-705. [Crossref] [PubMed]
- Muneyyirci-Delale O, Archer DF, Owens CD, et al. Efficacy and safety of elagolix with add-back therapy in women with uterine fibroids and coexisting adenomyosis. F S Rep 2021;2:338-46. [Crossref] [PubMed]
(English Language Editor: A. Kassem)



