
Predictive modeling of breast cancer-related lymphedema using machine learning algorithms
Highlight box
Key findings
• The machine learning (ML)-based eXtreme Gradient Boosting (XGBoost) model demonstrates good performance in predicting breast cancer-related lymphedema (BCRL), with an area under curve of 0.99 in the training set and 0.89 in the validation set.
What is known and what is new?
• Despite decreased incidence, effective treatment options for BCRL are still lacking, emphasizing the need for prevention. Traditional statistical methods (e.g., linear regression, logistic regression) have been used to analyze influencing factors, but they exhibit limitations such as poor fit for multiple variables, leading to lower accuracy.
• Among the ML-based models, including random forest, support vector machine, and XGBoost algorithms, the ML-based XGBoost model demonstrates the best performance in assisting healthcare professionals with rapidly and accurately assessing the risk of BCRL occurrence.
What is the implication, and what should change now?
• ML-based models, such as the XGBoost algorithm, can significantly improve the ability of healthcare professionals to rapidly and accurately assess the risk of BCRL, thereby facilitating early intervention and targeted preventive measures.
• Encourage ongoing research to refine ML models and explore new algorithms may offer even greater predictive accuracy.
Introduction
Background
Breast cancer has the highest incidence among female malignant tumors (1). According to the latest data from GLOBOCAN 2020 (2), breast cancer has surpassed lung cancer for the first time, becoming the most common cancer in women globally, with an estimated 2.3 million new cases, accounting for 11.7% of all cancer cases. Breast cancer-related lymphedema (BCRL) refers to the phenomenon of limb lymphedema caused by damage or blockage of the lymphatic vessels during the treatment of breast cancer, resulting in obstruction of lymphatic fluid drainage. It is one of the common complications after breast cancer surgery (3). According to incomplete statistics (4), approximately 20–30% of patients who undergo radical surgery for breast cancer develop upper limb lymphedema. Lymphedema can cause limb swelling, deformity, and upper limb functional impairment, significantly impacting the physical and mental health and quality of life of patients.
Rationale and knowledge gap
Although the current treatment for lymphedema is limited and cannot completely reverse the progression of lymphedema, Kuruvilla et al. (5) showed that early identification and intervention can significantly improve the prognosis and quality of life of patients. Early measures such as physical therapy, lifestyle adjustments (such as avoiding overwork and infection), and wearing pressure clothing can effectively slow the progression of lymphedema, relieve symptoms and reduce complications. In this context, machine learning (ML) algorithms can help identify high-risk individuals through risk stratification, enabling clinicians to take preventive measures early in the disease or even before symptoms appear. Early identification of the early symptoms of lymphedema is helpful for timely intervention, thereby slowing or preventing its progression. Disease risk prediction models are statistical assessment methods based on multiple risk factors of a disease. They assign scores based on the magnitude of influence and calculate the probability of future events with mathematical formulas. Such models can effectively estimate the risk of outcome occurrence, aid in targeted interventions for different risk populations, and play a crucial role in improving patient prognosis.
Objective
Wu et al. (6) mainly used statistical methods such as linear regression and logistic regression to analyze influencing factors, but they have limitations, like poor fit for multiple variables or features, leading to lower model accuracy. Traditional methods have limitations in dealing with large and high-dimensional data sets, while ML can extract useful information from complex data more effectively. ML extracts features and patterns from large amounts of data through trained algorithms, without relying on manually defined rules, enabling more accurate prediction and classification as an important branch of artificial intelligence. Compared to traditional statistical methods, ML emphasizes automation and data-driven approaches, effectively addressing issues such as multivariable interactions and collinearity. ML has been widely applied in the medical field in recent years (7-9). Currently, there are limited literature reports on using ML to construct BCRL prediction models in breast cancer patients. Breast cancer is one of the most common cancers in women worldwide, and the incidence of BCRL is generally high among breast cancer survivors, which seriously affects the quality of life of patients. Therefore, early identification and prevention of lymphedema is essential to improve the overall treatment effect and quality of life of patients. We present this article in accordance with the TRIPOD reporting checklist (available at https://gs.amegroups.com/article/view/10.21037/gs-24-252/rc).
Methods
Research objects
This study was a retrospective study that selected clinical data of breast cancer patients admitted to Hainan Cancer Hospital from September 2018 to May 2024. (I) The inclusion criteria were as follows: patients diagnosed with unilateral breast cancer confirmed by magnetic resonance imaging (MRI) or ultrasound imaging, female, underwent surgical treatment, age >18 years, expected postoperative survival time of more than 1 year, and availability of complete case data. (II) The exclusion criteria were as follows: presence of distant metastasis at the time of diagnosis or tumor recurrence or metastasis within 1 year of follow-up, edema caused by other reasons (such as cardiac, renal, or hepatic causes), history of severe head, neck, or upper limb trauma or surgery, loss to follow-up during the follow-up process.
The study was conducted in accordance with the Declaration of Helsinki (as revised in 2013). The study was approved by ethics committee of Hainan Cancer Hospital [2024 (Scientific Research) No. 028] on 5th June, 2024. Informed consent was waived for this study due to the exclusive use of de-identified patient data, which posed no potential harm or impact on patient care.
Variables and data collection methods
The study variables were mainly divided into predictor variables and outcome variables. (I) Predictor variables include demographic and clinical data of patients, collected through electronic medical records and follow-up. These variables include age, body mass index (BMI), history of hypertension, history of diabetes, type of breast resection, axillary lymph node dissection, number of dissected lymph nodes, postoperative complications (infection, seroma, etc.), dominant hand for surgery, postoperative functional exercise (weekly exercise time >2 hours), postoperative endocrine therapy, chemotherapy, radiotherapy, tumor pathology type, tumor node metastasis (TNM) staging, axillary lymph node status, tumor location, and others. (II) Outcome variable: the result variable is BCRL. The medical staff informs the patient and his family in detail the method of measuring the arm circumference. The patient starts to measure the arm circumference by himself 1 month after the operation. The data of the arm circumference of the upper limbs are collected once a month through WeChat, telephone or quarterly or semi-annual specialist outpatient follow-up. BCRL diagnostic criteria: the circumference of the affected limb increased after breast cancer surgery, exceeding the corresponding healthy side by 2 cm (10). The occurrence of BCRL was taken as the follow-up outcome event, and the follow-up deadline was March 2024.
Building ML models
The included data were randomly sampled in a 7:3 ratio, with 70% allocated to the training set and 30% to the validation set. Based on whether patients developed BCRL, the training set was further divided into the BCRL group and the non-BCRL group. The training set underwent 5-fold cross-validation for 100 iterations to obtain optimal hyperparameters. The optimal training model was rained on the entire training set and then applied to the corresponding validation group to assess the model’s fitting and generalization ability. For the validation set, logistic regression, random forest (RF), support vector machine (SVM), and eXtreme Gradient Boosting (XGBoost) algorithm classifiers were utilized to construct predictive models. The area under the curve (AUC) was used to evaluate the discriminative ability of the predictive models, where a higher AUC indicates better discriminative performance. Model performance was evaluated using AUC, sensitivity, specificity, and F1 score. The calibration of the models was assessed with calibration curves and the Hosmer-Lemeshow (H-L) Chi-squared test, which reflected the consistency between predicted BCRL risk and actual risk across different risk strata.
Statistical analysis
The collected data were statistically analyzed using R software (version 4.3.1). All data were tested for normal distribution by Shapiro-Wilk test. The measurement data that met the normal distribution were expressed as (mean ± standard deviation) and the t-test was used for comparison while non-normally distributed variables are presented as median (inter-quartile range, IQR) and compared using Mann-Whitney U test. Categorical variables were presented as frequencies or rates, and comparisons were conducted using Chi-squared tests. The performance of different ML models was evaluated by AUC, sensitivity, specificity and F1 score through receiver operating characteristic (ROC) curve analysis. A significance level of P<0.05 was deemed statistically significant for identifying two-sided differences.
Results
Comparison of general data between training set and validation set
Two hundreds and forty patients who met the inclusion criteria were selected. According to a 7:3 ratio, 168 patients were assigned to the training set, and 72 patients were assigned to the validation set, as shown in Figure 1. The comparison of general data between the training set and validation set showed no statistically significant differences (P>0.05), as shown in Table 1.
Table 1
| Factor | Training set (n=168) | Validation set (n=72) | χ2/t | P value |
|---|---|---|---|---|
| Age (years, ) | 53.03±8.59 | 52.73±8.14 | 0.252 | 0.80 |
| BMI (kg/m2, ) | 25.57±2.23 | 25.12±1.74 | 1.524 | 0.13 |
| History of hypertension (yes/no) | 47/121 | 19/53 | 0.064 | 0.80 |
| History of diabetes (yes/no) | 31/137 | 12/60 | 0.109 | 0.74 |
| Use of calcium channel blockers (yes/no) | 35/133 | 20/52 | 1.376 | 0.24 |
| Breast-conserving surgery (yes/no) | 34/134 | 16/56 | 0.120 | 0.73 |
| Axillary lymph node dissection (yes/no) | 107/61 | 43/29 | 0.339 | 0.56 |
| Dissected lymph nodes (number, ) | 13.38±1.75 | 12.92±1.92 | 1.812 | 0.07 |
| Postoperative complications (yes/no) | 10/158 | 5/67 | 0.085 | 0.77 |
| Surgical hand is dominant (yes/no) | 27/141 | 11/61 | 0.024 | 0.88 |
| Postoperative functional exercise (yes/no) | 71/97 | 28/44 | 0.237 | 0.63 |
| Postoperative endocrine therapy(yes/no) | 101/67 | 45/27 | 0.120 | 0.73 |
| Postoperative chemotherapy (yes/no) | 127/41 | 57/15 | 0.359 | 0.55 |
| Postoperative radiotherapy (yes/no) | 97/71 | 43/29 | 0.082 | 0.78 |
| Pathological type (infiltrating ductal carcinoma/infiltrating lobular carcinoma/others) | 126/32/10 | 56/11/5 | 0.530 | 0.77 |
| TNM stage (stage I–II/stage IIIa) | 115/53 | 52/20 | 0.338 | 0.56 |
| Level of axillary lymph node dissection (level I–II/level III) | 131/37 | 59/13 | 0.481 | 0.49 |
| Tumor location (left/right) | 81/87 | 39/33 | 0.714 | 0.40 |
BMI, body mass index; TNM, tumor node metastasis.
Comparison of general data between BCRL group and non-BCRL group in training set
In the training set, there were 44 cases of BCRL and 124 cases without BCRL. A comparison of hypertension history, number of dissected lymph nodes, postoperative complications, postoperative functional exercise, chemotherapy, radiotherapy, TNM stage, and level of axillary lymph node dissection between the BCRL and non-BCRL groups showed statistically significant differences (P<0.05). There were no statistically significant differences (P>0.05) in other variables between the two groups, as shown in Table 2.
Table 2
| Factor | BCRL group (n=44) | Non-BCRL group (n=124) | χ2/t | P value |
|---|---|---|---|---|
| Age (years, ) | 56.57±9.50 | 52.77±12.28 | 1.863 | 0.06 |
| BMI (kg/m2, ) | 25.51±2.27 | 25.16±1.72 | 1.288 | 0.20 |
| History of hypertension (yes/no) | 24/20 | 23/101 | 20.891 | <0.001 |
| History of diabetes (yes/no) | 9/35 | 22/102 | 0.159 | 0.69 |
| Use of calcium channel blockers (yes/no) | 12/32 | 23/121 | 2.841 | 0.09 |
| Breast-conserving surgery (yes/no) | 9/35 | 25/99 | 0.002 | 0.97 |
| Axillary lymph node dissection (yes/no) | 34/10 | 73/51 | 4.755 | 0.03 |
| Dissected lymph nodes (number, ) | 18.20±1.85 | 13.02±1.85 | 15.957 | <0.001 |
| Postoperative complications (yes/no) | 8/36 | 2/122 | 15.931 | <0.001 |
| Surgical hand is dominant (yes/no) | 7/37 | 20/104 | 0.001 | 0.97 |
| Postoperative functional exercise (yes/no) | 11/33 | 60/64 | 7.280 | 0.007 |
| Postoperative endocrine therapy(yes/no) | 21/23 | 80/44 | 3.818 | 0.051 |
| Postoperative chemotherapy (yes/no) | 44/0 | 83/41 | 19.251 | <0.001 |
| Postoperative radiotherapy (yes/no) | 36/8 | 61/63 | 0.082 | <0.001 |
| Pathological type (infiltrating ductal carcinoma/infiltrating lobular carcinoma/others) | 33/7/4 | 93/25/6 | 1.295 | 0.52 |
| TNM stage (stage I–II/stage IIIa) | 22/22 | 93/31 | 9.399 | 0.002 |
| Level of axillary lymph node dissection (level I–II/level III) | 18/26 | 113/11 | 47.691 | <0.001 |
| Tumor location (left/right) | 21/23 | 60/64 | 0.006 | 0.94 |
BCRL, breast cancer-related lymphedema; BMI, body mass index; TNM, tumor node metastasis.
Comparison of prediction performance among different models
The AUC of logistic regression, RF, SVM and XGBoost in the training set were 0.55, 0.93, 0.93 and 0.99, respectively, and the AUC in the validation set were 0.78, 0.80, 0.79 and 0.89, respectively. The prediction and evaluation performance results of the four models are shown in Table 3. Among them, the AUC, sensitivity, specificity and recall rate of XGBoost are the best, and the performance of the model is the best. The ROC curves of the model in the training set and the verification set are shown in Figures 2,3.
Table 3
| Model | AUC | Sensitivity | Specificity | Recall | F1 score |
|---|---|---|---|---|---|
| Logistic regression | |||||
| Training set | 0.55 | 0.59 | 0.92 | 0.59 | 0.55 |
| Validation set | 0.78 | 0.47 | 0.88 | 0.47 | 0.78 |
| Random decision forests | |||||
| Training set | 0.93 | 0.94 | 0.96 | 0.95 | 0.96 |
| Validation set | 0.80 | 0.52 | 0.87 | 0.52 | 0.49 |
| Support vector machine | |||||
| Training set | 0.93 | 0.80 | 0.91 | 0.80 | 0.77 |
| Validation set | 0.79 | 0.49 | 0.85 | 0.51 | 0.47 |
| eXtreme gradient boosting | |||||
| training set | 0.99 | 0.90 | 0.92 | 0.91 | 0.89 |
| validation set | 0.89 | 0.88 | 0.94 | 0.92 | 0.91 |
AUC, area under the curve.


Calibration curves of different models on training set and validation set
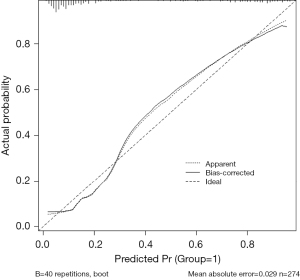
Among the four models, the XGBoost model is the best performing one. The calibration curves of the XGBoost model on the training set and validation set can be found in Figures 4,5, respectively. Calibration reflects the consistency between predicted probabilities and actual observed probabilities. The X-axis represents the predicted probabilities of BCRL, while the Y-axis represents the actual probabilities of BCRL. The solid line represents the actual predictive ability, and the diagonal line represents the ideal predictive ability. The closer the two lines are, the better. After conducting the H-L test on the training set and validation set, the chi-square statistics are 4.55 and 1.97, respectively, with corresponding P values of 0.829 and 0.165. These results suggest that the XGBoost model exhibits good calibration on both the training set and validation set. Therefore, the XGBoost model demonstrates good consistency with the ideal model.

Discussion
BCRL is a common complication in postoperative breast cancer patients. In this study, there were 44 cases of BCRL in the training set, with an incidence rate of 26.19% (44/168), which was significantly higher than the reported incidence rate of 26% by Erickson et al. (11). The results of this study showed significant differences in the history of hypertension, number of lymph nodes dissected, postoperative complications, postoperative functional exercises, chemotherapy, radiotherapy, TNM stage, and level of axillary lymph node dissection between the BCRL group and the non-BCRL group. This suggests that a history of hypertension, number of lymph nodes dissected, postoperative complications, postoperative functional exercises, chemotherapy, radiotherapy, TNM stage, and level of axillary lymph node dissection are influential factors for BCRL. Hypertension can lead to fluid and sodium retention in the body, resulting in subcutaneous edema. Additionally, the use of calcium channel blockers during medication can induce edema (12,13). Lymph node dissection is one of the major risk factors for BCRL and is estimated to carry a four-fold higher risk in comparison to sentinel lymph node biopsy. Axillary lymph node dissection is usually indicated in the presence of positive lymph nodes and leads to an increased number of excised lymph nodes (14). Axillary lymph node dissection is a surgical procedure that disrupts the lymphatic network and is still associated with lymphedema even after adjusting for the number of excised lymph nodes (15). Hua-Ping et al. stated (16) that the status of axillary lymph nodes has a significant impact on the treatment and prognosis of breast cancer patients, which may be related to a larger number of positive lymph nodes, a wider range of axillary lymph node dissection, and longer duration of radiotherapy, leading to damage to the axillary lymph nodes, upper limbs, and chest tissues. Numerous studies have found (17,18) that TNM stage IIIa indicates the progression of the disease to the middle and late stages, with lymph node metastasis and extensive surgical resection, which can exacerbate lymphatic tissue damage and increase the risk of upper limb lymphedema (19). Postoperative chemotherapy and radiotherapy can cause damage to normal tissue cells in addition to killing tumor cells, leading to venous occlusion, lymphangitis, and local muscle fibrosis, which impairs lymphatic reflux in the upper limbs and promotes the occurrence of upper limb lymphedema (20). Postoperative functional exercises can promote blood and lymphatic circulation in the body, helping to reduce the accumulation of lymphatic fluid in the interstitial spaces and thus reducing the occurrence and severity of lymphedema (21). Chen et al. (22) pointed out that BMI is an important factor leading to BCRL, but in the results of this study, there was no statistical difference in BMI between the two groups, suggesting that the reason may be that the evaluation time point of lymphedema may affect the results. If our study is evaluated in a short period of time after surgery, it may not fully reflect the results of long-term follow-up, while Chen et al. may include longer follow-up.
In recent years, with the continuous development of artificial intelligence, an increasing number of studies have suggested (23,24) that predictive models can be constructed to improve the clinical identification of high-risk BCRL. ML-based predictive models can learn from a large number of data samples, capture more complex patterns and correlations, and improve the accuracy of predictions. They can automatically adjust model parameters and weights to adapt to different data distributions and feature relationships, handle multiple features simultaneously, and identify correlations between different features, which helps to comprehensively assess the risk of BCRL occurrence in patients (24). In this study, four BCRL prediction models were constructed and validated based on ML methods, and the XGBoost model demonstrated the best predictive performance. This may be attributed to the strict inclusion criteria used in this study, reducing the burden on the model. Additionally, XGBoost utilizes an efficient parallel processing algorithm that can leverage multi-core processors for parallel computing during the training process, thereby accelerating model training and improving efficiency. This conclusion is consistent with the results reported by Xiu et al. (25). However, Vrdoljak et al. (26) pointed out that the RF model has the best performance in predicting BCRL. In this study, the prediction efficiency of the RF model is general. The reason may be that there may be category imbalance in the BCRL data set (that is, the proportion of patients with lymphedema and non-lymphedema is not balanced). The performance of the RF model on imbalanced datasets may be affected because it tends to favor most classes. In the future, this study will choose more detailed feature selection and feature engineering to improve data quality, and use grid search or random search to optimize model hyperparameters. Moreover, XGBoost introduces regularization terms to control model complexity and prevent overfitting. By penalizing the complexity of the trees, XGBoost can effectively improve the generalization ability of the model and prevent overfitting to the training data. It can automatically calculate feature importance, aiding clinical practitioners in understanding the prediction process and identifying features that significantly impact the results. This helps in feature selection and optimizing model performance. Therefore, the XGBoost model exhibits good performance in predicting BCRL by enhancing model generalization and interpretability.
There are certain limitations in this study. It was conducted in a single hospital with a small sample size, which may restrict the generalizability of the results due to the single-center setting. Additionally, some data were retrospectively extracted from medical records, which may not encompass all potential risk factors for BCRL. Therefore, further validation of the predictive models is necessary through multicenter, large-scale prospective studies.
Conclusions
In conclusion, the ML-based XGBoost model demonstrates good performance in predicting BCRL. The identified predictive variables include a history of hypertension, number of lymph nodes dissected, postoperative complications, postoperative functional exercises, chemotherapy, radiotherapy, TNM stage, and level of axillary lymph node dissection. These variables can assist healthcare professionals in rapidly and accurately assessing the risk of BCRL occurrence.
Acknowledgments
The authors express their appreciation to staff in Hainan Cancer Hospital, for their technical assistance.
Funding: None.
Footnote
Reporting Checklist: The authors have completed the TRIPOD reporting checklist. Available at https://gs.amegroups.com/article/view/10.21037/gs-24-252/rc
Data Sharing Statement: Available at https://gs.amegroups.com/article/view/10.21037/gs-24-252/dss
Peer Review File: Available at https://gs.amegroups.com/article/view/10.21037/gs-24-252/prf
Conflicts of Interest: All authors have completed the ICMJE uniform disclosure form (available at https://gs.amegroups.com/article/view/10.21037/gs-24-252/coif). The authors have no conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved. The study was conducted in accordance with the Declaration of Helsinki (as revised in 2013). The study was approved by ethics committee of Hainan Cancer Hospital [2024 (Scientific Research) No. 028] on 5th June, 2024. Informed consent was waived for this study due to the exclusive use of de-identified patient data, which posed no potential harm or impact on patient care.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Katsura C, Ogunmwonyi I, Kankam HK, et al. Breast cancer: presentation, investigation and management. Br J Hosp Med (Lond) 2022;83:1-7. [Crossref] [PubMed]
- Sung H, Ferlay J, Siegel RL, et al. Global Cancer Statistics 2020: GLOBOCAN Estimates of Incidence and Mortality Worldwide for 36 Cancers in 185 Countries. CA Cancer J Clin 2021;71:209-49. [Crossref] [PubMed]
- Donahue PMC, MacKenzie A, Filipovic A, et al. Advances in the prevention and treatment of breast cancer-related lymphedema. Breast Cancer Res Treat 2023;200:1-14. [Crossref] [PubMed]
- McLaughlin SA, Brunelle CL, Taghian A. Breast Cancer-Related Lymphedema: Risk Factors, Screening, Management, and the Impact of Locoregional Treatment. J Clin Oncol 2020;38:2341-50. [Crossref] [PubMed]
- Kuruvilla AS, Krajewski A, Li X, et al. Risk Factors Associated With Postmastectomy Breast Cancer Lymphedema: A Multicenter Retrospective Analysis. Ann Plast Surg 2022;88:S239-45. [Crossref] [PubMed]
- Wu X, Guan Q, Cheng ASK, et al. Comparison of machine learning models for predicting the risk of breast cancer-related lymphedema in Chinese women. Asia Pac J Oncol Nurs 2022;9:100101. [Crossref] [PubMed]
- Du J, Yang J, Yang Q, et al. Comparison of machine learning models to predict the risk of breast cancer-related lymphedema among breast cancer survivors: a cross-sectional study in China. Front Oncol 2024;14:1334082. [Crossref] [PubMed]
- Heo J, Yoon JG, Park H, et al. Machine Learning-Based Model for Prediction of Outcomes in Acute Stroke. Stroke 2019;50:1263-5. [Crossref] [PubMed]
- Lei T, Hu J, Riaz S. An innovative approach based on meta-learning for real-time modal fault diagnosis with small sample learning. Front Phys 2023;11:1207381.
- Sayko O, Pezzin LE, Yen TW, et al. Diagnosis and treatment of lymphedema after breast cancer: a population-based study. PM R 2013;5:915-23. [Crossref] [PubMed]
- Erickson VS, Pearson ML, Ganz PA, et al. Arm edema in breast cancer patients. J Natl Cancer Inst 2001;93:96-111. [Crossref] [PubMed]
- Drobot D, Zeltzer AA. Surgical treatment of breast cancer related lymphedema-the combined approach: a literature review. Gland Surg 2023;12:1746-59. [Crossref] [PubMed]
- Liang M, Chen Q, Peng K, et al. Manual lymphatic drainage for lymphedema in patients after breast cancer surgery: A systematic review and meta-analysis of randomized controlled trials. Medicine (Baltimore) 2020;99:e23192. [Crossref] [PubMed]
- Pappalardo M, Starnoni M, Franceschini G, et al. Breast Cancer-Related Lymphedema: Recent Updates on Diagnosis, Severity and Available Treatments. J Pers Med 2021;11:402. [Crossref] [PubMed]
- Shah C, Asha W, Vicini F. Current Diagnostic Tools for Breast Cancer-Related Lymphedema. Curr Oncol Rep 2023;25:151-4. [Crossref] [PubMed]
- Hua-Ping H, Jian-Rong Z, Zeng Q. Risk Factors Associated with Lymphedema among Postmenopausal Breast Cancer Survivors after Radical Mastectomy and Axillary Dissection in China. Breast Care (Basel) 2012;7:461-4. [Crossref] [PubMed]
- McEvoy MP, Gomberawalla A, Smith M, et al. The prevention and treatment of breast cancer- related lymphedema: A review. Front Oncol 2022;12:1062472. [Crossref] [PubMed]
- Paramanandam VS, Dylke E, Clark GM, et al. Prophylactic Use of Compression Sleeves Reduces the Incidence of Arm Swelling in Women at High Risk of Breast Cancer-Related Lymphedema: A Randomized Controlled Trial. J Clin Oncol 2022;40:2004-12. [Crossref] [PubMed]
- Bian J, Shen A, Yang W, et al. Financial toxicity experienced by patients with breast cancer-related lymphedema: a systematic review. Support Care Cancer 2023;31:354. [Crossref] [PubMed]
- Brahma B, Yamamoto T. Breast cancer treatment-related lymphedema (BCRL): An overview of the literature and updates in microsurgery reconstructions. Eur J Surg Oncol 2019;45:1138-45. [Crossref] [PubMed]
- Chen K, Beeraka NM, Zhang X, et al. Recent Advances in Therapeutic Modalities Against Breast Cancer-Related Lymphedema: Future Epigenetic Landscape. Lymphat Res Biol 2023;21:536-48. [Crossref] [PubMed]
- Chen ST, Lai HW, Wu WP, et al. The impact of body mass index (BMI) on MRI diagnostic performance and surgical management for axillary lymph node in breast cancer. World J Surg Oncol 2022;20:45. [Crossref] [PubMed]
- Clift AK, Dodwell D, Lord S, et al. Development and internal-external validation of statistical and machine learning models for breast cancer prognostication: cohort study. BMJ 2023;381:e073800. [Crossref] [PubMed]
- Sarkar S, Mali K. Breast Cancer Subtypes Classification with Hybrid Machine Learning Model. Methods Inf Med 2022;61:68-83. [Crossref] [PubMed]
- Xiu Y, Jiang C, Zhang S, et al. Prediction of nonsentinel lymph node metastasis in breast cancer patients based on machine learning. World J Surg Oncol 2023;21:244. [Crossref] [PubMed]
- Vrdoljak J, Boban Z, Barić D, et al. Applying Explainable Machine Learning Models for Detection of Breast Cancer Lymph Node Metastasis in Patients Eligible for Neoadjuvant Treatment. Cancers (Basel) 2023;15:634. [Crossref] [PubMed]



