
Propensity analysis reveals survival disparities between T1a and T1b well-differentiated thyroid cancer based on surgery
Highlight box
Key findings
• Propensity matching analysis demonstrates notable survival differences when stratifying by T1 tumor size subgroups.
• Refusing surgery was associated with an over 3-fold increased mortality risk in T1b well-differentiated thyroid cancer (WDTC), whereas no statistically significant survival difference was seen for T1a patients based on treatment choice.
What is known and what is new?
• Current treatment recommendations by 2015 American Thyroid Association (ATA) guidelines remain controversial; most reports group T1a with T1b cohorts, limiting insight on divergent size-based outcomes, and allowing clinical ambiguity to permeate treatment decisions.
• These novel findings indicate significant divergence in health outcomes based on tumor profile and highlight the need to consider personalized management approaches tailored to individual tumor characteristics.
What is the implication and what should change now?
• Given the rising early stage WDTC incidence and controversies around appropriate extent of treatment, surgery remains imperative for optimal outcomes in T1b WDTC patients.
• For select T1a patients, non-invasive monitoring strategies may provide comparable longevity while conserving thyroid function.
• Findings underscore the need for ATA guidelines to expand upon and clarify indications for active surveillance and to emphasize subtype-specific, evidence-based recommendations aligned with disease biology.
Introduction
Thyroid cancer represents the most rapidly increasing cancer diagnosis nationally, nearly tripling in incidence in the United States since 1975 even while related mortality has remained stable (1,2). This rise predominantly involves differentiated subtypes that include papillary and follicular histology, which comprise over 90% of cases and carry an excellent prognosis (1,3). However, optimal management for early-stage differentiated thyroid cancer (DTC) remains controversial given lack of prospective data and evolving practice patterns (4-6).
The American Thyroid Association (ATA) classifies low-risk papillary thyroid carcinomas as tumors 4 cm or smaller in diameter with no involvement beyond the thyroid, including spread to lymph nodes (7). 2009 ATA guidelines endorsed either lobectomy alone or total thyroidectomy without the use of adjuvant radioactive iodine (RAI), while low-risk tumors between 1–4 cm were deemed suitable for additional RAI in certain patient-specific contexts (7). Increasingly conservative recommendations were announced in the 2015 ATA guidelines, stating that a lobectomy alone was sufficient for treatment of all tumors <4 cm in diameter, among other de-escalation treatment recommendations (7).
As a result of the indolent nature of treatment risks associated with low-risk DTC, revised 2015 ATA guidelines consider active surveillance (AS) as a recommended treatment regimen, although less data supports this approach for T1b (8,9). AS is a strategy that entails close monitoring of the cancer with consistent symptom management without pursuing upfront invasive treatment such as surgical procedures (10). Patients that qualify as candidates for AS include those with well-defined solitary <1 or 1.5 cm intrathyroidal papillary thyroid carcinoma. Qualifying criteria also included older patient age, robust normal parenchyma surrounding the tumor, and absence of aggressive features or nodal disease (9,11,12). A 10-year Japanese study comparing immediate surgery with AS for patients meeting strict entry criteria found comparable outcomes, although 8% experienced size progression and 3.8% developed node metastasis on observation, while postsurgical groups suffered vocal cord palsy (4.1%) and para/hypoparathyroidism (16.7%) (13). In a prior prospective trial comparing AS for T1b tumors to T1a tumors, no significant differences were found in the development of lymph node metastasis or increased tumor burden between both groups, postulating that AS could be used for patients with T1b tumors (14).
These findings reveal complex tradeoffs between benefits and risks for early-stage patients considering treatment options. However, most reports group T1a with T1b cohorts, limiting insight on divergent size-based outcomes, and allowing clinical ambiguity to permeate treatment decisions. This investigation therefore leverages a large, national dataset to analyze the extent of treatment refusal and associated survival impacts specifically comparing T1a to T1b subtype DTC patients in order to inform optimized evidence-based recommendations aligned with disease biology. We present this article in accordance with the STROBE reporting checklist (available at https://gs.amegroups.com/article/view/10.21037/gs-24-327/rc).
Methods
Data source
This retrospective cohort study utilized data from the Surveillance, Epidemiology, and End Results (SEER) database (Registry 17) to investigate the factors associated with patients’ refusal of recommended cancer surgery. The SEER database is a nationally representative source that includes patients diagnosed with thyroid cancer. The SEER database contains a specific variable that captures whether surgery was recommended but not performed, refused by the patient, or performed. This variable enables identification of patients who were recommended surgery but refused it, as well as those who underwent surgical treatment. In this study, surgical treatment includes both partial and total thyroidectomy procedures, encompassing total thyroidectomy, subtotal thyroidectomy, lobectomy and/or isthmectomy, and local tumor destruction. The study period spanned from 2000 to 2019. Ethical approval was waived by the Tulane Human Research Protection Office (IRB #2023-449). This study was conducted in accordance with the Declaration of Helsinki (as revised in 2013).
Study population and variables
The study included well-differentiated thyroid cancer (WDTC) patients of any age with T1 stage tumors less than 2 cm in size, who either underwent cancer-directed surgery or refused the recommended surgery. Patients with missing tumor size data and those with nonspecific surgery information were excluded. Additionally, only patients diagnosed incidentally at autopsy or through death certification, as well as those with unspecified radiation, were excluded. The subset of patients with T1 stage as a or b were retained for analysis. Following exclusion of patients via the aforementioned criteria, 81,664 patients with positive histological confirmation of WDTC were selected for and included in the final data set. The variables analyzed spanned a wide range of demographic, clinical, as well as therapeutic factors, including age, sex, racial background, Hispanic/Latino ethnicity, urban or rural residency status, annual household income, histopathological subtype, prior malignancies, tumor size, and tumor-node-metastasis (TNM) staging based upon the American Joint Committee on Cancer (AJCC) 8th system. Extension of the cancer, nominally accounted for by subdivision into local, regional, and distant, was additionally considered. Surgical interventions targeting cancer and radiation therapy were investigated as well. Time-to-surgery was treated as a binary variable in this survival model (“early” <4 months vs. “late” ≥4 months) to stratify surgery patients for comparison within the surgery group only. This dichotomization was determined using receiver operator characteristic analysis to identify the optimal cutoff for predicting outcomes. Time-to-surgery was used specifically during the subset analysis of surgery patients. However, in comparing surgery operated vs. refused patients, time-to-surgery was not used, as there are no corresponding values for the non-surgery group. This covariate was removed from regression analyses to maintain the integrity of our primary comparison. Follow-up time was determined using a variable provided in the SEER database; this variable measures the time from diagnosis to either death or last follow-up, whichever occurred first.
Study outcomes
This study sought to comprehensively assess the outcomes of refusal of recommended surgery (n=99) or surgical intervention (n=81,565) in WDTC patients. Primary surgical treatment included all thyroidectomy procedure types. The primary surgical treatment as defined by the SEER database is a procedure that “modifies, controls, removes, or destroys cancerous tissue at the site of the cancer’s origin” and selects surgical procedure codes hierarchically based on the most definitive surgical procedure (15). Regarding the timeframe, SEER calculates months from diagnosis to treatment using the month and year treatment started and the month and year of diagnosis. Treatment could include surgery, radiation therapy, chemotherapy, hormone therapy, immunotherapy, and/or AS. Specifically, the months from diagnosis to treatment is calculated as: months from diagnosis to treatment = [(year initial treatment started * 12) + month initial treatment started] – [(year of diagnosis * 12) + month of diagnosis] (16).
The study also aimed to analyze disease outcomes, based on different cancer stages (T1a vs. T1b). First examining the relationship between variables by way of unmatched analysis, the data underwent matching modifications to determine outcomes after controlled correction.
Propensity matching analysis
To reduce bias and create balanced comparison groups, propensity score matching analysis was conducted using the R package ‘MatchIt’. Covariates adjusted for in the matching process included age, gender, race, ethnicity, residency, household income, TNM stage, and prior malignancies. Nearest neighbor matching with a control-to-treatment ratio of 4:1, a caliper of 0.2, and a threshold line of 0.1 was employed. No replacement was allowed, and logistic regression was used for distance computation. Sensitivity analyses were performed to assess the balance of covariates across different matching ratios to ensure robustness and validity of the results. QQ and jitter plots were used for visualization, and histograms were generated for covariate balance assessment.
Statistical analysis
The statistical analyses were performed using the R Statistical language (version 4.2.2; R Core Team, 2022) on macOS Ventura 13.3.1 and SPSS version 28.0 (IBM Corp., Armonk, NY, USA). A significance level of 0.05 was used for all analyses, and all tests were two-sided. Categorical variables were presented as frequencies and percentages, while continuous variables were reported as mean (standard deviation) or median (interquartile range) as appropriate. The data on treatment modalities and time to treatment were analyzed using descriptive statistics to determine the proportion of patients in each category. Descriptive statistics, such as Chi-squared or Fisher’s exact tests for categorical variables and Student’s t-test or Mann-Whitney U test for continuous variables, were used. Chi-squared tests indicated the association between treatment modalities and T1 stage subgroups (T1a and T1b), as well as between time to treatment and T1 stage subgroups. Thus, for demographic and clinical analysis, baseline characteristics were compared between the two groups using suitable statistical tests.
Logistic regression models were employed to identify independent predictor risk factors for recurrence and second primary malignancy, adjusting for potential confounders. Secondary malignancy analysis was completed by determining the incidence of recurrence in both groups and investigating the potential risk factors using multivariate regression models. Odds ratios (ORs) with 95% confidence intervals (CIs) were reported. Thyroid cancer-specific and overall survival analyses were conducted using the Kaplan-Meier method, and the log-rank test was used to assess differences in survival between groups. Prognostic factors for survival were identified using univariate and multivariate Cox proportional hazards regression models. Multivariate models were built using variables that were significant in the univariate analyses, and adjusted hazard ratios (aHRs) with 95% CIs were reported.
Results
Characteristics of the study population
Of the 81,664 total T1N0M0 thyroid cancer patients, 81,565 (99.9%) underwent surgery, while only 99 (0.1%) refused the recommended operation. Compared to the surgery group, the refusal cohort displayed a significantly higher composition of racial minorities (33.3% vs. 16.7% non-White, P<0.001) and higher income (45.5% vs. 33.6% with income ≥$75,000 annually, P=0.01). Refusers also more frequently had T1b tumors (66.7% vs. 38.7%, P<0.001; Table 1).
Table 1
| Characteristics | Levels | Total patients (N=81,664) | Surgery performed (N=81,565) | Patient refused (N=99) | P value |
|---|---|---|---|---|---|
| Age (years) | Mean ± SD | 49.9±14.5 | 49.9±14.5 | 51.7±16.6 | 0.21 |
| <55 years | 50,206 (61.5) | 50,145 (61.5) | 61 (61.6) | >0.99 | |
| ≥55 years | 31,458 (38.5) | 31,420 (38.5) | 38 (38.4) | ||
| Gender | Female | 65,754 (80.5) | 65,680 (80.5) | 74 (74.7) | 0.16 |
| Male | 15,910 (19.5) | 15,885 (19.5) | 25 (25.3) | ||
| Race | White | 68,019 (83.3) | 67,953 (83.3) | 66 (66.7) | <0.001 |
| Black | 4,924 (6.0) | 4,919 (6.0) | 5 (5.1) | ||
| API | 8,234 (10.1) | 8,210 (10.1) | 24 (24.2) | ||
| AI/AN | 487 (0.6) | 483 (0.6) | 4 (4.0) | ||
| Ethnicity | Not Hispanic/Latino | 69,779 (85.4) | 69,694 (85.4) | 85 (85.9) | >0.99 |
| Hispanic/Latino | 11,885 (14.6) | 11,871 (14.6) | 14 (14.1) | ||
| Metropolitan | Rural | 8,103 (9.9) | 8,093 (9.9) | 10 (10.1) | 0.87 |
| Urban | 73,561 (90.1) | 73,472 (90.1) | 89 (89.9) | ||
| Household annual income | <$75,000 | 54,234 (66.4) | 54,180 (66.4) | 54 (54.5) | 0.01 |
| ≥$75,000 | 27,430 (33.6) | 27,385 (33.6) | 45 (45.5) | ||
| Previous malignancies | No | 71,011 (87.0) | 70,928 (87.0) | 83 (83.8) | 0.37 |
| Yes | 10,653 (13.0) | 10,637 (13.0) | 16 (16.2) | ||
| T stage | T1a | 50,047 (61.3) | 50,014 (61.3) | 33 (33.3) | <0.001 |
| T1b | 31,617 (38.7) | 31,551 (38.7) | 66 (66.7) | ||
| N staging | N0 | 68,845 (84.3) | 68,757 (84.3) | 88 (88.9) | 0.27 |
| N1 | 12,819 (15.7) | 12,808 (15.7) | 11 (11.1) | ||
| M staging | M0 | 81,600 (99.9) | 81,501 (99.9) | 99 (100.0) | >0.99 |
| M1 | 64 (0.1) | 64 (0.1) | 0 | ||
| Extension | Localized | 68,509 (83.9) | 68,421 (83.9) | 88 (88.9) | 0.38 |
| Regional | 13,000 (15.9) | 12,989 (15.9) | 11 (11.1) | ||
| Distant | 155 (0.2) | 155 (0.2) | 0 |
Data is presented as number and percentage or mean ± SD. Two-sided Chi-squared or Student’s t-tests were used. Statistical significance was set at P value <0.05. SD, standard deviation; API, Asian or Pacific Islander, AI/AN, American Indian/Alaska Native.
Treatment approach in surgery cohort
Most patients (67%) received surgery alone. However, T1b tumors were twice as likely as T1a tumors to undergo additional therapies like RAI (T1b 49.7% vs. T1a 21.1%, P<0.001). Overall, 46.3% of the cohort had surgery with systematic adjuvant treatment, again more commonly for T1b than T1a subgroups (52.4% vs. 42.6%, P<0.001). Over two-thirds underwent treatment within 1 month of diagnosis, though T1a patients were more likely to have such expedient intervention (76.5% vs. 50.5% for T1b, P<0.001; Table S1).
Regarding the breakdown of partial vs. total thyroidectomy, 63,114 (77.4%) patients underwent total thyroidectomy, while 18,451 (22.6%) had a partial thyroidectomy performed. Partial thyroidectomy included 3,133 patients with subtotal thyroidectomy, 15,315 with lobectomy and/or isthmectomy, and 3 cases of local tumor destruction.
Survival outcomes
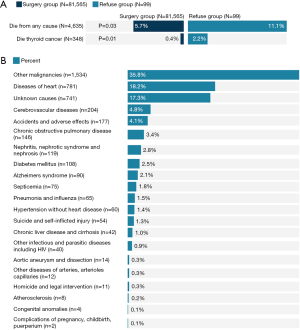
Vital status data revealed an overall mortality rate of 5.7% (n=4,635 deaths), significantly higher for the refusal group (11.1%, n=11) compared to the surgery group (5.7%, n=4,624, P=0.03). Thyroid cancer-specific mortality reached 2.2% (n=2) among refusers vs. 0.4% (n=346) for the surgery cohort (P=0.01). Other leading causes of death included other cancers (35.8%) and heart disease (18.2%) (Figure 1). Kaplan-Meier curves displayed lower median overall survival times for those refusing surgery (152.05 vs. 178.62 months, P<0.001). Similar trends appeared for thyroid cancer-specific survival (170.53 vs. 190.02 months, P=0.002; Figure 2).

Impact of surgery timing on survival
Survival times were also analyzed across different comparisons. The timing of surgery showed a trend toward better outcomes, with early surgery (defined as <4 months from diagnosis) demonstrating a slightly higher overall survival time (178.8±0.18 months) compared to late surgery (172.7±1.30 months, P=0.06). A similar trend was noted in thyroid cancer-specific survival, with early surgery patients having a slightly higher survival time of 190.1±0.05 months against 188.9±0.48 months for late surgery patients (P=0.13).
Risk factors for mortality
Our multivariate analysis identified several key predictors for increased mortality risk in patients. These include being 55 years or older (aHR =4.87), male gender (aHR =1.63), Black race (aHR =1.57), lower income levels (aHR =1.23), rural residency (aHR =1.28), presence of positive lymph nodes (aHR =1.13), a history of prior malignancy (aHR =2.81), and refusal of surgery (aHR =2.63) (all P<0.05; Table 2).
Table 2
| Risk factor | Comparison | aHR | 95% CI | P value |
|---|---|---|---|---|
| Age | ≥55 vs. <55 years old | 4.87 | 4.53–5.23 | <0.001 |
| Sex | Male vs. female | 1.63 | 1.53–1.73 | <0.001 |
| Race | Black vs. White | 1.57 | 1.42–1.75 | <0.001 |
| Race | API vs. White | 0.87 | 0.77–0.98 | 0.02 |
| Race | AI/AN vs. White | 1.20 | 0.83–1.75 | 0.33 |
| Ethnicity | Hispanic/Latino vs. none | 1.00 | 0.90–1.10 | 0.92 |
| Residency | Rural vs. urban | 1.28 | 1.18–1.39 | <0.001 |
| Household income | <$75,000 vs. ≥$75,000 | 1.23 | 1.16–1.32 | <0.001 |
| T stage | T1b vs. T1a | 0.96 | 0.90–1.02 | 0.15 |
| N stage | N1 vs. N0 | 1.13 | 1.04–1.24 | 0.006 |
| Prior primary malignancy | Prior malignancy vs. none | 2.81 | 2.64–2.99 | <0.001 |
| Surgery | Refused vs. operated | 2.63 | 1.45–4.76 | <0.001 |
Multivariate Cox regression hazards proportional test was used. aHRs and 95% CIs were reported. Statistical significance was set at P value <0.05. aHR, adjusted hazards ratio; CI, confidence interval; API, Asian or Pacific Islander; AI/AN, American Indian/Alaska Native.
Propensity matching analysis
After matching on demographics and clinical variables (Table 3 and Figure S1), surgery refusal remained associated with over twice the mortality risk (aHR =2.15, 95% CI: 1.01–4.57, P=0.046; Figure 3).
Table 3
| Characteristics | Levels | Total patients (N=495) | Surgery performed (N=396) | Patient refused (N=99) | P value |
|---|---|---|---|---|---|
| Age | <55 years | 269 (54.3) | 208 (52.5) | 61 (61.6) | 0.12 |
| ≥55 years | 226 (45.7) | 188 (47.5) | 38 (38.4) | ||
| Gender | Female | 371 (74.9) | 297 (75.0) | 74 (74.7) | >0.99 |
| Male | 124 (25.1) | 99 (25.0) | 25 (25.3) | ||
| Race | White | 332 (67.1) | 266 (67.2) | 66 (66.7) | 0.97 |
| Black | 28 (5.7) | 23 (5.8) | 5 (5.1) | ||
| API | 118 (23.8) | 94 (23.7) | 24 (24.2) | ||
| AI/AN | 17 (3.4) | 13 (3.3) | 4 (4.0) | ||
| Ethnicity | Not Hispanic/Latino | 441 (89.1) | 356 (89.9) | 85 (85.9) | 0.28 |
| Hispanic/Latino | 54 (10.9) | 40 (10.1) | 14 (14.1) | ||
| Metropolitan | Metropolitan >1 M pop | 444 (89.7) | 355 (89.6) | 89 (89.9) | >0.99 |
| Metropolitan >250 K–1 M pop | 51 (10.3) | 41 (10.4) | 10 (10.1) | ||
| Household annual income | Income <$75,000 | 276 (55.8) | 222 (56.1) | 54 (54.5) | 0.82 |
| Income ≥$75,000 | 219 (44.2) | 174 (43.9) | 45 (45.5) | ||
| Previous malignancies | No | 422 (85.3) | 339 (85.6) | 83 (83.8) | 0.64 |
| Yes | 73 (14.7) | 57 (14.4) | 16 (16.2) | ||
| T stage | T1a | 327 (66.1) | 261 (65.9) | 66 (66.7) | 0.91 |
| T1b | 168 (33.9) | 135 (34.1) | 33 (33.3) | ||
| N staging | N0 | 427 (86.3) | 339 (85.6) | 88 (88.9) | 0.51 |
| N1 | 68 (13.7) | 57 (14.4) | 11 (11.1) |
A nearest neighbor matching with a control-to-treatment ratio of 4:1 was employed. Data of matched groups is presented as number and percentage. Two-sided Chi-squared or Student’s t-tests were used. Statistical significance was set at P value <0.05. API, Asian or Pacific Islander; AI/AN, American Indian/Alaska Native; pop, population.

However, this risk was localized to T1b patients (aHR =3.44, 95% CI: 1.43–8.28, P=0.006) rather than the T1a subgroup (aHR =0.41, 95% CI: 0.049–3.46, P=0.42). Advanced age (≥55 years) emerged as a mortality predictor for both T1a (aHR =6.27, P=0.02) and T1b (aHR =7.13, P<0.001). Prior malignancy was also associated with higher T1b mortality (aHR =2.78, P=0.03; Table 4).
Table 4
| Risk factor for mortality | T1a | T1b | |||
|---|---|---|---|---|---|
| aHR (95% CI) | P value | aHR (95% CI) | P value | ||
| Age: ≥ 55 vs. <55 years old | 6.27 (1.32–29.8) | 0.02 | 7.13 (2.31–22.0) | <0.001 | |
| Sex: male vs. female | 2.98 (0.75–11.9) | 0.12 | 1.54 (0.69–3.43) | 0.29 | |
| Race: Black vs. White | 0.34 (0.03–3.32) | 0.35 | 0.32 (0.03–2.68) | 0.29 | |
| Race: API vs. White | 0.87 (0.16–4.63) | 0.87 | 0.69 (0.24–1.98) | 0.49 | |
| Residency: urban vs. rural | 0.53 (0.08–3.35) | 0.50 | 1.57 (0.37–6.74) | 0.54 | |
| Household income: high vs. low | 0.46 (0.11–1.88) | 0.28 | 0.43 (0.17–1.09) | 0.07 | |
| N stage: N1 vs. N0 | 1.62 (0.74–3.51) | 0.82 | 1.73 (0.58–5.12) | 0.32 | |
| Prior malignancy vs. none | 0.57 (0.11–2.98) | 0.50 | 2.78 (1.10–7.04) | 0.03 | |
| Surgery: refuse vs. operated | 0.41 (0.049–3.46) | 0.42 | 3.44 (1.43–8.28) | 0.006 | |
Multivariate Cox regression hazards proportional test was used. aHRs and 95% CIs were reported. Statistical significance was set at P value <0.05. T1b patients derive survival benefit from surgery, while select T1a patients may opt for active surveillance without significantly impacting longevity. aHR, adjusted hazards ratio; CI, confidence interval; API, Asian or Pacific Islander.
Kaplan-Meier curves showed T1a surgery patients had marginally longer survival (168.191±5.274 months) compared to those refusing surgery (163.269±4.639 months, P=0.39), while T1b surgery patients had significantly longer survival (174.224±3.861 months) than those refusing surgery (141.587±10.235 months, P=0.004; Figure 4).

Discussion
The current findings provide important insight into the factors influencing DTC health implications and survival, offering guidance for clinicians to devise personalized management and follow-up strategies for patients. Prior studies have investigated factors impacting surgery refusal in all stages of thyroid cancer patients and correlated with survival outcomes (17-19). However, little research has explored differences specifically between T1a and T1b WDTC subtypes, potentially warranting tailored treatment recommendations (20). The traditional approach to management of T1 tumors has consisted of recommendations for treatment conforming to ATA guidelines (20,21), yet evidence-based studies backing the distinction between treatment for T1a and T1b tumors continues to lag. Characterizing the available evidence for WDTC remains critical for understanding distinct treatment needs, particularly as it pertains to the influence of surgery refusal. Therefore, this study aimed to identify key outcome disparities in these WDTC subgroups to inform evidence-based guidelines promoting survival while mitigating risks.
Comparing those refusing vs. undergoing surgery revealed demographic and clinicopathological variations. T1b patients were more inclined to forgo surgery while T1a patients mostly opted for operation, potentially attributable to racial disparities as white individuals composed a greater fraction of the surgery cohort and racial minorities formed a larger fraction of the surgery refusal group reflecting trends of increased healthcare access and adherence to guidelines (22). These findings remain concerning given specific ATA guidelines advise against non-invasive strategies for those with T1b tumors. While this discrepancy undoubtedly deserves further clinical investigation into barriers to undergoing surgery for this subtype, the patterns derived from this study illuminate the need for providers to consider racial disparities when counseling patients, building trust, and facilitating informed choices, while also taking into consideration the need for personalization of treatment regimens.
Surgery was associated with improved overall survival and thyroid-cancer specific survival in patients with WDTC. Notably, patients who do opt for surgery typically do so within one month of diagnosis. Patients who are over 55 years old, male, black, live in rural areas, and make less than $75,000 in annual income tend to fair worse in terms of overall mortality, in addition to lymph node metastasis, history of a prior malignancy and refusal of surgery. Poor outcomes with systemic therapy may be attributed to the relatively good prognosis in terms of overall survival in the T1 group, discouraging the use of systemic therapy in the absence of thyroid cancer metastasis (23). These predictors warrant incorporation into clinical assessments for treatment planning and follow-up attuned to individual risks. Nonetheless, these factors must not overshadow the potential differences in presentation and needs between T1a and T1b groups.
When propensity matched analysis was performed, rendering many of the differences in demographic characteristics similar between those who underwent surgery and those who refused, splitting the T1 group into T1a and T1b demonstrated stark contrast in treatment outcomes. One previous study points to differences in outcomes and treatment of WDTC converging over time (1). However, this longer-term investigation revealed substantially improved survival for T1b patients undergoing surgery vs. refusal, unlike T1a patients showing no statistical distinction. Surgery did not significantly extend lifespan for T1a patients, questioning the viability of conflating the efficacy of surgery with non-invasive treatment options for this tumor subtype. Promisingly, prior work has proposed to expand criteria for AS (24), suggesting that, this powerful modality for patient care may benefit from further development should it be utilized as a primary treatment regimen in the context of WDTC. Despite poor outcomes among T1b patients relative to the T1a group when refusing recommended surgery, not all patients who refuse surgeon-recommended surgery should by default be selected for and monitored under AS protocols.
Of note, T1b patients, even after propensity score matching were more likely to have had a prior malignancy. Given a history of prior cancer, a greater proportion of T1b patients undergoing surgery would be expected due to indication of potential tumor aggressiveness (25). Yet, the opposite effect is observed in our study, where T1b patients are more likely than T1a patients to refuse surgery. As such, structuring recommendations with a greater understanding of differences in surgical outcomes between tumor types could profoundly improve the framework of T1-subtype management.
The difference between a T1a and T1b diagnosis significantly influences patient decision-making, potentially driven by provider recommendations based on the current set of ATA guidelines for surgery. As AS is gaining attention for its proposed suitability as an alternative to immediate surgery for selected patients, particularly in those with T1a tumors, it is crucial to thoroughly review recent evidence and ongoing trials related to AS for low-risk thyroid cancer. By assessing the efficacy, safety, and long-term outcomes of AS, guidelines can be refined and offer informed treatment options for T1a and T1b patients. The notion that T1b patients benefit from receiving surgery, yet have a higher refusal rate than T1a patients, sheds light on the concept that the difference in subclassification can affect patients’ decisions. With proper education, the difference in staging recommendations may be made more transparent for the patient, leading to more informed choices.
The findings of this study suggest that patients with T1b tumors benefit from surgery for improved overall survival and thyroid-cancer specific survival. However, surgery does not statistically improve the overall chance of survival for patients with T1a tumors. These findings call for clinical practice guidelines to specify the impact of surgery refusal in treatment recommendations with the outcomes specific to T1a and T1b tumors. By incorporating subtype-specific recommendations, clinicians can optimize patient management and improve survival rates. It is vital to evaluate the balance between cost and benefit of surgery vs. perpetual non-invasive strategies for T1a patients. Additionally, further research and analysis are warranted to assess the long-term survival outcomes, quality of life, and healthcare expenditures associated with AS compared to immediate surgery in this patient population. This analysis will provide a comprehensive understanding of the optimal management strategy for T1a patients.
This study does present several limitations. Although this is most likely a significant driving force behind decisions, there is no qualitative data provided that shows a difference in patient beliefs between the two groups. These beliefs could also potentially play significant roles in patient decisions, yet exploration of these variables in the current analysis is inherently limited by the scope of the SEER cancer registry database. Generally, potential reasons for refusal could include patient preference, perceived risks of surgery, fear of complications, underlying comorbid conditions, monetary concerns, and advice from non-medical sources. Perceived risks have been documented in a study evaluating patient decision making in AS vs. immediate surgery in papillary thyroid microcarcinoma patients, which included family history of thyroid-related or other cancers, family matters, trust in the medical system and physician advice, and treatment timing amidst miscellaneous life circumstances (26). These trends yield insight that can equip physicians with the knowledge of which patients are likely to refuse surgery, and in cases where surgery is pertinent, facilitate timely discourse regarding appropriate treatment regimens.
Additionally, this study design limits causality and generalizability. While the SEER database provides a large sample size and standardized data collection, it is still essential to consider the limitations of retrospective data collection; confounding by indication limits the utility of SEER data to be used to estimate outcomes by treatment, and controlling for income, race, and other demographic variables limit representation of the nuances involved in treatment decision making, particularly in regard to why individuals decide to prioritize one treatment over another. As such, robust clinical investigation into the social aspects of treatment decisions for WDTC would be of great value to future practice.
In the original dataset from the SEER database, a large discrepancy was found in sample size between the two groups: patients who underwent surgery and those who refused surgery. Because the cohort of surgery refusal patients was indeed much smaller proportionally to the overall composite population of the two groups combined, the study data required 4:1 propensity matching analysis to ensure a statistically sound method for group comparisons. This method runs the risk of incomplete matching; however, with new sample sizes of 396 and 99 in the surgery and refusal groups, respectively, generalizability of the study results is preserved due to new values for cohort sample sizes remaining sufficiently powered.
The narrowed focus on patients in the T1 tumor stage group in this study is a strength, allowing for a more specific understanding of the outcomes by direct comparison of WDTC subgroups made possible by SEER. Database driven approaches as exhibited in this context subsequently provide large-scale analysis of empirical evidence from which to deduce trends that deserve further investigation. To advance the interpretation of management and treatment surrounding WDTC, there is potential for future research to examine the impacts of different surgical approaches and adjuvant treatments in T1 thyroid cancer patients. Additionally, studies could explore the inclusion of time-to-surgery as a time-dependent variable and subsequently use landmark analysis by using an alternative data source to shed light on timing and outcomes of surgical intervention specifically. Subsequent work may also seek to identify the effect of social factors influencing surgical or surveillance decisions, in addition to assessing the cost-effectiveness and financial implications of different treatment strategies to improve outcomes. Qualitative data collection to capture in-depth reasons for refusal could offer greater understanding of patient behaviors and help design effective, patient-centered counseling and decision-making support.
Conclusions
This study contributes to the growing body of evidence on risk factors and treatment outcomes in WDTC, specifically the T1a and T1b subtypes. Our findings suggest that T1a and T1b thyroid cancers may benefit from different treatment approaches: While surgery appears to offer significant survival benefit for T1b patients, the same benefit was not observed for T1a patients in our study. These results indicate a need for further research into treatment strategies that consider tumor subtype. Future studies and guidelines may benefit from exploring how these subtype-specific outcomes relate to different surgical and non-surgical management options, to further optimize patient care.
Acknowledgments
We appreciate The Surveillance, Epidemiology, and End Results (SEER) Program providing us with the privilege to access thyroid cancer patients’ clinical and pathological data.
Funding: The project described was supported by
Footnote
Reporting Checklist: The authors have completed the STROBE reporting checklist. Available at https://gs.amegroups.com/article/view/10.21037/gs-24-327/rc
Peer Review File: Available at https://gs.amegroups.com/article/view/10.21037/gs-24-327/prf
Conflicts of Interest: All authors have completed the ICMJE uniform disclosure form (available at https://gs.amegroups.com/article/view/10.21037/gs-24-327/coif). E.K. serves as an Editor-in-Chief of Gland Surgery from May 2024 to April 2026. E.T. reports that funds were obtained from the School of Medicine Pilot Grant in support of this project. E.K. received funding by ThyCa, administered by the American Thyroid Association through grant number ThyroidGrant2021-0000000232. The other authors have no conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of this work are appropriately investigated and resolved. This study was conducted in accordance with the Declaration of Helsinki (as revised in 2013). The Tulane Human Research Protection Office has determined that the proposed research project (IRB #2023-449) does not involve identified human subjects and therefore does not require IRB review and approval.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Anderson KL Jr, Youngwirth LM, Scheri RP, et al. T1a Versus T1b Differentiated Thyroid Cancers: Do We Need to Make the Distinction? Thyroid 2016;26:1046-52. [Crossref] [PubMed]
- Davies L, Welch HG. Current thyroid cancer trends in the United States. JAMA Otolaryngol Head Neck Surg 2014;140:317-22. [Crossref] [PubMed]
- Pellegriti G, Frasca F, Regalbuto C, et al. Worldwide increasing incidence of thyroid cancer: update on epidemiology and risk factors. J Cancer Epidemiol 2013;2013:965212. [Crossref] [PubMed]
- Schmidbauer B, Menhart K, Hellwig D, et al. Differentiated Thyroid Cancer-Treatment: State of the Art. Int J Mol Sci 2017;18:1292. [Crossref] [PubMed]
- Zhang TT, Li CF, Wen SS, et al. Effects of tumor size on prognosis in differentiated thyroid carcinoma smaller than 2 cm. Oncol Lett 2019;17:4229-36. [Crossref] [PubMed]
- Twining CL, Lupo MA, Tuttle RM. Implementing Key Changes in the American Thyroid Association 2015 Thyroid Nodules/Differentiated Thyroid Cancer Guidelines Across Practice Types. Endocr Pract 2018;24:833-40. [Crossref] [PubMed]
- Gordon AJ, Dublin JC, Patel E, et al. American Thyroid Association Guidelines and National Trends in Management of Papillary Thyroid Carcinoma. JAMA Otolaryngol Head Neck Surg 2022;148:1156-63. [Crossref] [PubMed]
- Haugen BR, Alexander EK, Bible KC, et al. 2015 American Thyroid Association Management Guidelines for Adult Patients with Thyroid Nodules and Differentiated Thyroid Cancer: The American Thyroid Association Guidelines Task Force on Thyroid Nodules and Differentiated Thyroid Cancer. Thyroid 2016;26:1-133. [Crossref] [PubMed]
- Krajewska J, Kukulska A, Samborski K, et al. Lobo-isthmectomy in the management of differentiated thyroid cancer. Thyroid Res 2023;16:4. [Crossref] [PubMed]
- Krajewska J, Kukulska A, Oczko-Wojciechowska M, et al. Early Diagnosis of Low-Risk Papillary Thyroid Cancer Results Rather in Overtreatment Than a Better Survival. Front Endocrinol (Lausanne) 2020;11:571421. [Crossref] [PubMed]
- Addasi N, Fingeret A, Goldner W. Hemithyroidectomy for Thyroid Cancer: A Review. Medicina (Kaunas) 2020;56:586. [Crossref] [PubMed]
- Nabhan F, Dedhia PH, Ringel MD. Thyroid cancer, recent advances in diagnosis and therapy. Int J Cancer 2021;149:984-92. [Crossref] [PubMed]
- Miyauchi A. Clinical Trials of Active Surveillance of Papillary Microcarcinoma of the Thyroid. World J Surg 2016;40:516-22. [Crossref] [PubMed]
- Sakai T, Sugitani I, Ebina A, et al. Active Surveillance for T1bN0M0 Papillary Thyroid Carcinoma. Thyroid 2019;29:59-63. [Crossref] [PubMed]
- SEER Training Modules. Cancer Registration & Surveillance Modules National Cancer Institute: U. S. National Institutes of Health. Available online: https://training.seer.cancer.gov/
- surveillance, Epidemiology, and End Results Program. SEER*Stat Database: Incidence - SEER Research Data National Cancer Institute, DCCPS, Surveillance Research Program2000-2019. Available online: www.seer.cancer.gov
- Hussein MH, Toraih EA, Ohiomah IE, et al. Navigating Choices: Determinants and Outcomes of Surgery Refusal in Thyroid Cancer Patients Using SEER Data. Cancers (Basel) 2023;15:3699. [Crossref] [PubMed]
- van Gerwen M, Sinclair C, Rahman M, et al. The impact of surgery refusal on thyroid cancer survival: a SEER-based analysis. Endocrine 2020;70:356-63. [Crossref] [PubMed]
- Khan ZF, Kutlu O, Picado O, et al. Margin Positivity and Survival Outcomes: A Review of 14,471 Patients with 1-cm to 4-cm Papillary Thyroid Carcinoma. J Am Coll Surg 2021;232:545-50. [Crossref] [PubMed]
- Ullmann TM, Papaleontiou M, Sosa JA. Current Controversies in Low-Risk Differentiated Thyroid Cancer: Reducing Overtreatment in an Era of Overdiagnosis. J Clin Endocrinol Metab 2023;108:271-80. [Crossref] [PubMed]
- Matrone A, Campopiano MC, Nervo A, et al. Differentiated Thyroid Cancer, From Active Surveillance to Advanced Therapy: Toward a Personalized Medicine. Front Endocrinol (Lausanne) 2019;10:884. [Crossref] [PubMed]
- Haider AH, Scott VK, Rehman KA, et al. Racial disparities in surgical care and outcomes in the United States: a comprehensive review of patient, provider, and systemic factors. J Am Coll Surg 2013;216:482-92.e12. [Crossref] [PubMed]
- Bartz-Kurycki MA, Oluwo OE, Morris-Wiseman LF. Medullary thyroid carcinoma: recent advances in identification, treatment, and prognosis. Ther Adv Endocrinol Metab 2021;12:20420188211049611. [Crossref] [PubMed]
- Won HR, Jeon E, Heo DB, et al. Age-Dependent Clinicopathological Characteristics of Patients with T1b Papillary Thyroid Carcinoma: Implications for the Possibility of Active Surveillance. Ann Surg Oncol 2023;30:2246-53. [Crossref] [PubMed]
- Zhou P, Tian S, Li J, et al. Paradoxes in thyroid carcinoma treatment: analysis of the SEER database 2010-2013. Oncotarget 2017;8:345-53. [Crossref] [PubMed]
- Sawka AM, Ghai S, Yoannidis T, et al. A Prospective Mixed-Methods Study of Decision-Making on Surgery or Active Surveillance for Low-Risk Papillary Thyroid Cancer. Thyroid 2020;30:999-1007. [Crossref] [PubMed]


