
Preparation and characterization of a rat uterine decellularized scaffold
Highlight box
Key findings
• We successfully prepared a decellularized uterine scaffold that may be used as an alternative platform for uterine tissue engineering research.
What is known, and what is new?
• Many studies have been conducted on the acellular reconstitution of organs, such as the heart, liver, kidney, and lungs, using decellularization technology.
• Our study established a feasible strategy and evaluated a process for the preparation of a uterine decellularized scaffold that retains the extracellular matrix and vascular microenvironment, and has good biological activity.
What is the implication, and what should change now?
• Our natural uterine scaffold based on decellularization technology could have significant value in uterine tissue engineering research. In the future, we intend to combine induced pluripotent stem cells with our scaffold to explore the possibility of constructing an artificial uterus.
Introduction
Infertility is a special reproductive health defect and is currently a popular area of research in the field of reproductive medicine. Due to the coexistence of physiological and psychological factors, infertility can have significant negative effects for families and society (1). With the rapid development of reproductive medicine, in vitro fertilization technology, the core technology of reproductive medicine, has benefited the vast majority of infertile women. However, women with congenital uterine malformations and extensive adhesions in the uterine cavity, and those who have undergone hysterectomy may still experience infertility due to uterine absence or functional defects (2).
Surrogacy, another form of human-assisted reproductive technique, can help women become mothers in a genetic sense; however, it also has many disadvantages related to the high costs of surrogacy, the health status of the surrogate mothers, and the ethical, moral, and social legal issues (3-5). For such women, uterine transplantation is technically feasible, but its clinical application and development are also limited by donor shortages, immune rejection, and mainstream public opinions (6). Thus, new treatment methods for infertility caused by uterine defects promptly need to be explored, and the research on organ regeneration may provide a potential and promising treatment option. With the increasingly widespread application of decellularization technology in tissue regeneration engineering, natural extracellular matrix (ECM) scaffold structures have played an important carrier role in the study of organ regeneration (7,8). In the research of uterine tissue engineering, decellularized uterine scaffolds have become a new hotspot in recent years. This study aimed to explore the ideal perfusion strategy and evaluation system for successfully preparing natural uterine decellularized ECM biological scaffolds using decellularized perfusion technology to provide a good application carrier for the research of uterine tissue regeneration engineering. We present this article in accordance with the ARRIVE reporting checklist (available at https://gs.amegroups.com/article/view/10.21037/gs-24-474/rc).
Methods
Experimental animals
Clean-level, healthy, adult, female Sprague-Dawley (SD) rats (n=30), weighing 250–300 g, were provided by the Shanghai Experimental Animal Center of the Chinese Academy of Sciences, and raised in an specific pathogen free (SPF)-level experimental environment at the Experimental Animal Center of Wenzhou Medical University. The experiment started in December 2019 and ended in May 2021. The animal experiments were performed under a project license (No. wydw2016-0224) granted by Experimental Animal Ethics Committee of Wenzhou Medical University, in compliance with institutional guidelines for the care and use of animals. A protocol was prepared before the study without registration.
Main experimental equipment and reagents
The main experimental equipment used in this study were shown in Table 1, and also the following reagents were used:
- 0.1 M phosphate-buffered saline (PBS): 8 g sodium chloride, 0.2 g potassium chloride, 1.44 g disodium hydrogen phosphate, 0.24 g potassium dihydrogen phosphate, and 800 mL deionized water. The pH was adjusted with 1 M of HCl or NaOH to a constant volume of 1 L.
- 0.02% trypsin/0.05% ethylenediaminetetraacetic acid (EDTA) solution: 0.25% trypsin/0.5% EDTA solution was diluted by 0.1 M PBS for 10 times.
- 0.2 M PBS: 16 g sodium chloride, 0.4 g potassium chloride, 2.88 g disodium hydrogen phosphate, 0.48 g potassium dihydrogen phosphate, and 800 mL deionized water. The pH was adjusted with 1 M of HCl or NaOH to a constant volume of 1 L.
- 0.1% sodium dodecyl sulfate (SDS) solution: 1 g SDS, add 1,000 mL of 0.1 M PBS.
- 1% Triton X100/0.05% EDTA solution: Triton X100 10 mL, EDTA 500 mg, add 970 mL of 0.1 M PBS.
- Enzyme-linked immunosorbent assay (ELISA) kit for transforming growth factor beta (TGF-β), basic fibroblast growth factor (bFGF) and epidermal growth factor (EGF).
Table 1
| Equipment name | Source location |
|---|---|
| Microsurgical instruments | Shanghai Jinzhong Metal Products Factory, China |
| 8F, 24F, 28F silicone tubes | Haimen Shengbang Experimental Equipment Co., Ltd., China |
| Orange cap pipettes and blue cap bottles | Haimen Shengbang Experimental Equipment Co., Ltd., China |
| BTl00-2J peristaltic pump | Baoding Lange Company, Sino US joint venture |
| Optical microscope | Olympus, Japan |
| Scanning electron microscopy | Hitachi, Japan |
| Ultra CLEAN platform | Suzhou Purification Equipment Co., Ltd., China |
| Thermostatic water bath | Hero, Denmark |
| Multi-functional animal operating table | Institute of Surgery, First Affiliated Hospital of Wenzhou Medical College |
Harvesting of the rat uterine and the decellularization procedure
The adult female SD rats were intraperitoneally injected with 3% pentobarbital sodium (1 mL/kg), and a median incision was then made into the abdomen. After heparin was systemically injected through the inferior vena cava, the intestinal canal was wrapped with wet gauze and pushed upwards to expose the lower abdomen. The abdominal aorta was transected below the level of the ovarian artery, and the external iliac artery and vein were found. Other branches of the external iliac artery and vein were ligated and severed, and only the uterine artery and vein were preserved. A number 10 polyethylene tube was inserted through the broken end of the external iliac artery to the opening of the uterine branch of the external iliac artery. The tube was fixed properly, and the vagina was disconnected at the level of the cervix to completely remove the uterus and blood vessels connected to it. The uterine tissue was immersed in 0.1 mM PBS solution and freeze thawed at −80 ℃ overnight, and an optimized process combination was used to sequentially infuse 0.02% pancreatic enzyme/0.05% EDTA solution through the catheter (30 minutes). After treatment with 3% Triton X100/0.05% EDTA (for 240 minutes) and 0.1% SDS solution (for 30 minutes), The uterine tissue was washed by deionized water and 0.2 mM PBS solution for 15 minutes, respectively, to complete the decellularized process, and then sterilized with 0.01% peracetic acid/4% ethanol solution, penicillin/streptomycin/amphotericin B solution. The above perfusion processes were all performed using a peristaltic pump, and the flow rate was 5 mL/minutes at room temperature, and strict attention was paid to ensure a sterile operation. At the end of the experiment, the rats were euthanized by injecting high potassium.
General observation
The color and morphological changes of the fresh uterine tissue and the overall organs at various time points (e.g., 90, 180, and 330 minutes) after perfusion were observed. After the infusion process was completed, the vascular network structure of the decellularized uterine stent was observed by infusing 4% methylene blue solution
DNA quantification
In both the normal group and the decellularized group, the uterus had an average dry weight of 30 mg. After enzymatic hydrolysis with protease K, genomic DNA was extracted [according to the instructions of the tissue genomic DNA extraction kit (DP304)]. The DNA absorbance value of the proposed sample was detected by an ELISA at a wavelength of 260 nm, and the tissue DNA content (ng/mg) was calculated at a five-fold absorbance value.
Hematoxylin and eosin (H&E) staining
The uteruses of the normal and decellularized groups were fixed with 4% paraformaldehyde, dehydrated with alcohol gradient, made transparent with xylene, soaked in wax, embedded, and sliced. After routine H&E staining, the normal and decellularized uteruses were observed under light microscopy.
Immunohistochemical staining
Immunohistochemical staining was used to detect the expression of collagen I, collagen IV, fibronectin, and laminin in the fresh and decellularized uterine wax tissue sections in accordance with the manufacturer’s instructions. After the tissue sections were blocked with 3% bovine serum protein, rabbit anti-rat laminin antibody, rabbit anti-rat type I collagen antibody, rabbit anti-rat type VI collagen antibody, and rabbit anti rat-fibronectin antibody (diluted at 1:200 with 0.1 M PBS) were added in batches and incubated at room temperature for 2 hours. Biotinylation goat anti-rabbit secondary antibody (diluted by 1:500 drops) was added and incubated at 37 ℃ for 30 minutes. After diaminobenzidine (DAB) coloration, H&E staining, alcohol dehydration, and gum sealing, the distribution and content of each type of protein was observed under the light microscope.
Transmission electron microscopy (TEM) observation
The normal and decellularized uteruses were cut into 1.0 mm3-sized tissue blocks, fixed overnight with 2.5% glutaraldehyde at 4 ℃, washed with PBS, fixed with osmic acid, stained with uranium acetate, dehydrated with acetone gradient, soaked with epoxy resin embedding agent, and embedded. Ultrathin sections were made and observed under TEM.
Collagen quantification by hydroxyproline (HxP) assay
An improved HxP assay was used to analyze the collagen contents, and it was estimated that HxP content accounts for 13.4% of the total collagen content. The lysate contained 80–100 mg of freeze-dried tissues and was mixed with 6 M hydrochloric acid (1,000 µL). After hydrolysis was performed at 100 ℃ for 5 hours, the mixture was centrifuged, and 50 µL of the hydrolysate was then added to 450 µL of sodium hydroxide citric acid buffer. This solution was further diluted with 4.75 mL of citric acid buffer. Next, 500 µL of each sample was incubated with 250 µL of chloramine-T reagent, and 250 µL of 6.2 M perchloric acid was added. After incubation with the solution, 250 µL of para-dimethylaminobenzaldehyde was added, and the samples were incubated at 60 ℃ for 15 minutes. A standard curve was generated using HxP (Sigma-Aldrich). The absorbances of the samples were measured at a wavelength of 550 nm in a 96-well plate using an ELISA reader (Tecan, Männedorf, Switzerland).
Quantitative analysis of cytokines in decellularized uteruses
Total protein was extracted from natural and decellularized uteruses using an ELISA kit (R&D Systems, USA). The concentrations of the cytokines, including EGF, TGF-β, and bFGF, were measured with an ELISA reader (Tecan, Männedorf, Switzerland) at 450 nm.
Statistical analysis
The quantitative data are expressed as the mean ± standard deviation. Significant differences among the groups were examined using the Wilcoxon rank-sum test for two-group comparisons, and an analysis of variance followed by post-hoc analysis for multiple group comparisons using SPSS 16.0 software (IBM, USA). A probability (P) value <0.05 was considered statistically significant.
Results
Establishment of the perfusion pathway
As Figure 1 shows, the abdominal cavity was opened, the external iliac artery in the right lower abdomen was located, and a number 10 polyethylene tube was inserted to the beginning of the uterine artery branch. The tube was then fixed properly, and the remaining branches were ligated.
Decellularization of the uterine scaffold
Before perfusion, the general appearance of the normal uterus was linear and tubular with a thin layer of connective tissue membrane covering the surface, and adipose connective tissue visible on the inner side, closely connected to each other. As the decellularization perfusion progressed, the structure of each layer of the uterus gradually became loose, and the tissue gradually became white and transparent. Finally, a clear and transparent decellularized uterine tissue was observable with the naked eye (Figure 2).
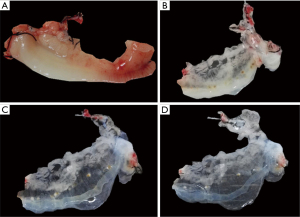
Methylene blue was infused, and the uterine scaffold gradually gained color, and the methylene blue gradually spread from the main vessel to each branch to the end branch. As Figure 3 shows, the decellularized vascular structure of the scaffold remained intact, and the outer capsule of the scaffold was clearly observable.

DNA quantification
A genomic DNA extraction kit (DP304) was used to identify and analyze the DNA content of the fresh uterine tissue and decellularized scaffold tissue. The results showed that the DNA content decreased from 1,515.3±243.9 ng/mg in the fresh uterus to 45.6±7.3 ng/mg in the decellularized scaffold, and the DNA residue was less than 5% of that of the normal uterine tissue, and the difference was statistically significant (P<0.001) (Figure 4).

H&E staining
Under H&E staining, the parenchymal cells of each layer of the uterine wall could be seen in the normal uterine tissue. The tissue had a regular morphology, compact arrangement, and clear structure, and showed deep staining of the cell nucleus, and shallow staining of the cytoplasm (Figure 5A,5B). Overall, the decellularized uterine scaffold appeared transparent and “honeycomb like”, and the ECM components were grid-like and stained. The morphology of the vascular main stem and small branch structures was preserved intact, and no deeply stained nuclear structure was found (Figure 5C,5D).

Immunohistochemical staining of ECM components
As Figure 6 shows, collagen I, collagen IV, fibronectin, and laminin were detected in a normal uterus. These four major collagen components were well preserved in the decellularized uterine scaffolds, and the fiber network morphology formed by each type of collagen was basically consistent with that of a normal uterus.

Measurement of total collagen content
To evaluate the retention of the ECM components in our perfusion process, we used a tissue sample acid hydrolysis HxP test kit to detect the HxP acid value of the fresh uterus and the decellularized scaffold to evaluate the total collagen content (HxP content accounts for 13.4% of the total collagen content). As Figure 7 shows, the results indicated that compared with the fresh uterine tissue (which contained 0.57±0.12 µg/mg), the HxP content of the decellularized scaffolds was significantly increased (to 0.88±0.10 µg/mg), and the difference between the two was statistically significant (P=0.001).

Electron microscope observations
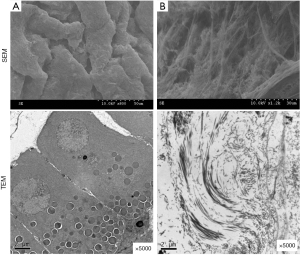
Under scanning electron microscopy (SEM), the parenchymal cells of the normal uterus were observed to be closely arranged. Under TEM, a rough endoplasmic reticulum could be seen in the cells. The nuclear boundary was clear, the basement membrane was complete, and the gap was narrow. After the cells were removed, the vascular network structure could be seen clearly by SEM, including various types of collagens and the basement membrane of the ECM. Under TEM, the cell structure and large organelle in the cells had disappeared (Figure 8).
Bioactivity evaluation of the decellularized scaffolds
To evaluate the biological activity of the decellularized scaffolds, we detected the content of cytokines EGF, bFGF, and TGF-β in the fresh uterine tissue and decellularized scaffolds. The results showed that after decellularization perfusion, the content of the cytokines EGF, bFGF, and TGF-β in the scaffold was decreased compared to that in the fresh tissues, however, most of these cytokines were retained. Specifically, 54% of the TGF-β was retained, 65.7% of the EGF was retained, and 85% of the bFGF was retained. The results indicate that the decellularized scaffold we prepared had good biological activity (Figure 9).

Discussion
The clinical application of uterine transplantation is in its infancy, however, it is more in line with medical ethics and ethics for women who become infertile due to uterine deficiency or uterine dysfunction than other methods, such as surrogacy (9). The severe shortage of donors and immune rejection have limited the clinical application of uterine transplantation. Research on uterine tissue engineering based on decellularized scaffolds may be an ideal method to resolve the shortage of donors. Due to the crucial role of the natural ECM obtained from decellularized biological scaffolds in mediating biophysical stimuli, biochemical signal transduction, and the formation of spatial stereostructures required for cell differentiation and maturation (10), research has been conducted on the decellularized reconstitution of organs such as the heart, liver, kidney, and lungs using decellularization technology (11-13). However, little domestic and international research has been conducted on the preparation and application of decellularized scaffolds for the uterus. This study used decellularization technology to optimize perfusion strategies and successfully prepared natural decellularized scaffolds of the rat uterus. After comprehensive evaluation, we found that the decellularized scaffolds retain ECM components, have good immunogenicity and biological activity, and could be used as an alternative biological carrier for uterine tissue regeneration engineering research.
It is generally believed that the following steps are key to the successful production of decellularized scaffolds: (I) thoroughly washing out the cells and cellular structural residues within the perfusion body; and (II) preserving the ECM components inside the perfusion body as much as possible (14). The traditional decellularized process focuses on mechanical agitation and freeze-thaw fragmentation. It often takes several days or even weeks to achieve decellularization, and this process cannot guarantee that the ECM components will be completely preserved without damage.
In recent years, domestic and foreign scholars have conducted in-depth research on the use of physical methods, chemical reagents, enzymology, the osmotic pressure method, and other different methods, and established different perfusion methods. Notably, different combinations of processes can achieve different decellularization effects (15,16). Based on current reports, we believe that we need to perform freeze-thaw and trypsin hydrolysis before perfusion, and the main chemical reagents are SDS and Triton X-100. SDS is an ionic eluent that is more effective for thicker organs such as the kidneys and heart, while Triton X-100 is a deionized eluent that is more efficient for thinner tissues such as blood vessels and skin (17). Because both have a certain destructive power on the ECM, it is crucial to optimize the perfusion ratio of both.
Kasravi et al. successfully prepared a rat whole liver acellular scaffold using different concentration gradients of SDS as eluents through the vascular perfusion pathway (18). However, their acellular efficiency was low and the process of decellularization took a long time to completed (up to 5 days). Moreover, while SDS, as an ionic eluent, had a good acellular effect, it also caused significant damage to the ECM structure. Next, Jalan-Sakrikar et al. proposed a new decellularized perfusion strategy using TritonX-100 as the main reagent at a concentration of 3%. They found that TritonX-100, which is a mild non-ionic eluent, not only achieved similar decellularized effects to those of SDS, but also reduced damage to the ECM (19). Thus, to minimize the damage of eluting agents to the scaffold in this experiment, we optimized the perfusion process and developed a decellularization strategy using TritonX-100 as the main eluting agent, supplemented with SDS short-term perfusion. The obtained decellularized scaffolds were identified and evaluated, and the results showed that after H&E staining and a comparative analysis of the DNA content, no cells or cell residues were found in the scaffolds. The identified DNA content was 45.6±7.3 ng/mg, which differed significantly from that of the normal control.
ECM components are important mediators for promoting cell differentiation and proliferation, and serve as the foundation for the natural biochemical properties of decellularized scaffolds. Our detection of the total collagen content and immunohistochemical analysis of four major collagen components indicated that the ECM components inside the scaffold were perfectly preserved after decellularization. The results of the methylene blue staining and electron microscopy observations also showed a clear vascular network and spatial microenvironment inside the decellularized scaffold, further proving the unique physical properties of the scaffold. In addition, multiple research reports have shown that the ECM also contains many growth factors (GFs) and bio-inducible factors that promote cell adhesion and tissue structure integration, repair, and proliferation (20,21). To further evaluate the biological activity of the scaffold, we detected the GFs (i.e., EGF, bFGF, and TGF) inside the scaffold. The results indicated that the decellularized scaffold effectively retained its internal EGF, bFGF, and TGF-β biological active factors. These molecules are essential in angiogenesis and can promote angiogenesis, cell development, neuronal growth, and the immune regulation of regeneration (22). However, our decellularized uterine scaffold also removes endothelial cells of the vessels, there will be lots of limitations in vivo transplantation studies due to the potential for thrombosis, In the future, research is needed on endothelial cell modification of decellularized scaffolds, and we hope to rebuild regenerated uterine tissue based on this decellularized scaffold.
Conclusions
We employed a novel perfusion strategy and successfully established an ideal decellularized uterine scaffold. Notably, the scaffold retained an ECM and vascular microenvironment that promote cell differentiation and maturation, and showed good biological activity. Thus, our scaffold should have good application in the research of uterine tissue regeneration engineering.
Acknowledgments
Funding: The study was supported by a grant from
Footnote
Reporting Checklist: The authors have completed the ARRIVE reporting checklist. Available at https://gs.amegroups.com/article/view/10.21037/gs-24-474/rc
Data Sharing Statement: Available at https://gs.amegroups.com/article/view/10.21037/gs-24-474/dss
Peer Review File: Available at https://gs.amegroups.com/article/view/10.21037/gs-24-474/prf
Conflicts of Interest: All authors have completed the ICMJE uniform disclosure form (available at https://gs.amegroups.com/article/view/10.21037/gs-24-474/coif). The authors have no conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved. The animal experiments were performed under a project license (No. wydw2016-0224) granted by Experimental Animal Ethics Committee of Wenzhou Medical University, in compliance with institutional guidelines for the care and use of animals.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Snow M, Vranich TM, Perin J, et al. Estimates of infertility in the United States: 1995-2019. Fertil Steril 2022;118:560-7. [Crossref] [PubMed]
- Aghajanova L, Altmäe S, Sokalska A. Editorial: Uterine factors associated with fertility impairment. Front Endocrinol (Lausanne) 2023;14:1307237. [Crossref] [PubMed]
- Horsey K, Gibson G, Lamanna G, et al. First clinical report of 179 surrogacy cases in the UK: implications for policy and practice. Reprod Biomed Online 2022;45:831-8. [Crossref] [PubMed]
- van Oosbree A, Von Wald T. Gestational Surrogacy and Ethical Considerations. S D Med 2023;76:72-5. [PubMed]
- Qi Q, Gu X, Zhao Y, et al. The status of surrogacy in China. Biosci Trends 2023;17:302-9. [Crossref] [PubMed]
- Brännström M, Racowsky C, Richards EG, et al. Absolute uterine infertility a cornelian dilemma: uterine transplantation or surrogacy? Fertil Steril 2023;119:918-29. [Crossref] [PubMed]
- Huang G, Zhao Y, Chen D, et al. Applications, advancements, and challenges of 3D bioprinting in organ transplantation. Biomater Sci 2024;12:1425-48. [Crossref] [PubMed]
- Lim LY, Ding SSL, Muthukumaran P, et al. Tissue engineering of decellularized pancreas scaffolds for regenerative medicine in diabetes. Acta Biomater 2023;157:49-66. [Crossref] [PubMed]
- Testa G, McKenna GJ, Johannesson L. The History of Uterus Transplantation, Rewritten. Ann Surg 2022;275:833-5. [Crossref] [PubMed]
- Kafili G, Kabir H, Jalali Kandeloos A, et al. Recent advances in soluble decellularized extracellular matrix for heart tissue engineering and organ modeling. J Biomater Appl 2023;38:577-604. [Crossref] [PubMed]
- Morrissey J, Mesquita FCP, Hochman-Mendez C, et al. Whole Heart Engineering: Advances and Challenges. Cells Tissues Organs 2022;211:395-405. [PubMed]
- Khazaei MR, Ibrahim R, Faris R, et al. Decellularized kidney capsule as a three-dimensional scaffold for tissue regeneration. Cell Tissue Bank 2024;25:721-34. [Crossref] [PubMed]
- Ramzan F, Salim A, Khan I. Osteochondral Tissue Engineering Dilemma: Scaffolding Trends in Regenerative Medicine. Stem Cell Rev Rep 2023;19:1615-34. [Crossref] [PubMed]
- Shang Y, Wang G, Zhen Y, et al. Application of decellularization-recellularization technique in plastic and reconstructive surgery. Chin Med J (Engl) 2023;136:2017-27. [Crossref] [PubMed]
- Borges MF, Maurmann N, Pranke P. Easy-to-Assembly System for Decellularization and Recellularization of Liver Grafts in a Bioreactor. Micromachines (Basel) 2023;14:449. [Crossref] [PubMed]
- Ibi Y, Nishinakamura R. Kidney Bioengineering for Transplantation. Transplantation 2023;107:1883-94. [Crossref] [PubMed]
- Afzal Z, Huguet EL. Bioengineering liver tissue by repopulation of decellularised scaffolds. World J Hepatol 2023;15:151-79. [Crossref] [PubMed]
- Kasravi M, Ahmadi A, Babajani A, et al. Immunogenicity of decellularized extracellular matrix scaffolds: a bottleneck in tissue engineering and regenerative medicine. Biomater Res 2023;27:10. [Crossref] [PubMed]
- Jalan-Sakrikar N, Brevini T, Huebert RC, et al. Organoids and regenerative hepatology. Hepatology 2023;77:305-22. [Crossref] [PubMed]
- Liu C, Pei M, Li Q, et al. Decellularized extracellular matrix mediates tissue construction and regeneration. Front Med 2022;16:56-82. [Crossref] [PubMed]
- Mavrogonatou E, Papadopoulou A, Pratsinis H, et al. Senescence-associated alterations in the extracellular matrix: deciphering their role in the regulation of cellular function. Am J Physiol Cell Physiol 2023;325:C633-47. [Crossref] [PubMed]
- Davis GE, Kemp SS. Extracellular Matrix Regulation of Vascular Morphogenesis, Maturation, and Stabilization. Cold Spring Harb Perspect Med 2023;13:a041156. [Crossref] [PubMed]



