
A preliminary study on the diagnostic value of contrast-enhanced ultrasound and micro-flow imaging for detecting blood flow signals in breast cancer patients
Highlight box
Key findings
• Contrast-enhanced ultrasound and micro-flow imaging (CEUS-MFI) is a new and promising imaging method for visualizing microvasculature in breast masses. CEUS-MFI improved the diagnostic capacity for breast cancer.
What is known and what is new?
• The distribution and morphology of microvessels are very important for the differentiation of benign and malignant breast masses. Presently, there is no reliable microvascular imaging technology to differentiate between malignant and benign breast masses.
• The application of CEUS-MFI can more clearly show the distribution and morphology of breast masses to improve the diagnostic accuracy compared with CEUS and color Doppler flow imaging.
What is the implication, and what should change now?
• CEUS-MFI can serve as a new microvascular imaging method for breast masses. There are some limitations in this study, with the relatively small sample size, a further large-scale, multicenter, randomized controlled trial is necessary to verify the clinical value of this new microvascular imaging.
Introduction
Breast cancer (BC), is the most common malignancy in women older than 40 years old, and is the dominant cause of cancer-related death worldwide. According to Global Cancer Statistics, BC is responsible for 11.6% of all new cancer diagnoses and 6.8% of all tumor-related deaths (1). Thus, early diagnosis is vital for the treatment and survival of patients. Ultrasound is an important imaging technique for evaluating morphological changes in breast lesions. Conventional ultrasound applies the Breast Imaging Reporting and Data System (BI-RADS) to conduct ultrasound diagnosis by revealing the ultrasonic features of breast masses (2,3). The BI-RADS is broadly used and offers a standardized interpretation for determining treatment suggestions; it includes a comprehensive description of the morphological characteristics of ultrasound, but lacks a description of vascular characteristics (4).
Among the features of breast lesions, blood flow is a crucial assessment criterion.
Angiogenesis plays a key role in the growth, formation and proliferation of BC cells. Breast malignancies usually involve rich and chaotic blood vessels. The distribution and morphology of microvessels are very important for the differentiation of benign and malignant breast masses (5,6). Color Doppler flow imaging (CDFI) is a conventional method for displaying blood flow in breast lesions, but it is difficult to distinguish microvessels (<0.1 mm in diameter) within tissue (7,8). Contrast-enhanced ultrasound (CEUS) can be used to evaluate the blood flow distribution in tumors effectively and provide perfusion information, which is helpful for enhancing diagnostic performance (9,10). The value of CEUS in the evaluation of breast masses is limited, owing to obvious overlaps in the perfusion types of malignant and benign masses. Micro-flow imaging (MFI) is a new ultrasound flow imaging technique in which advanced algorithms are used to suppress tissue motion while presenting microvessels and making images closer to real intralesional flow (11). Several studies have indicated that the MFI is superior to the CDFI for exploring microvessel signals and displaying vascular architecture (12,13). However, with increasing depth, the ability of the MFI to detect the blood vessels in the mass decreases.
Contrast-enhanced ultrasound and micro-flow imaging (CEUS-MFI) is an emerging technique that possesses the advantages of both CEUS and MFI. This technique offers the feasibility of simultaneously and continuously visualizing the microvascular architecture of breast lesions while observing the perfusion of blood vessels. However, as far as we know, the application of this technique has rarely been reported. In this study, we aimed to estimate the performance of CEUS-MFI in revealing microvascular characteristics within breast lesions, in comparison with that of CEUS and CDFI. We present this article in accordance with the STARD reporting checklist (available at https://gs.amegroups.com/article/view/10.21037/gs-24-264/rc).
Methods
Study design and participants
This prospective study was approved by the Institutional Review Board of The First Affiliated Hospital, College of Medicine, Zhejiang University Hospital (No. bc2022013). The study was conducted in accordance with the Declaration of Helsinki (as revised in 2013). Each patient provided written informed consent. From June 2022 to August 2022, a total of 106 consecutive patients who were evaluated as BI-RADS category 4 were included in our study. The exclusion criteria for patients were as follows: less than 18 years old; pregnant or lactating; had a history of breast surgery; had other malignancies; had endocrine system disorders that may influence the results of our research; had immune system diseases, serious cognitive impairment or a history of psychiatric disorders or had contraindications for ultrasound contrast agents. All patients subsequently underwent CDFI, CEUS, CEUS-MFI and histological examination. In this study, all breast tissue for pathological diagnosis was obtained from puncture biopsy or surgical excision.
Ultrasound examinations
Grayscale ultrasound was conducted with a high-frequency transducer (L18-4 phased array probe frequency 4–18 MHz) and a Philips EPIQ7 (Philips Medical Systems, Bothell, WA, USA), instrument equipped with CEUS and CEUS-MFI software. First, patients were placed in a supine position while breathing calmly and with their arms raised. Second, the breasts were thoroughly scanned via ultrasound. Once lesions were found, the general features of the breast masses which were determined according to the BI-RADS lexicon (14) were detected, and the best lesion image were selected on the largest plane. Subsequently, CDFI was performed to estimate the blood vessels around the region of interest.
CEUS and CEUS-MFI were performed as follows: the cut surface of a lesion with rich blood was selected for CEUS, which was conducted in double-amplitude contrast mode and 4.8 mL of the contrast agent SonoVue (Bracco, Milan, Italy) was rapidly injected via the antecubital vein, followed by 5.0 mL of sodium chloride solution. Subsequently, the whole examination process (at least 3 minutes) was recorded for analysis. The operation processes used for CEUS and CEUS-MFI were the same. CEUS-MFI requires switching to MFI mode on the basis of CEUS when the number of microbubbles is adequate.
Imaging analysis and diagnostic criteria
All breast masses were subjected to ultrasound examination by a single investigator (J.D.) with more than 12 years of experience. All the ultrasound images were evaluated by two sonographers; both of them (Q.C., H.W.) had more than 8 years of experience in reading ultrasound scans and CEUS images. They did not participate in ultrasound scans of breast tissue and did not know relevant information about the patients when reading the images. A third experienced radiologist evaluated the images in cases of disagreement. Before this study, these three sonographers were trained on predefined diagnostic criteria for vascular distribution and morphology.
According to previous studies (15,16), the intralesional vascular architecture depicted by CDFI and CEUS-MFI was classified into five types: (I) avascular pattern, which was short of blood vessels; (II) line-like pattern, which has few straight or slightly curved vessels inside the lesion; (III) branch-like pattern, which has flow signals inside the lesions with several microvessels branching; (IV) root hair-like pattern, which consisted of twisted and chaotic blood vessels inside the lesion with fewer than 2 thick and twisted vessels surrounding the lesion; (V) crab claw-like pattern, which was dominated by radial vessels and more than 2 thick and twisted vessels surrounding the lesion (Figure 1). In this study, the avascular pattern, line-like pattern and branch-like pattern were defined as benign, and the malignant root hair-like pattern and crab claw-like pattern were defined as malignant.
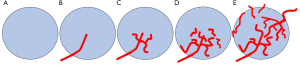
CEUS was used to identify breast lesions by analyzing qualitative features through the use of the 5-point score (17). Score 1: there was no-, hypo-enhancement of the mass, and the boundary between the lesion and adjacent parenchyma was clear; score 2: the mass and adjacent parenchyma were synchronized and iso-enhanced, and the boundary was not clear; score 3: early homogeneous/heterogeneous enhancement of mass with regular shape (round or oval), sharp edges, size equal to or less than conventional ultrasound; score 4: early heterogeneous enhancement, mass size larger than conventional ultrasound, irregular shape, with or without perfusion defects, with or without crab-claw-like enhancement; score 5: early, heterogeneous and typical crab-claw-like enhancement, irregular margins, mass size larger than conventional ultrasound, with or without perfusion defects. A score of 1–2 was considered benign, and a score of 3–5 was considered malignant.
Histopathological examination, which is considered the gold standard further confirmed the diagnosis.
Statistical analysis
Continuous variables are expressed as the mean ± standard deviation, while categorical variables were expressed as proportions. Student’s t-test and the Chi-squared test were used to estimate the statistical significance of differences between the benign and malignant group. Statistical tests of sensitivity and specificity were conducted by using the McNemar test for correlated proportions. Indeterminate results were considered false-positive or false-negative and incorporated into the final analysis. All the statistical analyses were conducted using SPSS 26.0 (IBM Corporation, Armonk, NY, USA). A two-sided P value <0.05 indicated statistical significance.
Results
General characteristics and pathologic results of breast lesions
This study included 106 patients with breast lesions (Figure 2). Histopathological examination revealed that 34 (32.1%) lesions were benign and 72 (67.9%) lesions were malignant. The detailed histopathological diagnoses are summarized in Table 1. As shown in Table 2, location was not significantly different between the benign and malignant groups (P=0.34). There were significant differences in size, age, and BI-RADS classification between the two groups (P=0.002, P=0.01 and P<0.001, respectively).
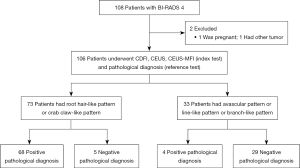
Table 1
| Histologic diagnosis | Number |
|---|---|
| Benign | 34 |
| Fibroadenoma | 12 |
| Intraductal papilloma | 11 |
| Hyperplasia | 7 |
| Chronic inflammation | 3 |
| Adenosis | 1 |
| Malignant | 72 |
| Invasive carcinoma | 48 |
| Intraductal carcinoma | 7 |
| Ductal carcinoma in situ | 3 |
| Intraductal papillary carcinoma | 5 |
| Invasive micropapillary carcinoma | 3 |
| Invasive lobular carcinoma | 2 |
| Medullary carcinomas | 2 |
| Mucinous carcinoma | 2 |
| Total | 106 |
Table 2
| Parameters | Benign | Malignant | P |
|---|---|---|---|
| Age (years) | 40.95±8.25 | 46.51±9.62 | 0.01 |
| Location | 0.34 | ||
| Left | 16 | 40 | |
| Right | 18 | 32 | |
| Size (mm) | 16.1±8.9 | 27.2±11.5 | 0.002 |
| BI-RADS classification | <0.001 | ||
| 4a | 20 | 8 | |
| 4b | 10 | 23 | |
| 4c | 4 | 41 |
Data are expressed as mean ± standard deviation or numbers. BI-RADS, Breast Imaging Reporting and Data System.
The microvascular patterns of breast masses showed by CEUS-MFI
As shown in Table 3, CEUS-MFI clearly delineated the microvascular structural patterns, which were classified into 5 patterns, of all 106 masses. There was significant difference in microvascular architectural patterns between benign and malignant breast masses (P<0.001).
Table 3
| Features | Benign (n=34) | Malignant (n=72) | P |
|---|---|---|---|
| Microvascular architectural pattern, n (%) | <0.001 | ||
| Avascular | 2 (5.9) | 0 (0.0) | |
| Line-like | 7 (20.6) | 2 (2.8) | |
| Branch-like | 20 (58.8) | 2 (2.8) | |
| Root hair-like | 4 (11.8) | 33 (45.8) | |
| Crab claw-like | 1 (2.9) | 35 (48.6) | |
CEUS-MFI, contrast-enhanced ultrasound and micro-flow imaging.
Diagnostic performance of the CDFI, CEUS and CEUS-MFI
In this study, the CEUS-MFI showed more microvascular structures than did the CEUS and CDFI (Figures 3,4). According to the definitions of benign and malignant breast masses in this study, the sensitivity, specificity, and accuracy of CEUS-MFI were 94.4%, 85.3%, and 91.5%, respectively. The sensitivity, specificity, and accuracy of CEUS were 88.9%, 82.4%, and 86.8%, respectively. The sensitivity, specificity, and accuracy of the CDFI were 61.1%, 76.5% and 66.0%, respectively, as listed in Table 4. There were significant differences in accuracy between the CEUS-MFI and CEUS (P=0.01). There were significant differences in accuracy between the CEUS-MFI and CDFI (P<0.001).
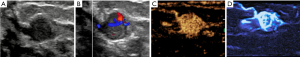
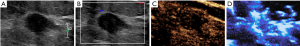
Table 4
| Method | Sensitivity (%) | Specificity (%) | Accuracy (%) |
|---|---|---|---|
| CDFI | 61.1 (44/72) | 76.5 (26/34) | 66.0 (70/106) |
| CEUS | 88.9 (64/72) | 82.4 (28/34) | 86.8 (92/106) |
| CEUS-MFI | 94.4 (68/72) | 85.3 (29/34) | 91.5 (97/106) |
Data are expressed as percentages (numbers). CDFI, color Doppler flow imaging; CEUS, contrast-enhanced ultrasound; CEUS-MFI, contrast-enhanced ultrasound and micro-flow imaging.
In this study, 4 breast masses (3 fibroadenomas, 1 chronic inflammation) were diagnosed as malignant by CEUS due to rich blood supply and blurred boundaries, while CEUS-MFI avoided misdiagnosis by reassessing microvascular architectural patterns. Four breast masses (2 mucinous carcinomas, 2 medullary carcinoma) were diagnosed as benign by CEUS due to non-enhancement and clear boundary, while CEUS-MFI avoided missed diagnosis by reassessing microvascular structure.
Re-assessment of ultrasound (US) BI-RADS 4a masses using CEUS-MFI
CEUS-MFI re-assessed 28 US BI-RADS 4a masses (20 benign and 8 malignant masses). Nineteen US BI-RADS 4a masses were downgraded by the features of CEUS-MFI without missing any malignant tumors, with biopsy rate decreased from 100% (28/28) to 32.1% (9/28).
Discussion
Understanding tumor angiogenesis is helpful for diagnosing of malignant lesions, evaluating therapeutic response and determining patient prognosis. The detection of vascularization in breast lesions is significantly associated with malignancy (18,19). The results of this study confirmed that the CEUS-MFI may have better diagnostic value than the CEUS or CDFI for distinguishing malignant and benign breast masses.
CDFI is a commonly used technique for noninvasive detection of blood vessels and blood flow information in breast masses. It can observe the degree of vascularity inside and around breast lesions (20). Conventional CDFIs are usually limited by their angular dependency and poor signal-to-noise ratios, and low-velocity flows cannot be evaluated (7). A previous study demonstrated that the detection of vascularity by CDFI cannot be used as a major indicator of BC (19). Our study revealed that the CDFI yielded high false-positive and false-negative results for distinguishing benign and malignant masses. This result is in agreement with that of a previous study (21). Hence, the ability of the CDFI to evaluate microvessels was very limited.
Among the several blood flow imaging methods used for ultrasound, CEUS is the most commonly used imaging tool for the diagnosis of BC and can distinguish between benign and malignant breast masses (22,23). The benign features of CEUS included sharp margins, no enhancement or uniform centrifuge enhancement. In contrast, malignant lesions have blurred edges, uneven centripetal enhancement, filling defects, and vascular distortion (24). In our study, we also detected similar perfusion characteristics of breast lesions. Compared with those of CDFI, the sensitivity, specificity and accuracy of CEUS were improved by 27.8%, 5.9% and 20.8%, respectively. However, with the development of CEUS for detecting blood flow signals, there has been an increasing number of studies on the overlap between the characteristics of benign and malignant lesions (17). Benign inflammatory lesions with abundant blood flow or fibroadenomas may be mistaken for malignant tumors. Similarly, hypovascular malignancies such as ductal carcinoma in situ (DCIS) may be misjudged as benign (25). The overlap of benign and malignant lesion features led to a decrease in the diagnostic effectiveness of CEUS. Therefore, the combination of perfusion features in breast masses with the morphology and distribution characteristics of blood vessels observed by MFI can provide additional information for the differential diagnosis of BCs to a certain extent.
CEUS-MFI is an emerging imaging technique that combines the strengths of both MFI and CEUS. The technique involves interspersed MFI send/receive pulse sequences between the pulse sequences needed to constitute CEUS frames, but the amplitude of the CEUS-MFI pulses is much lower than that of regular MFI pulses to minimize microbubble destruction. The small amount of microbubble destroyed by CEUS-MFI may have a little influence on the results of microvascular structure detection. This technique can record the dynamic perfusion process of tissue and continuously reveal the newly formed microvascular morphology and distribution of breast masses at the same time. The structure of newly formed blood vessels in BC tissue is different from that in normal tissue because of the special branching pattern of these vessels. Several studies have demonstrated different patterns of blood flow distribution in benign and malignant lesions. A lack of blood vessels, few straight or slightly curved vessels, and a branch-like pattern were considered benign, whereas twisted and chaotic blood vessels and radial vessels were considered malignant (12,15). There was only one previous study evaluating liver tumor microvascular structure using the CEUS-MFI method; compared with CEUS, CEUS-MFI can better describe vascular branching details and microvascular structure (26). In this study, 3 patients with highly enhanced fibroadenomas with poorly defined borders and 1 patient with chronic inflammatory breast masses were classified as malignant by CEUS, while CEUS-MFI was used to diagnose benign breast masses due to the presence of flow signals inside the lesions with branching of several microvessels. Two patients with mucinous carcinomas showed no change in size and hypoenhancement after CEUS, two patients with medullary carcinoma showed no change in size and homogenous enhancement with clear boundaries after CEUS; these were all defined as benign lesions, while CEUS-MFI was diagnosed as a malignant breast mass due to the multiple twisted surrounding blood vessels. The pathological results were the same as those of CEUS-MFI. Our results also indicated that CEUS-MFI is superior to CEUS and CDFI for comprehensively revealing the vascular details of breast tumors. Therefore, CEUS-MFI contributes to accurately recognizing the types of certain breast lesions by revealing the structural features of mass microvessels. The higher sensitivity, specificity and accuracy may be related to the fact that in CEUS-MFI, microflow imaging is conducted on the basis of CEUS.
When evaluating a low-suspect mass, radiologists are always reluctant to recommend a biopsy. In this study, we found that the features of CEUS-MFI could help decrease biopsy rate by 67.9% of US BI-RADS 4a without malignant masses missed. Our findings suggest that CEUS-MFI should be routinely used to assess the vascularity of US BI-RADS 4a. However, our study included only a small number of BI-RADS 4a masses. In US BI-RADS 4b and US BI-RADS 4c, CEUS-MFI is one of the important means to identify benign and malignant lesions.
In a word, pathological examination or surgical treatment may be avoided for some BI-RADS category 4 masses that do not need clinical treatment. It is showed that the microvascular pattern of CEUS-MFI was a very credible feature, and it might improve the accuracy and confidence of radiologists in the diagnosis of breast masses.
There are some limitations in this study. First, this was a small preliminary study conducted at one center. Second, the sample size of our study might be small, and the pathological categories were not comprehensive enough; moreover, some infrequent categories of BC were lacking. Third, all the breast lesions were BI-RADS category 4. The exclusion of BI-RADS category 3 or 5 breast lesions may have resulted in selection bias. Fourth, all ultrasound examinations performed by one sonographer may have affected the reliability of the results. Finally, specific ultrasound machines provide CEUS-MFI imaging. These limitations need to be avoided in further studies.
Conclusions
In summary, our preliminary study indicated that CEUS-MFI imaging is a new and useful ultrasound technique for evaluating microvascular types and vessel distribution pattern in breast lesions. CEUS-MFI improves the diagnostic ability for benign and malignant breast masses.
Acknowledgments
Funding: The work was supported by grants from
Footnote
Reporting Checklist: The authors have completed the STARD reporting checklist. Available at https://gs.amegroups.com/article/view/10.21037/gs-24-264/rc
Data Sharing Statement: Available at https://gs.amegroups.com/article/view/10.21037/gs-24-264/dss
Peer Review File: Available at https://gs.amegroups.com/article/view/10.21037/gs-24-264/prf
Conflicts of Interest: All authors have completed the ICMJE uniform disclosure form (available at https://gs.amegroups.com/article/view/10.21037/gs-24-264/coif). All authors report the grants from the National Natural Science Foundation of China (No. 81971623), the Taizhou Social Development Science and Technology Plan Project (No. 23ywb18). Q.C. is an employee of the Zhejiang CuraWay Medical Technology Co., Ltd. The authors have no other conflicts of interest to declare.
Ethical Statement:
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Sung H, Ferlay J, Siegel RL, et al. Global Cancer Statistics 2020: GLOBOCAN Estimates of Incidence and Mortality Worldwide for 36 Cancers in 185 Countries. CA Cancer J Clin 2021;71:209-49. [Crossref] [PubMed]
- Spick C, Bickel H, Polanec SH, et al. Breast lesions classified as probably benign (BI-RADS 3) on magnetic resonance imaging: a systematic review and meta-analysis. Eur Radiol 2018;28:1919-28. [Crossref] [PubMed]
- Luo J, Cao Y, Nian W, et al. Benefit of Shear-wave Elastography in the differential diagnosis of breast lesion: a diagnostic meta-analysis. Med Ultrason 2018;1:43-9. [Crossref] [PubMed]
- Kim S, Lee HJ, Ko KH, et al. New Doppler imaging technique for assessing angiogenesis in breast tumors: correlation with immunohistochemically analyzed microvessels density. Acta Radiol 2018;59:1414-21. [Crossref] [PubMed]
- Zhan J, Diao XH, Jin JM, et al. Superb Microvascular Imaging-A new vascular detecting ultrasonographic technique for avascular breast masses: A preliminary study. Eur J Radiol 2016;85:915-21. [Crossref] [PubMed]
- Chen L, Zhan J, Diao XH, et al. Additional Value of Superb Microvascular Imaging for Thyroid Nodule Classification with the Thyroid Imaging Reporting and Data System. Ultrasound Med Biol 2019;45:2040-8. [Crossref] [PubMed]
- Schroeder RJ, Bostanjoglo M, Rademaker J, et al. Role of power Doppler techniques and ultrasound contrast enhancement in the differential diagnosis of focal breast lesions. Eur Radiol 2003;13:68-79. [Crossref] [PubMed]
- Zhu YC, Zu DM, Zhang Y, et al. A comparative study on superb microvascular imaging and conventional ultrasonography in differentiating BI-RADS 4 breast lesions. Oncol Lett 2019;18:3202-10. [Crossref] [PubMed]
- Lekht I, Gulati M, Nayyar M, et al. Role of contrast-enhanced ultrasound (CEUS) in evaluation of thermal ablation zone. Abdom Radiol (NY) 2016;41:1511-21. [Crossref] [PubMed]
- Hu W, Dong Y, Zhang X, et al. The clinical value of Arrival-time Parametric Imaging using contrast-enhanced ultrasonography in differentiating benign and malignant breast lesions. Clin Hemorheol Microcirc 2020;75:369-82. [Crossref] [PubMed]
- Han H, Ji Z, Ding H, et al. Assessment of blood flow in the hepatic tumors using non-contrast micro flow imaging: Initial experience. Clin Hemorheol Microcirc 2019;73:307-16. [Crossref] [PubMed]
- Yongfeng Z, Ping Z, Wengang L, et al. Application of a Novel Microvascular Imaging Technique in Breast Lesion Evaluation. Ultrasound Med Biol 2016;42:2097-105. [Crossref] [PubMed]
- Park AY, Seo BK, Cha SH, et al. An Innovative Ultrasound Technique for Evaluation of Tumor Vascularity in Breast Cancers: Superb Micro-Vascular Imaging. J Breast Cancer 2016;19:210-3. [Crossref] [PubMed]
- Mercado CL. BI-RADS update. Radiol Clin North Am 2014;52:481-7. [Crossref] [PubMed]
- Xiao XY, Chen X, Guan XF, et al. Superb microvascular imaging in diagnosis of breast lesions: a comparative study with contrast-enhanced ultrasonographic microvascular imaging. Br J Radiol 2016;89:20160546. [Crossref] [PubMed]
- Diao X, Zhan J, Chen L, et al. Role of Superb Microvascular Imaging in Differentiating Between Malignant and Benign Solid Breast Masses. Clin Breast Cancer 2020;20:e786-93. [Crossref] [PubMed]
- Boca Bene I, Dudea SM, Ciurea AI. Contrast-Enhanced Ultrasonography in the Diagnosis and Treatment Modulation of Breast Cancer. J Pers Med 2021;11:81. [Crossref] [PubMed]
- Zhu Q, Cronin EB, Currier AA, et al. Benign versus malignant breast masses: optical differentiation with US-guided optical imaging reconstruction. Radiology 2005;237:57-66. [Crossref] [PubMed]
- del Cura JL, Elizagaray E, Zabala R, et al. The use of unenhanced Doppler sonography in the evaluation of solid breast lesions. AJR Am J Roentgenol 2005;184:1788-94. [Crossref] [PubMed]
- Moustafa AF, Cary TW, Sultan LR, et al. Color Doppler Ultrasound Improves Machine Learning Diagnosis of Breast Cancer. Diagnostics (Basel) 2020;10:631. [Crossref] [PubMed]
- Huang C, Zhang W, Gong P, et al. Super-resolution ultrasound localization microscopy based on a high frame-rate clinical ultrasound scanner: an in-human feasibility study. Phys Med Biol 2021;66: [Crossref] [PubMed]
- Cao X, Xue J, Zhao B. Potential application value of contrast-enhanced ultrasound in neoadjuvant chemotherapy of breast cancer. Ultrasound Med Biol 2012;38:2065-71. [Crossref] [PubMed]
- Saracco A, Szabó BK, Tánczos E, et al. Contrast-enhanced ultrasound (CEUS) in assessing early response among patients with invasive breast cancer undergoing neoadjuvant chemotherapy. Acta Radiol 2017;58:394-402. [Crossref] [PubMed]
- Zhao H, Xu R, Ouyang Q, et al. Contrast-enhanced ultrasound is helpful in the differentiation of malignant and benign breast lesions. Eur J Radiol 2010;73:288-93. [Crossref] [PubMed]
- Wan C, Du J, Fang H, et al. Evaluation of breast lesions by contrast enhanced ultrasound: qualitative and quantitative analysis. Eur J Radiol 2012;81:e444-50. [Crossref] [PubMed]
- Han H, Ji Z, Huang B, et al. The Preliminary Application of Simultaneous Display of Contrast-Enhanced Ultrasound and Micro-Flow Imaging Technology in the Diagnosis of Hepatic Tumors. J Ultrasound Med 2023;42:729-37. [Crossref] [PubMed]


