
Endoscopic transoral approach to accessory parotid tumors-an updated approach and literature review
Highlight box
Key findings
• There were 10 patients who underwent endoscopic transoral approach for accessory parotid tumors at West China Hospital of Stomatology and the First Affiliated Hospital of Xiamen University from 2022 to 2024.
• Among 119 articles identified through database searches, 7 were included in the final analysis, providing medical information of 30 patients.
• There were five patients reporting postoperative complications: swelling, hydrops, and transient facial palsy.
• The average operation time of endoscopic transoral approach to accessory parotid tumors was 54.70±7.73 minutes.
What is known and what is new?
• Surgical resection is considered the best treatment option for an accessory parotid mass. The conventional surgical method requires a large incision while the resulting facial scars often fail to meet patients’ aesthetic expectations.
• Our study conducts a retrospective investigation of patients who underwent an endoscopic transoral approach for accessory parotid tumors to avoid facial scars.
What is the implication, and what should change now?
• Endoscopic resection of accessory parotid mass through a transoral approach avoids an external scar, has good aesthetics, high patient satisfaction, and fewer postoperative complications.
IntroductionOther Section
Background
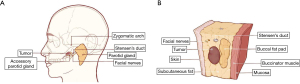
The accessory parotid gland (APG) is a distinct salivary gland located between Stensen’s duct and the zygomatic arch, separate from the main parotid gland (1) (Figure 1). It drains saliva into the Stensen’s duct through several small tributaries. According to the literature, only 21% to 32.1% of the general population have APGs (2). The incidence of accessory parotid tumors is rare, occurring in 1% to 7.7% of cases (3). Nearly 75% of these tumors are benign, with pleomorphic adenoma being the most common type. Among malignant accessory parotid tumors, mucoepidermoid carcinoma is the most prevalent (1,4).
Patients complaining of a mid-cheek mass should be suspected with accessory parotid tumors (5,6). Surgical resection is considered the best treatment option for an accessory parotid mass (3). To protect the nerves and ducts around the APG, the conventional surgical method requires a large incision in the face, full exposure of the operative area, blunt separation, and complete removal of the tumors under the naked eye (7,8). The most common surgical approaches are a preauricular S-shaped incision and a face-lift incision (4,9) (Figure 2). However, the resulting facial scars often fail to meet patients’ aesthetic expectations. Although these approaches provide adequate exposure of the operative field, they also pose a risk of complications such as facial nerve injury, Frey’s syndrome, salivary fistula, and auriculotemporal nerve injury, which can lead permanent loss of sensation in this area (10-12).

Rationale and knowledge gap
Surgeons use a transoral approach to avoid scarring on the face. However, due to the narrow cavity and limited operative field, tumors cannot be completely dissected under the naked eye, increasing the risk of damage to the facial nerves and Stensen’s duct (1). With the popularization of endoscopic surgery in head and neck oncology, surgeons have begun performing endoscopic-assisted APG tumor resections using approaches such as the high submandibular approach, preauricular minimal incision, and so on (10,13). Compared to traditional surgery, endoscopic salivary gland surgery has less blood loss and better cosmesis (14). Although these techniques reduce scarring, provide a clear surgical field, and result in fewer complications, an extraoral incision is still required.
Objective
In this study, we aim to present the endoscopic transoral resection surgical technique and clinical outcomes of accessory parotid tumors.
MethodsOther Section
Patients
The study was conducted in accordance with the Declaration of Helsinki (as revised in 2013). The study was approved by the Ethics Committee of West China Stomatology Hospital, Sichuan University (No. WCHSIRB-D-2020-311-R1) and individual consent for this retrospective analysis was waived. Participants were informed of the nature of the data collected and the scope of this survey project in the invitation letter, consent is implied if a participant complete and submit their response. We did not create a separate form to specifically document consent. Patients between January 2022 and April 2024 at the First Affiliated Hospital of Xiamen University and West China Hospital of Stomatology, Sichuan University, were included in this retrospective observational study. The inclusion criteria were: (I) patients presenting with a mass in the APG; (II) surgical process performed via an endoscopic transoral approach; and (III) agreement to long-term follow-up. Patients who did not undergo an endoscopic transoral approach and those unwilling to participate in the study were excluded. After obtaining informed consent, data on demographic information, tumor size, pathology, operation time, follow-up details, and postoperative complications were collected from the patient’s medical records. To ensure privacy, personal information such as patients’ names, telephone numbers, ID numbers, and home addresses were removed.
Surgery
The operative procedures were recorded and analyzed. They were then divided into different components to develop a standardized treatment protocol. The preoperative examination, operative steps from incision design to wound closure, and postoperative care were described in a standardized manner through a classic case.
Review
The literature screening process was conducted in accordance with the Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) guidelines (15) (Figure 3). A systematic and structured literature search was carried out in the PubMed, China National Knowledge Infrastructure (CNKI), and Web of Science databases using the following search terms: “accessory parotid gland” in combination with “resection”. The databases were searched up to April 2024. The inclusion criteria for the literature were: (I) clinical studies or case reports on endoscopic transoral resection; and (II) provision of detailed information about the patient’s operation. Reviews, meta-analyses, technical presentations, and studies lacking detailed information were excluded. After removing duplicates, two investigators (G.W. and H.T.) independently screened all titles and abstracts according to the criteria. Potentially eligible full-text articles were then reviewed independently. In case of disagreements, a third investigator (B.Y.) was consulted for the final decision. Basic information about the patients, tumor size, pathology, follow-up, and complications were extracted and summarized alongside our cases.
Statistical analysis
Demographic characteristics, operation time, and tumor characteristics were reported as means ± standard deviations or medians. Statistical analyses were conducted using IBM SPSS Statistics (version 26, IBM, Armonk, NY, USA).
ResultsOther Section
Patients demographics
From the beginning of January 2022 to the end of April 2024, ten patients underwent endoscopic transoral accessory parotid mass excisions. The details and clinical outcomes of these patients are listed in Table 1. There are five male patients and five female patients. Ages ranged from 21 to 71 years, with a mean age of 42.50±15.56 years. One patient underwent preoperative magnetic resonance imaging (MRI), while five patients had preoperative spiral computed tomography (CT) of the head and neck. None of the patients received a fine-needle aspiration biopsy. The primary complaint of these patients was the presence of a slow-growing, painless mass located in the middle of the cheek.
Table 1
| Sex | Age (years) | Size (cm) | Operative time (min) | Pathology | Span | Complication |
|---|---|---|---|---|---|---|
| M | 34 | 3.0×1.5×1.0 | 65 | Fibroangioma | 3 months | Swelling |
| F | 50 | 2.0×1.5×1.5 | 55 | Pleomorphic adenoma | 3 months | None |
| M | 41 | 1.9×1.0×1.0 | 50 | Pleomorphic adenoma | 3 months | None |
| F | 40 | 2.0×1.5×1.0 | 58 | Pleomorphic adenoma | 3 months | None |
| M | 43 | 1.5×1.1×1.3 | 70 | Angiocavernoma | 3 months | None |
| F | 63 | 1.5×1.2×1.0 | 48 | Parotid cyst | 3 months | Hydrops |
| F | 21 | 1.5×1.0×1.0 | 46 | Pleomorphic adenoma | 3 months | Hydrops |
| M | 72 | 2.5×1.5×1.0 | 50 | Basal cell adenomas | 3 months | None |
| M | 30 | 2.1×1.3×1.0 | 55 | Mucoepidermoid carcinoma | 3 months | None |
| F | 31 | 1.0×1.0×0.9 | 50 | Pleomorphic adenoma | 3 months | Hydrops |
M, male; F, female.
Tumor characteristics
Lesion diameters varied from 1 to 3 cm, averaging 1.90±0.57 cm. There were 50% of the patients diagnosed with pleomorphic adenoma. In addition, there was one case of parotid cyst, fibroangioma, angiocavernoma, and basal cell adenomas. Unfortunately, one patient was diagnosed with mucoepidermoid carcinoma according to intraoperative frozen biopsy, and a complete parotid lobectomy was performed with traditional standard S-shaped incision instead.
Case information
A 43-year-old man presented with asymptomatic mid-cheek masses that had been slowly growing for 21 years. MRI and contrast-enhanced CT revealed an abnormal mass in the APG (Figure 4A-4E). The size of the mass was about 1 cm × 1 cm, with clear boundaries. On clinical examination, a painless, mobile, medium-sized mass was palpated in the patient’s right cheek area. Examination of facial nerve function revealed no pathologic alterations, and his ability to blink, frown, and smile was unaffected (Figure 4F). After discussing the risks of the procedure and the possibility of avoiding an external scar with the patient, we proposed performing surgery via an endoscopic transoral approach with superficial nerve monitoring.

Surgical specifics
The operation was performed by a team consisting of the chief surgeon, an endoscopy assistant, a surgical assistant, and a scrub nurse. After administering appropriate anesthesia to the patient, the preparatory work involved placing electrodes in the orbicular muscle of the eye and mouth for facial nerve monitoring. The patient’s head was then rotated, and his mouth was opened to provide a clear view of the oral cavity. Anesthetic (lidocaine) and vasoconstrictor (epinephrine) were initially injected into the mid-cheek at a ratio of 1:100,000 to optimize better hemostasis (16).
During the operation, the first assistant held the endoscope camera while the chief surgeon performed the surgery (Figure 5). The first step was to mark the body surface projection of the Stensen’s duct and its opening site to facilitate easy location throughout the procedure (Figure 6A). An incision of 2.5–3 cm was made parallel to the Stensen’s duct, extending through the oral mucosa and subcutaneous layer to expose the buccinator. After cutting the buccinator, the oral mucosal flap was elevated using a goiter retractor, allowing easy dissection of the surgical plane under the endoscope (4 mm, 0 degrees rigid scope; KARL STORZ SE & Co. KG, Tuttlingen, Germany) (Figure 6B).

The endoscope’s focal length and magnification were adjusted to obtain a clear field. The inner side of the tumor was seen to be surrounded by fat tissue. The buccal fat pad was then removed using an ultrasonic scalpel under the endoscope (Figure 6C) Stensen’s duct was dissected and found above the accessory parotid mass. The upper and lower buccal branches of the face nerve, located on either side of the mass, were preserved after complete dissection with the aid of a nerve stimulator (Figure 6D). Blunt and sharp dissections along the tumor capsule were performed under the endoscope. Finally, the tumor was pulled outward to sever its connections with the surrounding tissue (Figure 6E).
After rinsing the operative cavity, the wound was sutured with 3-0 absorbable suture material, and a drainage strip was inserted. The strip was removed after 2 days. The mass measured 15 mm × 11 mm × 13 mm (Figure 6F), and the histologic diagnosis was angiocavernoma.
Clinical outcomes
The surgery times for the 10 patients ranged from 46 to 70 minutes, with an average operation time of 54.70±7.73 minutes. A drainage strip was placed in the wound post-surgery, and a pressure bandage was applied on the first day. The drainage strip was removed on the second day, and intraoral sutures were taken out one week after surgery. At the 2-week follow-up, 10% of patients reported persistent swelling in the operative area, which gradually resolved after one week of intravenous antibiotic treatment. Hydrops occurred in 30% of the patients and this was managed with re-drainage and pressure dressing. At 3 months after surgery, none of the patients complained hydrops or swelling. Importantly, there were no instances of facial nerve damage among the patients.
Literature review
After searching the databases, a total of 119 papers were obtained from which 22 papers were selected for full-text screening based on titles and abstracts. Fifteen articles were excluded, because they did not answer the focused question. Ultimately, seven studies were included in our analysis. Among the seven articles, two were observational studies and five were case reports (2,3,5,17-20). The case information from these articles was extracted, resulting in a total of 30 patients included in the study. The detailed information of the 30 patients were listed in Table 2. Of the 30 patients, 15 were males and 15 were females, with a mean age of 39.30±10.32 years old. Preoperative examination included ultrasound, CT, MRI, and FNAC (fine-needle aspiration cytology). CT and MRI were primarily used to assess the extent and size of the lesions, as well as their proximity to adjacent structures. FNAC was instrumental in determining the lesions’ nature preoperatively. The maximum diameter of the masses ranged from 1 to 4 cm, with a median size of 2.8 cm. Consistent with previous studies, 83.33% of the pathological results were pleomorphic adenomas. Additionally, there were cases of two parotid cyst, two basal cell adenomas, and one fibroma. According to Kim et al.’s study, the average operation times for the transoral approach was 47.5±9.93 minutes, significantly shorter than the parotidectomy approach group at 82.72±15.86 minutes (2). Besides, the operation times for the transoral approach closely matched our results. Other studies did not specify operation times. The follow-up period ranged from 2 weeks to 13 months, with 6 months being sufficient to observe postoperative complications. Only one experienced transient facial palsy.
Table 2
| Studies | Sex | Age (years) | Preoperative examination | Size (cm) | Pathology | Span | Complication |
|---|---|---|---|---|---|---|---|
| Lenzi 2022 (5) | F | 76 | Ultrasound, FNAC, MRI | 3.0×3.0×2.0 | Pleomorphic adenoma | 6 months | None |
| Mani 2019 (18) | F | 43 | Ultrasound, FNAC | 1.0×1.0 | Pleomorphic adenoma | 8 months | None |
| Woo 2016 (19) | M | 55 | FNAC, CT | 4.0×4.0 | Pleomorphic adenoma | 6 months | None |
| Schmutzhard 2007 (3) | F | 42 | MRI | 2.5×1.3×1.8 | Pleomorphic adenoma | 6 months | None |
| Voora 2024 (17) | M | 37 | FNAC, MRI | 4.0×3.0 | Pleomorphic adenoma | 2 weeks | None |
| Kim 2020 (2) | F:M (9:11) | 34.3±8.61 | FNAC, CT | 2.8±0.89 | Pleomorphic adenoma:basal cell adenomas:parotid cyst:fibroma (16:1:2:1) | 13.4±1.27 months | Transient facial palsy (5%) |
| Wang 2023 (20) | F | 35 | Ultrasound, CT or MRI | 2.5 | Pleomorphic adenoma | 6 months | None |
| M | 45 | Ultrasound, CT or MRI | 2.8 | Pleomorphic adenoma | 6 months | None | |
| F | 48 | Ultrasound, CT or MRI | 1.3 | Pleomorphic adenoma | 6 months | None | |
| F | 61 | Ultrasound, CT or MRI | 2.8 | Pleomorphic adenoma | 6 months | None | |
| M | 57 | Ultrasound, CT or MRI | 3.5 | Basal cell adenomas | 6 months | None |
F, female; FNAC, fine-needle aspiration cytology; MRI, magnetic resonance imaging; M, male; CT, computed tomography.
DiscussionOther Section
Key findings, explanations & comparison to prior research
The incidence of APG tumors is relatively rare, and surgical resection is currently the best treatment option (1). The standard S-shaped incision is commonly used for excision of parotid tumors (1,7). This technique involves making a preauricular long incision, lifting the buccal flap to fully expose the accessory parotid tissue, and resecting the tumor under direct vision (6). This fully exposed field allows for the precise dissection and preservation of the facial nerve and Stensen’s duct.
However, the standard S-shaped incision leaves an external scar, which often leads to patient dissatisfaction with their appearance (Figure 2A). To address this issue, various minor incisions have been developed for the resection of accessory parotid tumors, including face-lift incision (9), modified Blair’s incision (21), preauricular right angle incision (22), and the mid-cheek incision (23) (Figure 2B-2E). These approaches improve aesthetics by reducing the length of the incisions or by concealing their location. Nevertheless, these methods often require complex plastic closure, which at the same time is not aesthetically pleasing (24). The narrower surgical field makes it more challenging for the surgeon to clearly dissect the facial nerve and Stensen’s duct, thereby increasing the risk of nerve and duct injury.
The anatomy of the accessory parotid tumor is relatively centralized, with the lateral aspect covered by skin and subcutaneous tissue, and the internal aspect covered by the buccal fat pad and mucous membrane (2,25) (Figure 1B). Therefore, the transoral approach can easily reach the anatomical level of the APG. To avoid surface scars, some surgeons perform the resection of accessory parotid tumors through a transoral approach (17). This method involves an incision in the middle of the buccal mucosa, eliminating external scar. Nevertheless, the transoral incision has its drawbacks. The narrow operating space in the oral cavity increases the risk of damaging the facial nerve and Stensen’s duct, leading to a higher incidence of complications (26). Thus, a paradox arises between minimizing complications and achieving aesthetic outcomes.
With the development of endoscopic techniques in head and neck oncology, the application of endoscopy to the transoral approach has gradually been put into practice (5). Endoscopic techniques provide clear visualization in narrow spaces (27,28). With the assistance of an endoscope, surgeons can see the adjacent tissues of the mass and can dissect and preserve important anatomical structures such as Stensen’s duct and facial nerve, which reduces the complications and improves aesthetics (2,18).
In this study, of the 40 patients who underwent an endoscopic transoral approach for accessory parotid tumors, only five reported postoperative complications, and all recovered within a period after surgery without permanent damage (Tables 1,2). Three patients exhibited hydrops within the surgical wound one-week post-operation, presumably due to the persistence of unhealed dead space following tumor excision. These patients were managed with oral drainage and sustained compressive bandaging over the course of one week. As the dead space resolved, the hydrops progressively diminished and normalized by the end of the first month post-surgery. Elevating and utilizing the buccal fat pad to fill the operative cavity, instead of excising it, supplemented by postoperative compression, may serve as an effective strategy to minimize dead space. The efficacy of this approach, however, requires further empirical validation. More importantly, the absence of facial scars resulted in high patient satisfaction with the surgery.
Strengths and limitations
Of course, as an updated approach, there are still some areas for improvement. First, the indications of this operation are narrow and only applicable to benign lesions. For malignant tumors, to reduce the risk of recurrence, it is necessary to extend the resection during surgery, which involves removing the APG along with the superficial lobe or the entire lobe of the parotid gland (29). Unfortunately, an intraoral incision cannot achieve this extent of resection. Therefore, the surgeon should evaluate the lesion using MRI, ultrasound, or fine needle aspiration to choose the most appropriate approach based on the type of the lesion (2,30). Second, the oral cavity is rich in bacteria, and intraoral incisions have a higher risk of infection compared to extraoral incisions (31). Oral wound infection is characterized by redness, swelling, and pain around the wound. If not promptly treated, it may lead to deeper space infections and, in severe cases, progress to sepsis and septic shock (32). Therefore, patients need to pay careful attention to postoperative oral hygiene, and routine oral care should be performed to avoid wound infection (5,33). Postoperative oral rinse can help prevent infection, and we typically use 20 mL of kangfuxin liquid mixed with 250 mL of 0.9% normal saline. Additional gargling with chlorhexidine mouthwash is necessary. On the first day after surgery, a pressure bandage should be applied to the operative area to prevent infection from hydrops in the dead space. What is more, monitoring postoperative blood routine and C-reactive protein levels is essential for detecting inflammation. Antibiotics should only be administered when there are clear signs of infection. Thirdly, the endoscopic transoral approach demands a high level of skill from the surgeon, who must be proficient in using endoscopic instruments and accustomed to operating under indirect vision. Finally, this study is retrospective and may contain potential information biases due to inaccuracies in data records and patients’ memory. Therefore, prospective cohort studies or randomized controlled studies are needed. Regression analyses should be also conducted to examine relevant risk factors, such as smoking, alcohol consumption, and overall health status. Despite these challenges, compared to traditional approaches, the endoscopic transoral approach offers significant advantages in reducing postoperative complications and improving patient aesthetics, making it a promising technique.
ConclusionsOther Section
Endoscopic resection of accessory parotid mass through a transoral approach avoids an external scar, has good aesthetics, high patient satisfaction, and fewer postoperative complications. Preoperatively, the surgeon should complete the essential examination and select appropriate patients to carry out the operation. During the surgery, it is necessary to fully expose the field to preserve Stensen’s duct and facial nerve branches under the endoscope. Postoperatively, it is also important to pay attention to oral hygiene to avoid infection in the operative area. Prospective cohort studies or randomized controlled studies of endoscopic transoral approach to accessory parotid tumors are needed to further analyze the risk factors associated with the surgery.
AcknowledgmentsOther Section
Funding: None.
FootnoteOther Section
Data Sharing Statement: Available at https://gs.amegroups.com/article/view/10.21037/gs-24-294/dss
Peer Review File: Available at https://gs.amegroups.com/article/view/10.21037/gs-24-294/prf
Conflicts of Interest: All authors have completed the ICMJE uniform disclosure form (available at https://gs.amegroups.com/article/view/10.21037/gs-24-294/coif). The authors have no conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved. The study was conducted in accordance with the Declaration of Helsinki (as revised in 2013). The study was approved by the Ethics Committee of West China Stomatology Hospital, Sichuan University (No. WCHSIRB-D-2020-311-R1) and individual consent for this retrospective analysis was waived.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
ReferencesOther Section
- Pasick LJ, Tong JY, Benito DA, et al. Surgical management and outcomes of accessory parotid gland neoplasms: A systematic review. Am J Otolaryngol 2020;41:102610. [Crossref] [PubMed]
- Kim JP, Lee DK, Moon JH, et al. Endoscope-assisted transoral accessory parotid mass excision: Multicenter prospective observational study. Laryngoscope 2020;130:1218-26. [Crossref] [PubMed]
- Schmutzhard J, Schwentner IM, Andrle J, et al. Resection of accessory parotid gland tumors through a peroral approach with facial nerve monitoring. J Craniofac Surg 2007;18:1419-21. [Crossref] [PubMed]
- Ma H, Jin S, Du Z, et al. Pathology and management of masses in the accessory parotid gland region: 24-year experience at a single institution. J Craniomaxillofac Surg 2018;46:183-9. [Crossref] [PubMed]
- Lenzi R, Matteucci J, Muscatello L. Endoscopic transoral approach to accessory parotid gland. Auris Nasus Larynx 2022;49:511-4. [Crossref] [PubMed]
- Dell' Aversana Orabona G. Midcheek mass: 10 year of clinical experience. J Craniomaxillofac Surg 2014;42:e353-8. [Crossref] [PubMed]
- Choi HJ, Lee YM, Kim JH, et al. Wide excision of accessory parotid gland with anterior approach. J Craniofac Surg 2012;23:165-8. [Crossref] [PubMed]
- Ramachar SM, Huliyappa HA. Accessory parotid gland tumors. Ann Maxillofac Surg 2012;2:90-3. [Crossref] [PubMed]
- Luksic I, Mamic M, Suton P. Management of accessory parotid gland tumours: 32-year experience from a single institution and review of the literature. Int J Oral Maxillofac Surg 2019;48:1145-52. [Crossref] [PubMed]
- Hasegawa K, Sukegawa S, Ono S, et al. Endoscopic-assisted resection of pleomorphic adenoma in the accessory parotid gland. J Med Invest 2021;68:376-80. [Crossref] [PubMed]
- Klotz DA, Coniglio JU. Prudent management of the mid-cheek mass: revisiting the accessory parotid gland tumor. Laryngoscope 2000;110:1627-32. [Crossref] [PubMed]
- Yankov YG. Auriculotemporal Nerve and Frey’s Syndrome. J of IMAB 2023;29:5240-4.
- Li B, Zhang L, Zhao Z, et al. Minimally invasive endoscopic resection of benign tumours of the accessory parotid gland: an updated approach. Br J Oral Maxillofac Surg 2013;51:342-6. [Crossref] [PubMed]
- Moori PL, Rahman S. Endoscopic versus conventional parotid gland excision: a systematic review and meta-analysis. Br J Oral Maxillofac Surg 2021;59:272-80. [Crossref] [PubMed]
- Page MJ, McKenzie JE, Bossuyt PM, et al. The PRISMA 2020 statement: an updated guideline for reporting systematic reviews. BMJ 2021;372:
- Yankov YG, Stanislavova K, Stoev LL et al. Salivary duct carcinoma of the parotid gland: a review article. Varna Medical Forum 2023;12:46-54.
- Voora RS, Stramiello J, Funk E, et al. Transoral Excision of a Large Accessory Parotid Gland Tumor. Ear Nose Throat J 2024;103:156-8. [Crossref] [PubMed]
- Mani S, Mathew J, Thomas R, et al. Feasibility of Transoral Approach to Accessory Parotid Tumors. Cureus 2019;11:e4003. [Crossref] [PubMed]
- Woo SH. Endoscope-assisted transoral accessory parotid mass excision. Head Neck 2016;38:E7-12. [Crossref] [PubMed]
- Wang QX, Yin XL. Clinical applications of benign tumor resection of accessory parotid gland through transoral approach: A report of 5 cases. J Oral Maxillofac Surg 2023;33:347-9.
- Xie L, Zhang D, Lu MM, et al. Minimally invasive endoscopic-assisted resection of benign tumors in the accessory parotid gland: 5 case studies. Head Neck 2012;34:1194-7. [Crossref] [PubMed]
- Bu j, Huang XL, Chen P, et al. Operative appraoches for resection of tumors in the accessory parotid gland. J Modern Stomatol 2009;23:567-9.
- Huvenne W, De Vriese C, Bogaert J, et al. Review of publications on the possible advantages of a direct cheek incision for accessory parotid gland masses. Br J Oral Maxillofac Surg 2020;58:e248-53. [Crossref] [PubMed]
- Yankov YG, Stoev L, Stanislavova K, et al. An Unusual Case of Metachronous Tumors of Prostate and Parotid Gland: A Diagnostic Dilemma. Cureus 2023;15:e48477. [Crossref] [PubMed]
- Osborne RF. Transoral Parotidectomy. Oral Maxillofac Surg Clin North Am 2021;33:169-75. [Crossref] [PubMed]
- Ruohoalho J, Mäkitie AA, Aro K, et al. Complications after surgery for benign parotid gland neoplasms: A prospective cohort study. Head Neck 2017;39:170-6. [Crossref] [PubMed]
- Schubert W, Jenabzadeh K. Endoscopic approach to maxillofacial trauma. J Craniofac Surg 2009;20:154-6. [Crossref] [PubMed]
- Pedroletti F, Johnson BS, McCain JP. Endoscopic techniques in oral and maxillofacial surgery. Oral Maxillofac Surg Clin North Am 2010;22:169-82. [Crossref] [PubMed]
- Newberry TR, Kaufmann CR, Miller FR. Review of accessory parotid gland tumors: pathologic incidence and surgical management. Am J Otolaryngol 2014;35:48-52. [Crossref] [PubMed]
- Stenner M, Preuss SF, Hüttenbrink KB, et al. Accessory parotid gland lesions: case report and review of literature. Eur Arch Otorhinolaryngol 2008;265:1135-8. [Crossref] [PubMed]
- Belusic-Gobic M, Zubovic A, Predrijevac A, et al. Microbiology of wound infection after oral cancer surgery. J Craniomaxillofac Surg 2020;48:700-5. [Crossref] [PubMed]
- La Via L, Sangiorgio G, Stefani S, et al. The Global Burden of Sepsis and Septic Shock. Epidemiologia (Basel) 2024;5:456-78. [Crossref] [PubMed]
- Ishimaru M, Matsui H, Ono S, et al. Preoperative oral care and effect on postoperative complications after major cancer surgery. Br J Surg 2018;105:1688-96. [Crossref] [PubMed]




