
A nomogram for individualized prediction for cervical lymph node metastasis of papillary thyroid carcinoma
Highlight box
Key findings
• The biggest highlight of this study is that the overall echogenicity of the thyroid gland is less correlated with metastasis to the cervical lymph nodes in the prediction model when it exhibits diffuse lesions.
What is known and what is new?
• Papillary thyroid carcinoma is considered the “gentlest” malignancy, metastasis in the cervical lymph nodes is considered a risk factor for distant metastasis, recurrence and reduced survival. Clinical metastasis of thyroid cancer mostly starts from the central zone lymph nodes (zone VI).
• We found that the overall echogenicity of the thyroid gland, the number of malignant nodules, the location of the nodules, nodule left-right diameter, the relationship between the nodules and the thyroid peritoneum, and the elasticity score of the nodules were independent predictors of thyroid cancer-related cervical lymph node metastasis by the prediction model analysis.
What is the implication, and what should change now?
• In the future, we can consider adding more predictive factors, not only limited to the analysis of ultrasound images. It is believed that this nomogram can improve the accuracy of ultrasound in the diagnosis of papillary thyroid carcinoma patients and to assist clinical decision-making on the treatment and follow-up of papillary thyroid carcinoma patients to a certain extent.
Introduction
In recent years, along with the rapid development of medicine, ultrasound examination technology has also been developed. In addition, the awareness of human health is increasing, and the detection rate of thyroid nodules is becoming higher. Among them, there is no shortage of malignant thyroid nodules.
It is well known that thyroid cancer has several pathological types, among which papillary carcinoma is the most common and is considered the “gentlest” malignancy (1). Because of the slow progression of the disease, thyroid surgery for the detection of thyroid malignancies is nowadays considered as a possible overdiagnosis (2). As an important endocrine organ of the body, the thyroid gland secretes and synthesizes thyroxine that regulates human growth and participates in the metabolism of the body. After aggressive surgical treatment, patients need exogenous thyroxine supplementation and need to adjust the intake of exogenous hormones according to the hormone levels in the body, which undoubtedly greatly affect the quality of life of patients (3). The most common postoperative complications of thyroid surgery are mainly hypocalcemia due to hypoparathyroidism and damage to the external branch of superior laryngeal nerve and recurrent laryngeal nerves (4-6).
On the other hand, some patients with malignant thyroid tumors have certain high-risk factors, such as a family history of papillary thyroid carcinoma, a history of radiation exposure to the neck, or a highly suspicious thyroid nodule detected by ultrasound along with a lymph node with metastatic potential in the relevant region of the neck (7).
In contrast, metastasis in the central group of cervical lymph nodes is considered a risk factor for distant metastasis, recurrence and reduced survival (8-10). Considering that the clinical metastasis of papillary thyroid carcinoma mostly starts from the central group of cervical lymph nodes (zone VI) and then spreads to the lateral group of cervical lymph nodes (zones II–V), very few patients may skip the central group of cervical lymph nodes and metastasize directly to the lateral group of cervical lymph nodes, achieving “jump metastasis” (11). However, the American Thyroid Association (ATA) guidelines also state that in adult patients with papillary thyroid cancer, thyroidectomy alone without prophylactic central group of cervical lymph nodes dissection should be performed when no cervical lymph node metastasis is clinically detected (12).
There is still great disagreement about which groups of patients with ultrasound-detected papillary thyroid carcinoma can be considered for long-term close follow-up and which patients should undergo lobectomy of the affected lobe of the thyroid gland and ipsilateral central lymph node dissection. It has been reported that the metastasis rate of lymph nodes in the central region of patients with papillary thyroid carcinoma is as high as 30–80%, and the metastasis of lymph nodes in the lateral cervical region also reaches 40% (13), and the identification of suspicious lymph nodes in the central region of the neck is particularly important. Due to the special location of the central zone, adjacent to the trachea and the complex anatomy of the thorax, the visualization effect is easily disturbed by the bones and the gas in the trachea, which makes the metastasis of lymph nodes in the central zone underestimated (7). Some scholars have applied the prediction model to the treatment process of prostate cancer and breast cancer and achieved good predictability (14). Therefore, we generalized and analyzed the image characteristics of thyroid malignant tumors on high-frequency ultrasound and correlated them with the pathology of patients’ cervical lymph nodes, and established a line graph prediction model, aiming to improve the accuracy of ultrasound in the diagnosis of papillary thyroid carcinoma patients and to assist clinical decision-making on the treatment and follow-up of papillary thyroid carcinoma patients to a certain extent. We present this article in accordance with the TRIPOD reporting checklist (available at https://gs.amegroups.com/article/view/10.21037/gs-24-277/rc).
Methods
General data
A total of 366 patients, including 270 (74%) females and 96 (26%) males, aged 18–67 years, with a mean age of 40.1±10.7 years, were included in this study. The above patients underwent thyroid ultrasound examination in the Department of Ultrasound Medicine of The First Affiliated Hospital of Nanjing Medical University between January 1, 2021 and October 31, 2021, and at least one thyroid nodule with TI-RADS class 4 or above was found, all of these patients underwent fine needle aspiration (FNA) for thyroid nodules, thyroid function test. They underwent at least sampling of lymph nodes in the central neck (zone VI) to determine whether to perform a lobectomy or total thyroidectomy dissection and to determine whether central lymph node dissection was required in The First Affiliated Hospital of Nanjing Medical University, and postoperative pathology confirmed primary thyroid cancer with cervical lymph node metastasis or not. None of the above patients had any previous history of thyroid surgery or radiation exposure to the neck. The study was conducted in accordance with the Declaration of Helsinki (as revised in 2013). The study was approved by the Ethics Committee of The First Affiliated Hospital of Nanjing Medical University (ethics No. 2023-SR-462). As all data were de-identified, individual consent for this retrospective analysis was waived by the committee.
Image acquisition
The patients in this study were examined with a Philips EPIQ5 or Siemens 3000 ultrasound machine. The examiners had to have more than three years of experience in thyroid scanning, and two doctors used a 12-5 Hz high-frequency linear ultrasound probe to scan the thyroid gland and the lymph nodes in the neck.
Information recording
The ultrasound characteristics of thyroid nodules suspected of malignancy were recorded in the following aspects: overall echogenicity of the thyroid gland, divided into normal, thickened, and diffuse lesions; number of malignant nodules, divided into single and multiple; nodule echogenicity, divided into hypoechoic, mixed echogenicity, isoechoic, and hyperechoic; nodule upper and lower diameter, left and right diameter, and anterior and posterior diameter, and when the malignant nodule is multiple, the diameter of the largest nodule was taken for, then when the malignant nodule is multiple, the largest nodule should be recorded; the lobe of the nodule, divided into left lobe, right lobe, isthmus, cone lobe; the location of the nodule, divided into upper, upper middle, middle, lower middle, lower pole, medial, lateral, dorsal, ventral and middle nodules; the relationship between the nodule and the thyroid capsule, divided into contiguous, non-contiguous; the internal structure of the nodule, divided into solid, mainly solid, mainly cystic, mixed echogenicity; aspect ratio of the nodule, divided into less than 1, greater than one and equal to one; the calcification of the nodule, divided into no calcification, microcalcification, coarse calcification, coarse and microcalcifications; nodule morphology, classified as regular, irregular; nodule boundary, classified as clear, unclear; nodule margin, classified as smooth, lobulated, angular, burr, unable to evaluate; nodule with or without acoustic halo, nodule posterior echogenicity, classified as no abnormality, attenuation; nodule blood flow, classified as no blood flow, punctate blood flow, strip blood flow, abundant blood flow; nodule elasticity score. The presence of ultrasound-visible cervical lymph node metastases was also recorded. After the patients underwent thyroid surgery, the pathological type of papillary thyroid carcinoma and cervical lymph node metastasis was recorded.
Statistical analysis
Categorical data were expressed as number; continuous data were expressed as mean ± standard deviation. Least absolute shrinkage and selection operator (LASSO) regression technique was used for predictor variable selection. Binary logistic regression analysis was used to develop predictive models and nomogram. Receiver operating characteristic (ROC) curves and Harrell’s consistency index (C-index) were used to assess model performance and discrimination and consistency; the goodness of fit of predictive models was evaluated through the use of the Hosmer-Lemeshow test, and the clinical utility of the models was assessed through clinical decision curve analysis (DCA). Statistical analysis was performed using R software (Version 4.2.2; https://www.R-project.org), and P<0.05 was considered statistically significant. Based on the results of the nomogram, we created a web page calculator that can be more intuitive and easier to predict cervical lymph node metastasis in papillary thyroid carcinoma.
Results
Characteristics of patients
A total of 366 patients, including 270 (74%) females and 96 (26%) males, aged 18–67 years, with a mean age of 40.15±10.71 years, were included in this study. Based on ultrasonography and Pathology, all patients were divided into the metastasis group and the no-metastasis group. All data of patients in both groups, including ultrasound and clinical characteristics, are shown in Table 1.
Table 1
| Characteristics | No metastasis (n=139) | Metastasis (n=227) | Total (n=366) |
|---|---|---|---|
| Age, years | 41.78±10.16 | 39.15±10.93 | 40.15±10.71 |
| Gender | |||
| Male | 33 | 63 | 96 |
| Female | 106 | 164 | 270 |
| Thyroid echo | |||
| Normal | 76 | 143 | 219 |
| Thickened | 17 | 37 | 54 |
| Diffuse lesions | 46 | 47 | 93 |
| No. of malignant nodules | |||
| Single | 93 | 127 | 220 |
| Multiple | 46 | 100 | 146 |
| Nodule echogenicity | |||
| Hypoechoic | 128 | 208 | 336 |
| Mixed echogenicity | 3 | 6 | 9 |
| Isoechoic | 8 | 12 | 20 |
| Hyperechoic | 0 | 1 | 1 |
| Upper and lower diameter, mm | 10.52±8.66 | 12.86±8.67 | 11.98±8.73 |
| Left and right diameter, mm | 8.62±6.52 | 10.78±7.46 | 9.96±7.19 |
| Anterior and posterior diameter, mm | 8.01±4.46 | 9.30±4.49 | 8.81±4.52 |
| The lobe of the nodule | |||
| Left | 66 | 96 | 162 |
| Right | 69 | 122 | 191 |
| Isthmus | 3 | 7 | 10 |
| Cone lobe | 1 | 2 | 3 |
| Location of the nodule | |||
| Location of the pole | |||
| Upper | 36 | 40 | 76 |
| Upper middle | 17 | 23 | 40 |
| Middle | 34 | 52 | 86 |
| Lower middle | 14 | 36 | 50 |
| Lower | 34 | 65 | 99 |
| Occupies almost the entire lateral lobe (including invasion of isthmus) | 4 | 11 | 15 |
| Ventral/dorsa | |||
| Ventral | 71 | 115 | 186 |
| Dorsal | 55 | 71 | 126 |
| Middle | 13 | 41 | 54 |
| Inside/outside | |||
| Inside | 53 | 103 | 156 |
| Outside | 46 | 67 | 113 |
| Middle | 40 | 57 | 97 |
| The relationship between the nodule and the thyroid capsule | |||
| Contiguous | 36 | 96 | 132 |
| Non-contiguous | 103 | 131 | 234 |
| Internal structure | |||
| Solid | 122 | 201 | 323 |
| Mainly solid | 14 | 19 | 33 |
| Mainly cystic | 0 | 1 | 1 |
| Mixed echogenicity | 3 | 6 | 9 |
| Aspect ratio | |||
| <1 | 70 | 134 | 204 |
| >1 | 68 | 91 | 159 |
| 1 | 1 | 2 | 3 |
| Calcification | |||
| No | 32 | 24 | 56 |
| Microcalcification | 105 | 199 | 304 |
| Coarse | 0 | 1 | 1 |
| Both | 2 | 3 | 5 |
| Shape | |||
| Regular | 47 | 45 | 92 |
| Irregular | 92 | 182 | 274 |
| Boundary | |||
| Clear | 121 | 193 | 314 |
| Unclear | 18 | 34 | 52 |
| Edge | |||
| Smooth | 15 | 12 | 27 |
| Lobulated | 31 | 67 | 98 |
| Angular | 17 | 31 | 48 |
| Burr | 58 | 83 | 141 |
| Boundaries not clear for assessment | 18 | 34 | 52 |
| Sound halo | |||
| Yes | 5 | 2 | 7 |
| No | 134 | 225 | 359 |
| Echo attenuation | |||
| Yes | 5 | 13 | 18 |
| No | 134 | 214 | 348 |
| Blood flow | |||
| No | 53 | 60 | 113 |
| Punctate | 57 | 94 | 151 |
| Strip | 7 | 26 | 33 |
| Abundant | 22 | 47 | 69 |
| Resilience score | |||
| 2 | 3 | 0 | 3 |
| 3 | 84 | 78 | 162 |
| 4 | 52 | 149 | 201 |
| Pathology | |||
| Classic | 123 | 196 | 319 |
| Follicular | 5 | 8 | 13 |
| Extra | 11 | 23 | 34 |
Data are presented as mean ± standard deviation or number.
Feature selection
Based on LASSO logistic regression, 11 independent predictors as overall thyroid echo, number of nodules, nodule location, left and right nodule diameters, relationship between the nodule and thyroid capsule, nodule morphology, whether the nodule was calcified or not, nodule blood flow, nodule elasticity score, presence or absence of halo in the nodule, and posterior echogenicity of the nodule were chosen from the 24 characteristics of the cohort of 366 patients in the data set and included in a binary logistic regression analysis (Figure 1).
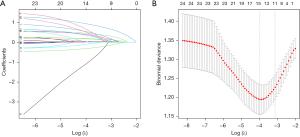
Development of personalized prediction models
The 11 features mentioned above were analyzed by binary logistic regression, and overall thyroid echogenicity, number of nodules, left-right diameter of nodules, location of nodules, relationship between nodules and thyroid capsule, and nodule elasticity scores were the independent predictors of lymph node metastasis associated with papillary thyroid carcinoma (Table 2).
Table 2
| Intercept and variable | β | Odds ratio (95% CI) | P value |
|---|---|---|---|
| (Intercept) | −5.49 | 0.004 (0.0006–0.025) | <0.001 |
| T-echo | −0.334 | 0.713 (0.5399–0.9400) | 0.02 |
| S-M | 0.686 | 1.985 (1.215–3.289) | 0.007 |
| L_diameter | 0.0493 | 1.0505 (1.01052–1.095) | 0.02 |
| Pole | 0.219 | 1.245 (1.069–1.4566) | 0.005 |
| Connected | 0.515 | 1.6727 (1.014–2.7901) | 0.046 |
| ResilienceScore | 1.373 | 3.950 (2.4907–6.3699) | <0.001 |
β is the regression coefficient. T_echo, overall thyroid echogenicity; S_M, single or multiple; L_diameter, left-right diameter of nodules; Pole, location of nodules; Connected, relationship between nodules and thyroid capsule; ResilienceScore, nodule elasticity scores; CI, confidence interval.
The above independent predictors were developed and presented as a nomogram (Figure 2).

Performance and validation of the nomogram
The area under the ROC curve for nomogram was 0.748 [95% confidence interval (CI): 0.698–0.798]; indicating that the discriminatory power of the nomogram was moderate for this data set (Figure 3). The validation result using the bootstrap method was 0.748, indicating good calibration of this model; The Hosmer-Lemeshow test resulted in a P value of 0.30; indicating good goodness-of-fit of this predictive model (Figure 4).


Clinical efficacy
Finally, the DCA of the nomogram for thyroid cancer-associated lymph node metastasis showed a range of net benefit thresholds of approximately 16–91% for the nomogram in this data set; suggesting that the nomogram predicting lymph node metastasis efficacy affecting thyroid cancer-associated lymph node metastasis was higher than the value of benefit for the full diagnosis of having lymph node metastasis and for the full diagnosis of having no lymph node metastasis (Figure 5).

Web calculator
Although the corresponding visualization results can be obtained through the nomogram; it still lacks convenience in clinical work. Therefore, based on the results of the nomogram, we built a webpage calculator that can predict the metastasis of cervical lymph nodes of papillary thyroid carcinoma more intuitively and conveniently (Figure 6A,6B).

Fill in the ultrasound characteristics of the patients’ thyroid nodules, including the overall background of the thyroid gland, the size of the thyroid nodules, distribution location, morphology, margins, aspect ratio, and the elasticity of the nodules. Click “Prediction” in the dynamic columnar diagram. Click “Prediction” in the dynamic line graph to view the prediction probability and confidence interval of cervical lymph node metastasis of thyroid cancer.
Discussion
In recent years, with the continuous improvement of high-frequency ultrasonography and the application of ultrasonography and ultrasound-guided fine-needle aspiration thyroid nodule aspiration biopsy techniques, the detection rate of thyroid cancer has been greatly improved (15). The American Cancer Society’s publication, cancer statistics in the United States 2020, states that thyroid cancer has become the fifth most common cancer in the female population. Unfortunately, this medical trend is also playing out in the Chinese society (12).
Although thyroid cancer progresses slowly, the metastasis rate of lymph nodes in the central region of patients with papillary thyroid carcinoma is as high as 30–80%, and the metastasis of lymph nodes in the lateral cervical region also reaches 40%, which is considered to be highly relevant to distant metastases, recurrence and prognosis of papillary thyroid carcinoma (9). Therefore, lymph node dissection in the central lymph node dissection of the neck is considered necessary for patients with clinically suspected neck lymph node metastases (16).
Ultrasound, as the preferred screening tool for thyroid nodules, has the advantages of convenience, speed, economy, safety, and reproducibility, but due to the complex anatomy of the neck and the limited penetration of ultrasound, ultrasound may underestimate the suspicious lymph nodes in the neck.
Therefore, we need to analyze the ultrasound characteristics of malignant thyroid nodules and screen out the image features that have correlation with cervical lymph node metastasis of thyroid cancer to establish a prediction model, so as to reduce the underdiagnosis rate of ultrasound and expect to be helpful to clinicians in choosing the treatment plan for patients.
We found that the overall echogenicity of the thyroid gland, the number of malignant nodules, the location of the nodules, nodule left-right diameter, the relationship between the nodules and the thyroid peritoneum, and the elasticity score of the nodules were independent predictors of thyroid cancer-related cervical lymph node metastasis by the prediction model analysis.
It is worth exploring that the overall echogenicity of the thyroid gland is less correlated with metastasis to the cervical lymph nodes in the columnar map prediction model when it exhibits diffuse lesions. In a previous study, more scholars believe that thyroid echo is less correlated with neck lymph node metastasis of papillary thyroid carcinoma (12). Here, the author suggests that this may be due to the fact that when the thyroid gland as a whole presents diffuse lesions, a small number of malignant nodules may be masked by abnormalities in the background thyroid echogenicity, and examiners often misclassify thyroid nodules as Hashimoto foci, thus causing a missed diagnosis. In contrast, normal thyroid background echogenicity allows for clearer and more accurate evaluation of thyroid nodule images.
The multiplicity of malignant thyroid nodes is closely associated with metastasis in the cervical lymph nodes, Wang et al.’s conclusions (16) are consistent with those of this study. Previous studies have suggested that age is a prognostic factor for cervical lymph node metastasis in papillary thyroid carcinoma, and the odds of cervical lymph node metastasis in papillary thyroid carcinoma gradually increase with age (16-20). In the present study, we did not find the predictive significance of age on neck lymph node metastasis. In the future, we need to further expand the sample size to provide more reliable information for our study.
Multiple malignant thyroid nodules are considered a specific form of intraglandular metastasis of papillary thyroid carcinoma, and our study also confirmed that patients with multiple manifestations of malignant nodules are more likely to develop metastasis in the cervical lymph nodes.
The isthmus is located in a special anatomical position, which is in front of the trachea and covered by the cervical musculature, and is a bridge between the left and right lobes of the thyroid gland. Therefore, thyroid cancer located in the isthmus can easily metastasize to the left and right lobes and invade the trachea, cervical musculature and lymph nodes, especially the central lymph nodes. This was confirmed by our findings (21,22).
Extraglandular metastasis of thyroid cancer is considered an independent risk factor for metastasis of thyroid cancer to the cervical lymph nodes (11), which echoes the findings of the present study. In this study, during the pre-scan of thyroid nodules, one of the ultrasound image features of thyroid nodules that we recorded showed the relationship between the nodule and the thyroid capsule, i.e., whether the nodule was attached to the thyroid capsule. Malignant nodules abutting the thyroid capsule are considered to be a pre-existing behavior of extra-glandular metastasis of thyroid cancer, predicting the possibility of distant metastasis of thyroid cancer.
In the nomogram prediction model we developed, the left-right diameters of thyroid nodules were considered an independent risk factor for lymph node metastasis in the neck. This is also consistent with previous experience (12). The growth of tumors is mostly aggressive, infiltrative and exophytic in nature. Despite known as the “mildest” tumor, papillary thyroid carcinoma is no exception. The left-right diameters of thyroid nodules are an important component of the aspect ratio of the nodule, and at the same time, the growth of the right and left diameters of the nodule represents expansion and extension in the horizontal direction, and is also an important indication that the nodule is invasive, which was well confirmed in our study.
The elasticity score of nodules is a quantitative representation of the softness of nodules. Both in terms of ultrasound quantification and clinical palpation, malignant nodules almost always have a high degree of hardness, while benign nodules tend to be softer in texture, which is complemented by our prediction results (1).
Of course, there are some shortcomings in the present study, one being the limitation of the sample size, which needs to be expanded in the future to provide stronger support for the previous study. In addition, the limitations in the selection of possible influencing factors are also a drawback of this study. In the future, we can consider adding more predictive factors, not only limited to the analysis of ultrasound images, but also to include some clinical factors, such as the level of thyroglobulin, which is considered to be an important indicator for postoperative disease monitoring in papillary thyroid carcinoma patients; or the situation of BRAF gene, because there are many patients in the clinic, the FNA of preoperative nodules due to low cell volume, diagnosis needs to be aided by genetic testing. The BRAF gene test is undoubtedly a support and supplement to cytologic pathology at this time. And then operator dependency of ultrasound and because different ultrasound machines are used in clinical practice, the accuracy of the study was affected.
Conclusions
We developed a nomogram to predict metastasis in the cervical lymph nodes of papillary thyroid carcinoma, and the nomogram showed good performance for predictive aspects. As a prediction model that has emerged in recent years, nomogram is used in clinical settings to identify plausible predictors from generalized analysis of large amounts of data. It is believed that with more and more data support, it can provide an important reference basis for the treatment plan of more “difficult” patients.
Acknowledgments
We acknowledge Dabao Zhao and Yiming Zhao for their unconditional support.
Funding: None.
Footnote
Reporting Checklist: The authors have completed the TRIPOD reporting checklist. Available at https://gs.amegroups.com/article/view/10.21037/gs-24-277/rc
Data Sharing Statement: Available at https://gs.amegroups.com/article/view/10.21037/gs-24-277/dss
Peer Review File: Available at https://gs.amegroups.com/article/view/10.21037/gs-24-277/prf
Conflicts of Interest: All authors have completed the ICMJE uniform disclosure form (available at https://gs.amegroups.com/article/view/10.21037/gs-24-277/coif). The authors have no conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved. The study was conducted in accordance with the Declaration of Helsinki (as revised in 2013). The study was approved by the Ethics Committee of The First Affiliated Hospital of Nanjing Medical University (ethics No. 2023-SR-462). As all data were de-identified, individual consent for this retrospective analysis was waived by the committee.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Tong Y, Li J, Huang Y, et al. Ultrasound-Based Radiomic Nomogram for Predicting Lateral Cervical Lymph Node Metastasis in Papillary Thyroid Carcinoma. Acad Radiol 2021;28:1675-84. [Crossref] [PubMed]
- McLeod DSA, Zhang L, Durante C, et al. Contemporary Debates in Adult Papillary Thyroid Cancer Management. Endocr Rev 2019;40:1481-99. [Crossref] [PubMed]
- Zhang X, Zhang F, Li Q, et al. Iodine nutrition and papillary thyroid cancer. Front Nutr 2022;9:1022650. [Crossref] [PubMed]
- Ywata de Carvalho A, Chulam TC, Kowalski LP. Longterm Results of Observation vs Prophylactic Selective Level VI Neck Dissection for Papillary Thyroid Carcinoma at a Cancer Center. JAMA Otolaryngol Head Neck Surg 2015;141:599-606. [Crossref] [PubMed]
- Viola D, Materazzi G, Valerio L, et al. Prophylactic central compartment lymph node dissection in papillary thyroid carcinoma: clinical implications derived from the first prospective randomized controlled single institution study. J Clin Endocrinol Metab 2015;100:1316-24. [Crossref] [PubMed]
- Tartaglia F, Blasi S, Giuliani A, et al. Central neck dissection in papillary thyroid carcinoma: results of a retrospective study. Int J Surg 2014;12:S57-62. [Crossref] [PubMed]
- Lyu YS, Pyo JS, Cho WJ, et al. Clinicopathological Significance of Papillary Thyroid Carcinoma Located in the Isthmus: A Meta-Analysis. World J Surg 2021;45:2759-68.
- Chow SM, Law SC, Chan JK, et al. Papillary microcarcinoma of the thyroid-Prognostic significance of lymph node metastasis and multifocality. Cancer 2003;98:31-40. [Crossref] [PubMed]
- Hay ID, Hutchinson ME, Gonzalez-Losada T, et al. Papillary thyroid microcarcinoma: a study of 900 cases observed in a 60-year period. Surgery 2008;144:980-7; discussion 987-8. [Crossref] [PubMed]
- Roti E. Thyroid papillary microcarcinoma: a descriptive and meta-analysis study. Eur J Endocrinol 2008;159:659-73. [Crossref] [PubMed]
- Liu LS, Liang J, Li JH, et al. The incidence and risk factors for central lymph node metastasis in cN0 papillary thyroid microcarcinoma: a meta-analysis. Eur Arch Otorhinolaryngol 2017;274:1327-38. [Crossref] [PubMed]
- Sun F, Zou Y, Huang L, et al. Nomogram to Assess the Risk of Central Cervical Lymph Node Metastasis in Patients With Clinical N0 Papillary Thyroid Carcinoma. Endocr Pract 2021;27:1175-82. [Crossref] [PubMed]
- Sherman SI. Thyroid carcinoma. Lancet 2003;361:501-11. [Crossref] [PubMed]
- Delpech Y, Bashour SI, Lousquy R, et al. Clinical nomogram to predict bone-only metastasis in patients with early breast carcinoma. Br J Cancer 2015;113:1003-9. [Crossref] [PubMed]
- Davies L, Welch HG. Increasing incidence of thyroid cancer in the United States, 1973-2002. JAMA 2006;295:2164-7. [Crossref] [PubMed]
- Wang Y, Guan Q, Xiang J. Nomogram for predicting central lymph node metastasis in papillary thyroid microcarcinoma: A retrospective cohort study of 8668 patients. Int J Surg 2018;55:98-102. [Crossref] [PubMed]
- Xiang D, Xie L, Xu Y, et al. Papillary thyroid microcarcinomas located at the middle part of the middle third of the thyroid gland correlates with the presence of neck metastasis. Surgery 2015;157:526-33. [Crossref] [PubMed]
- Lin Y, Cui N, Li F, et al. The model for predicting the central lymph node metastasis in cN0 papillary thyroid microcarcinoma with Hashimoto's thyroiditis. Front Endocrinol (Lausanne) 2024;15:1330896. [Crossref] [PubMed]
- Zhang J, Zhou X, Yao F, et al. TIPARP as a prognostic biomarker and potential immunotherapeutic target in male papillary thyroid carcinoma. Cancer Cell Int 2024;24:34. [Crossref] [PubMed]
- Wu F, Huang K, Huang X, et al. Nomogram model based on preoperative clinical characteristics of unilateral papillary thyroid carcinoma to predict contralateral medium-volume central lymph node metastasis. Front Endocrinol (Lausanne) 2023;14:1271446. [Crossref] [PubMed]
- Lee YC, Na SY, Chung H, et al. Clinicopathologic characteristics and pattern of central lymph node metastasis in papillary thyroid cancer located in the isthmus. Laryngoscope 2016;126:2419-21. [Crossref] [PubMed]
- Chang YW, Lee HY, Kim HS, et al. Extent of central lymph node dissection for papillary thyroid carcinoma in the isthmus. Ann Surg Treat Res 2018;94:229-34. [Crossref] [PubMed]


