
Case series of secretory carcinoma in the parotid glands
Highlight box
Key findings
• Magnetic resonance imaging (MRI) image features of secretory carcinoma (SC) include clear borders and round or oval shapes, but lack specificity. Immunohistochemistry (IHC) is used for SC diagnosis with high accuracy.
What is known and what is new?
• SC is a rare tumor primarily affecting adults, but also occur in children. The prognosis is usually good, but T4 tumors require more aggressive treatment and monitoring. The diagnostic options for SC are limited, necessitating better preoperative methods. Useful IHC markers include: IHC markers including S-100, mammaglobin, and calponin, and Ki67, vimentin, and P63 can be used to predict the degree of malignancy; MRI image features include well-defined borders and round or oval shapes, but lack of specificity; its pathologic features include solid, laminar, sieve-like, and papillary arrangements with interstitial fibrosis and inflammatory cell infiltration.
• In this study, we analyzed the treatment outcome, clinicopathological features, and prognostic factors of SC, and systematically evaluated and meta-analyzed the diagnostic value of S-100/mammaglobin/SOX10/DOG1 IHC in identifying SCs. Diagnosis and treatment of NTRK fusion cancers were reviewed, and small-molecule tropomyosin receptor kinase (TRK) inhibitor-based therapy for NTRK fusion cancers was evaluated.
What is the implication, and what should change now?
• Diagnostic methods are limited, and more effective methods are needed to manage preoperative diagnosis. Therefore, future research on diagnostic methods for SC should be strengthened to improve the accuracy and efficiency of diagnosis. For T4 tumors, more aggressive treatment and close monitoring are needed to improve patient prognosis.
Introduction
Mammary analogue secretory carcinoma (MASC) of the salivary gland was first reported in 2010 with a known ETV6 gene rearrangement (1). the World Health Organization (WHO) classified MASC as a distinct type of salivary gland epithelial tumor and refers to it simply as secretory carcinoma (SC) in 2017. As a rare tumor of the salivary gland, with non-specific morphological and clinical features, SC lacks an effective method to manage preoperative diagnosis. In other words, it is a big challenge to differentiate SC from other familiar salivary tumors, such as follicle cell carcinoma, mucoepidermoid carcinoma, and adenocarcinoma (2). SC is a type of tumor that is extremely rare, accounting for less than 0.3% of all salivary gland tumors (3). Despite being predominantly found in adults, it can also occur in children. There is no significant difference in the occurrence of this disease between males and females. However, in parotid SC, male gender, T3–T4 tumors, and recurrent disease are considered unfavorable predictors of survival. T4 tumors require more aggressive treatment and close monitoring (4). Despite this, the long-term prognosis for those diagnosed with this disease is generally favorable.
Inspired by the recent descriptions of SC, we retrospectively collected all the salivary tumor cases in Guangdong Provincial People’s Hospital, aiming to identify this new salivary tumor in a retrospective study and correlate histomorphologic features with the immune profile and clinical outcomes of the patients. In collecting treatment methods and outcomes of five cases, we found that preoperative diagnosis is of vital significance to make an accurate diagnosis, provide the correct treatment, and to avoid the need for secondary treatment that may harm patient’s physical and mental health.
Thus, our work aimed to provide valuable information and diagnostic evidence for peers, as well as explore the possibility of preoperative diagnosis. We present this article in accordance with the AME Case Series reporting checklist (available at https://gs.amegroups.com/article/view/10.21037/gs-24-422/rc).
Case presentation
Case selection and patient clinical information
A total of five cases of SC had been treated at Guangdong Provincial People’s Hospital from 2018 to 2020. The clinical data of the patients were analyzed, including age, preoperative examinations, tumor size and site, lymph nodes and distant metastases, surgical options, and recurrences. All procedures performed in this study were in accordance with the ethical standards of the institutional and/or national research committee(s) and with the Helsinki Declaration (as revised in 2013). Written informed consent for publication of this case series and accompanying images was not obtained from the patients or the relatives after all possible attempts were made.
Patient clinical information
Surgical procedures were performed on all five cases. Clinical data from these cases were collected from the surgical pathology records, which included information such as age at the time of initial tumor diagnosis, gender, preoperative assessments, tumor size and location, treatment methods used, instances of tumor recurrence, and any involvement of the lymph nodes. In all cases, we scheduled patients for follow-up appointments every six to twelve months, the maximum follow-up period was up to 35 months and the minimum was 17 months (see Table 1 for details). Except for case 1, which was considered to be a low-grade malignant tumor before surgery, the other four cases were considered to be benign parotid tumors before surgery. Due to the lack of knowledge about SC at that time, all five cases were operated according to the surgical plan for benign tumors; case 1 could not ensure complete removal of the tumor because it was found to have broken through the periampullary area, and postoperative radiotherapy was performed in order to prevent local recurrence; whereas case 5 was found to have a higher degree of malignancy after postoperative pathological confirmation, and thus underwent a second operation with unilateral cervical lymph node dissection. All five patients had a positive prognosis, based on the telephone follow-ups conducted.
Table 1
| No. | Age (years) | Sex | Tumor size (cm) | Regional lymph nodes | Metastasis | Local resection | Neck dissection | Facial nerve | Post-surgical radiation (Y/N) | Outcome | Observation (months) |
|---|---|---|---|---|---|---|---|---|---|---|---|
| 1 | 37 | M | 3.0×2.3 (T2) | N0 | M0 | Sub | SND | Pres | Y | Alive | 35 |
| 2 | 34 | M | 3.5×2.5 (T2) | N0 | M0 | Sub | – | Pres | N | Alive | 30 |
| 3 | 40 | M | 1.0×1.4 (T1) | N0 | M0 | Sub | – | Pres | N | Alive | 26 |
| 4 | 30 | F | 1.8×1.5 (T1) | N0 | M0 | Sub | – | Pres | N | Alive | 18 |
| 5 | 24 | M | 2.4×3.1 (T2) | N2 (I, IV) | M0 | Sub | SND | Pres | Y | Alive | 17 |
SC, secretory carcinoma; Y, yes; N, no; M, male; F, female; Sub, subtotal parotidectomy; SND, selective neck dissection; Pres, preservation.
Imaging study
All pre-operative magnetic resonance imaging (MRI) images were collected and analyzed to diagnose tumors based on size, shape, boundary, and signal level in order to find the possibility of preoperative diagnosis.
Histochemical and immunohistochemical analysis of biomarkers
All the pathological sections were reviewed and evaluated by two independent pathologists. They recorded all the antibodies and expression situations. Calponin, CK7, CK, SMA, S-100, P63, mammaglobin, CD117, Ki67, and vimentin, which are recognized as characteristic indicators (5), were detected through selected immunohistochemistry (IHC) stains in all five cases. However, fluorescence in situ hybridization (FISH) was not performed in any of the 5 cases.
Clinical characteristics
The total of 5 patients, 1 female and 4 males, with an average age of 33 years (range, 24–40 years), presented with painless, firm masses in the parotid area without any associated numbness or discomfort (as shown in Table 1).
During an MRI examination, all of the patients were diagnosed with either T1 or T2 stage of the disease. Only one case showed lymph node involvement, and none of the patients displayed any signs of distant metastasis. All patients underwent local excision of the mass with preservation of the facial nerve, and two of them underwent cervical lymph node dissection and postoperative radiotherapy only one of the two patients with cervical dissection had postoperative pathology suggesting positive lymph nodes. Since the SC presented as a well-defined mass, the benignity or malignancy of the mass was not clear preoperatively, no margins were taken during the resection of the mass. However, all five patients showed no recurrence or metastasis, based on the telephone follow-ups conducted. With a requirement for follow-up every six months to one year, the maximum and minimum follow-up periods were up to 35 months and 17 months, respectively. All five of the patients have been cooperative during the follow-up visits up to this point. No complications or adverse events such as facial paralysis occurred in all patients after surgery.
Imaging characteristics
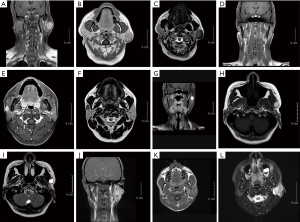
MRI was conducted before the surgery 4 out of the 5 cases. All of the tumors were described as oval or roundish masses located in the superficial lobe of the parotid gland with clear boundaries (as shown in Figure 1). Although the image features of SC have been analyzed by various institutions, no significant specificity has been reported. Our cases can be classified into two types based on their images: the first type is a benign tumor that appears as a partially cystic irregular mass or lobulated (Figure 1A-1I). Case 5, which was finally diagnosed as a malignant mass, was irregular in shape with less cystic composition (Figure 1J-1L). A previous article explored the possibility of detecting SC by ultrasound (6), and the author concluded that most SC ultrasound images can be described as irregular, cystic nodules with a solid part of the papillary protrusion. Combining the author’s conclusion and the features of our images, a lobulated and irregular mass in the parotid gland may suggest a low-grade tumor.
Pathological characteristics
In these cases, the cross-section appeared as gray-yellow or gray-red in color. A total of 3 cases had a distinct border with the surrounding tissue, whereas only 1 case had an unclear border (Figure 2).
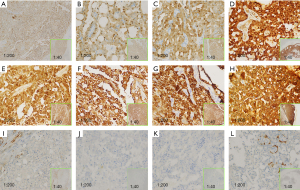
From the hematoxylin and eosin (HE) staining, a solid lamellar, cribriform, and papillary arrangement was observed (Figure 3) along with interstitial fibrosis and infiltration of inflammatory cells. The neoplastic cells were uniform and round in shape with small nucleoli, abundant cytoplasm, and basophilic features. Cystic changes were noted in 3 cases, lobulated in 1 case, and hyaline in 1 case. The tumor tissue, infiltrating the salivary gland, presented multi-nodular growth with partial cystic change lined with columnar epithelium. The focal mucilage could been found in the cyst cavity. The tumor cell cytoplasm was rich. The big cyst in the periphery was intermittently broken showing hemorrhage and fibroblastic reactions. The stroma presented the obvious hyaline change; basophilic or eosinophilic secretions were seen in the lumen. The perisalivary gland tissue was compressed and atrophied.

IHC of SC showed strong positivity for S-100, mammaglobin, CK7, and vimentin, yet was negative for calponin (besides case 4). In nearly all of the cases, Ki-67 positivity ranged at 10% as well as case 1 ranged at 15% (Table 2).
Table 2
| No. | S-100 | Mammaglobin | Calponin | Ki67 | CK5/6 | CK7 | P63 |
|---|---|---|---|---|---|---|---|
| 1 | ++ | +++ | − | 15%+ | Focal + | +++ | − |
| 2 | Diffuse + | Diffuse + | − | − | Diffuse + | − | |
| 3 | ++ | +++ | − | 10%+ | +++ | − | |
| 4 | +++ | +++ | Focal + | 10%+ | Focal + | +++ | Partly + + |
| 5 | ++ | +++ | − | 10%+ | Focal + | +++ | − |
S-100, mammaglobin, and calponin are commonly used immunohistochemical indicators; Ki67, vimentin, and P63 can be used to predict the degree of malignancy. The results suggest that none of the Ki67 are higher than 15%, all vimentin are positive, and P63 is not positive, which is in line with the current knowledge of the SC study; and CK5/6 suggests that there may be a possible association with secretory cancer of the breast. SC, secretory carcinoma.
Discussion
SC is a type of malignant tumor that occurs in the salivary gland. It is usually of low malignancy (7). SC has similar morphology and IHC to that of breast secretory cancer which was first reported in 2010 by Skálová. In 2017, the WHO classified SC as a distinct type of salivary gland epithelial tumor and refers to it simply as Secretory Carcinoma (8). The five cases included in this study accorded with some typical histological features of SC, and met the diagnostic criteria for SC, which is characterized by slow-growing, painless tumors that lack obvious specificity.
To make an accurate postoperative diagnosis, IHC and molecular studies are necessary to distinguish SC from other salivary gland tumors.
In the clinical setting, the lack of effective detection methods is the biggest obstacle in the treatment of SC. Despite the small number of cases we collected, it is still important to gather information on these rare diseases. In our case, all patients were diagnosed with IHC after their surgery. In case 5, the high degree of malignancy was only discovered after postoperative pathologic confirmation, so a second operation was performed to remove a unilateral cervical lymph node, which was clearly detrimental to the patient’s physical and mental health. For those who prefer to avoid surgery, fine needle aspiration (FNA) is a good option to make a preoperative diagnosis (9). Additionally, MRI and contrast-enhanced MRI are highly accurate in detecting soft tissue lesions and can provide a definite basis for the diagnosis and clinical treatment of SC. Combining FNA with malignant signs features (such as irregular, lobulated, and partially cystic) may help in the diagnosis and prognosis (9).
SC is a type of lesion that occurs in the salivary glands due to the fusion of NTRK genes. The pathogenic mechanism of salivary glands involves the expression of TRK. When the TRK receptor is activated by NTRK4 fusion, it becomes an extensive carcinogenic factor because it is not regulated or controlled by nerve growth factor ligands (10).
Typically, SC can be identified by staining for S-100 and mammaglobin (11), which was observed in all of our patients. The detection of the ETV6-NTRK3 fusion gene can increase the detection rate (12), but it may not be feasible for all hospitals. A recent report suggests that diffuse and/or focal pan-TRK nuclear staining can be a sensitive and specific marker for SC of the breast (13). Since SC and SC have similar IHC, this method can also help diagnose SC.
In tumors that frequently have carcinogenic NTRK fusion, several verification methods can be used, including IHC, reverse transcription polymerase chain reaction (RT-PCR), and DNA-based next-generation sequencing (NGS). They should first be classified based on histological categories (14). The use of IHC to evaluate the expression of TRK fusion protein is widely used in clinical laboratories (15). It is relatively inexpensive and has a fast turnover time, usually within 24 hours. For IHC, it has high sensitivity in detecting TRK. Positive S-100, mammaglobin, CK7, and vimentin, as well as negative DOG-1 and calponin, are the features of SC, and low-level Ki67 should not be missed. The pathogenesis of NTRK gene fusion has been verified to be related to the excessive expression of activation bonding variants and TRKS (16). This indicates that in SC, the pan-TRK IHC can be used as a preliminary screening, but if it is negative, additional FISH or RNA horizontal fusion detection should be used.
SC is challenging to differentiate from other salivary gland tumors. Key differential diagnoses include (17): (I) acinic cell carcinoma (AiCC): shares overlapping features with SC, like microcystic and tubular structures. AiCC typically shows minimal S-100 and mammaglobulin expression, while SC co-expresses both. A panel of markers can distinguish them; (II) low-grade salivary gland ductal carcinoma (LGSDC): occurs mostly in larger glands and shows diffuse S-100 and mammaglobulin expression. Differentiation lies in the presence of myoepithelial cells surrounding LGSDC nests, marked by p63, Calponin, or SMA; (III) mucinous epidermoid carcinoma (MEC): comprises intermediate and squamous cells. Identification relies on p63 positivity and S-100 negativity, with a CRTC1-MAML2 fusion specific to MEC; (IV) polymorphic low-grade malignant adenocarcinoma (PLGA): found in minor salivary glands, lacks vesicular cytoplasm, and shows rare co-expression of S-100 and mammaglobulin.
All five cases underwent surgery, and two received radiotherapy. Higher Ki67 indices, nodules, and invasive growth are associated with increased risk of poor prognosis in traditional breast-like secretion cancer (18).
Based on the National Comprehensive Cancer Network (NCCN) Guidelines Version 2, 2023 (19), resection is recommended as the primary treatment for salivary tumors, including SC. Radiotherapy can be used as an additional treatment after surgery if the tumor is at T1 or T2 with adverse indications. However, for tumors larger than T2, neck lymph node dissection is necessary.
The retrospective study was limited by the small sample size of patients and the absence of genetic FISH. However, given the rarity of the disease, there is still a significant need for further research and exploration of this condition.
Conclusions
Our clinical and pathological findings align with those of previous studies. It can be challenging to differentiate between SC and benign or lower-grade malignant salivary gland tumors based solely on clinical characteristics and MRI images. Therefore, diagnosis requires the use of IHC and gene detection. SC tumors often exhibit a low level of malignancy and have a favorable prognosis. However, further research is necessary to enhance the clinical accuracy of diagnosis and guide appropriate treatment for SC.
Acknowledgments
Funding: None.
Footnote
Reporting Checklist: The authors have completed the AME Case Series reporting checklist. Available at https://gs.amegroups.com/article/view/10.21037/gs-24-422/rc
Peer Review File: Available at https://gs.amegroups.com/article/view/10.21037/gs-24-422/prf
Conflicts of Interest: All authors have completed the ICMJE uniform disclosure form (available at https://gs.amegroups.com/article/view/10.21037/gs-24-422/coif). P.I.C. serves as an unpaid editorial board member of Gland Surgery from March 2023 to February 2025. The other authors have no conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved. All procedures performed in this study were in accordance with the ethical standards of the institutional and/or national research committee(s) and with the Helsinki Declaration (as revised in 2013). Written informed consent for publication of this case series and accompanying images was not obtained from the patients or their relatives after all possible attempts were made.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Skálová A, Vanecek T, Sima R, et al. Mammary analogue secretory carcinoma of salivary glands, containing the ETV6-NTRK3 fusion gene: a hitherto undescribed salivary gland tumor entity. Am J Surg Pathol 2010;34:599-608. [Crossref] [PubMed]
- Baghai F, Yazdani F, Etebarian A, et al. Clinicopathologic and molecular characterization of mammary analogue secretory carcinoma of salivary gland origin. Pathol Res Pract 2017;213:1112-8. [Crossref] [PubMed]
- Zardawi IM, Hook P. Mammary analogue secretory carcinoma of minor salivary glands. Pathology 2014;46:667-9. [Crossref] [PubMed]
- Janik S, Faisal M, Marijić B, et al. Prognostic factors in mammary analogue secretory carcinomas of the parotid gland: Systematic review and meta-analysis. Head Neck 2022;44:792-804. [Crossref] [PubMed]
- Skalova A. Mammary analogue secretory carcinoma of salivary gland origin: an update and expanded morphologic and immunohistochemical spectrum of recently described entity. Head Neck Pathol 2013;7:S30-6. [Crossref] [PubMed]
- Su H, Lv G, Su Y, et al. Ultrasound Manifestations of Mammary Analogue Secretory Carcinoma of the Parotid Gland. Chinese Journal of Ultrasound in Medicine 2022;4:457-60.
- Yosefof E, Boldes T, Dan D, et al. Salivary Gland Secretory Carcinoma; Review of 13Years World-Wide Experience and Meta-Analysis. Laryngoscope 2024;134:1716-24. [Crossref] [PubMed]
- Skálová A, Gnepp DR, Lewis JS Jr, et al. Newly Described Entities in Salivary Gland Pathology. Am J Surg Pathol 2017;41:e33-47. [Crossref] [PubMed]
- Kala PS, Gupta M, Thapliyal N. Efficacy of Fine-Needle Aspiration Cytology in Diagnosing Secretory Carcinoma of Salivary Gland: A Systematic Review and Meta-Analysis. Acta Cytol 2024;68:83-106. [Crossref] [PubMed]
- Jiang T, Wang G, Liu Y, et al. Development of small-molecule tropomyosin receptor kinase (TRK) inhibitors for NTRK fusion cancers. Acta Pharm Sin B 2021;11:355-72. [Crossref] [PubMed]
- Sharma G, Kamboj M, Narwal A, et al. Diagnostic Utility of Expression Pattern of S100/Mammaglobin/SOX10/ DOG 1 Immunohistochemistry in Differentiation of Secretory and Acinic Cell Carcinoma: A Systematic Review and Meta-Analysis. Indian J Otolaryngol Head Neck Surg 2024;76:208-18. [Crossref] [PubMed]
- Lee DH, Kim JH, Yoon TM, et al. Outcomes of treatment of mammary analogue secretory carcinoma of the parotid gland. Br J Oral Maxillofac Surg 2020;58:158-62. [Crossref] [PubMed]
- Harrison BT, Fowler E, Krings G, et al. Pan-TRK Immunohistochemistry: A Useful Diagnostic Adjunct For Secretory Carcinoma of the Breast. Am J Surg Pathol 2019;43:1693-700. [Crossref] [PubMed]
- Solomon JP, Benayed R, Hechtman JF, et al. Identifying patients with NTRK fusion cancer. Ann Oncol 2019;30:viii16-22. [Crossref] [PubMed]
- Sun J, Liu S, Fu K, et al. Clinicopathological characteristics and outcomes of 23 patients with secretory carcinoma of major salivary glands. Sci Rep 2021;11:22639. [Crossref] [PubMed]
- Hechtman JF. NTRK insights: best practices for pathologists. Mod Pathol 2022;35:298-305. [Crossref] [PubMed]
- Zhao M, Zhao DH, Cheng G, et al. Clinicopathologic and molecular genetic analysis of secretory carcinoma of salivary gland. Zhonghua Kou Qiang Yi Xue Za Zhi. 2018;53:533-8. [Crossref] [PubMed]
- Sun J, Wang L, Tian Z, et al. Higher Ki67 Index, Nodal Involvement, and Invasive Growth Were High Risk Factors for Worse Prognosis in Conventional Mammary Analogue Secretory Carcinoma. J Oral Maxillofac Surg 2019;77:1187-202. [Crossref] [PubMed]
- NCCN Clinical Practice Guidelines in Oncology: Head and Neck Cancers (Version 2.2023). Available online: https://www.nccn.org/guidelines/guidelinesdetail?category=1&id=1437)


