
Comparison of clinical characteristics and pathologic complete response rate after neoadjuvant chemotherapy in women under 35 years and older women with breast cancer
Highlight box
Key findings
• Age is the main factor affecting the achievement of pathologic complete response (pCR) in patients with breast cancer (BC). Young women with breast cancer (YWBC) are more likely to achieve pCR.
What is known and what is new?
• Age has been confirmed as a very aggressive biological factor associated with the poor prognosis of BC patients.
• This study shows that chemotherapy is more effective in YWBC.
What is the implication, and what should change now?
• Research on YWBC helps to deepen the understanding of their biological characteristics and molecular mechanisms and provides an essential basis for developing clinical treatment and prevention strategies. Measures are needed to improve the early diagnosis rate, optimize treatment options, and strengthen interdisciplinary cooperation in the future.
Introduction
In the past two decades, the incidence of cancer worldwide has increased significantly (1). In the most recent decade of data collection [2010–2019], the incidence of breast cancer (BC) increased by 0.5% annually (2). In contrast, BC mortality has been declining steadily since peaking in 1989, despite a slower decrease in recent years (1.3% annually from 2011 to 2020) than in the previous decade (1.9% annually from 2002 to 2011) (3). China is one of the countries with the fastest-growing incidence of BC. The BC patients in China tend to be younger. The mean age of onset in China is 40–55 years old (4). After many years of research, age has been shown to be a biological factor affecting the prognosis and survival of BC patients; age stratification has been widely used in decision-making for subsequent treatment.
The definition of young women with breast cancer (YWBC) in Western countries and China is inconsistent. The European Society of Medical Oncology (ESMO) believes that the age threshold of YWBC is 40 years. The Chinese BC consensus and guidelines define the age for YWBC threshold as below 35 years (5). To determine the definition of YWBC, Han et al.’s predictive analysis of 9,885 BC patients found a sharply increased risk of BC death in women under 35 years old, suggesting that age younger than 35 years is a good dividing line to define young-onset BC (6). YWBC have an inferior quality of survival, and the risk of death and recurrence is very high (7).
As a particular BC group, YWBC are slightly different from other patients, and doctors and patients face relatively increased difficulties and challenges (8). Due to the young age of illness onset, the economic foundation of YWBC is insufficient to be eligible for complete medical insurance treatment, thus affecting the prognosis (9). Moreover, in general, the mammary glands of YWBC in China are very dense, with a higher chance of missed disease (10). The optimal treatment for this particular population remains controversial. Some studies have shown that although young patients have poor subsequent survival, they respond well to chemotherapy (11,12). Neoadjuvant chemotherapy (NAC) has become the standard of care for the treatment of patients with locally advanced BC, and pathologic complete response (pCR) correlates with patient survival. It may serve as a surrogate for survival outcomes (13-15).
Our study aimed to analyze and compare the clinicopathological variables of Chinese non-metastatic YWBC (≤35 years) with that of other age groups and to evaluate the pCR rate after receiving NAC between different age groups. We present this article in accordance with the STROBE reporting checklist (available at https://gs.amegroups.com/article/view/10.21037/gs-24-293/rc).
Methods
Study sample
We conducted a retrospective cohort study of patients diagnosed with BC at Harbin Medical University Cancer Hospital from 1 January 2012 to 31 December 2019 who underwent surgery after completing NAC. Before the treatment, the patients and their families were explained the purpose and intention of using the clinical and pathological data for clinical research. The study was conducted in accordance with the Declaration of Helsinki (as revised in 2013). The study was approved by the Ethics Committee of Harbin Medical University (No. KY2019-17). Individual consent for this retrospective analysis was waived.
NAC admittance criterion
NAC is recommended for patients who meet one of the following conditions: (I) large lesions (>5 cm); (II) axillary lymph node metastasis; (III) positive human epidermal growth factor receptor 2 (HER-2) expression; (IV) triple-negative breast cancer (TNBC); (V) those with a desire to preserve breasts but with a large ratio of tumor size to breast volume.
Inclusion and exclusion criteria
A total of 1,419 patients were obtained for the analysis, and screened according to the following inclusion criteria: (I) female patients; (II) patients who completed NAC before surgery and the cycle is complete; (III) BC was confirmed by pathological confirmation before chemotherapy; (IV) patients with sufficient detailed clinical and pathological data; (V) patients with T1–T3 tumors according to the American Joint Committee on Cancer (AJCC) tumor-node-metastasis (TNM) staging system. The exclusion criteria were as follows: (I) patients lacking complete data; (II) patients with multiple tumors; (III) patients missing the data of age of diagnosis and survival status; (IV) patients with occult BC; (V) male patients; (VI) patients whose treatment was interrupted, including those who transferred to another institution. Finally, 879 patients were selected for analysis. A flowchart is shown in Figure 1.

Clinical and pathological variables
Study variables included patient age, surgical mode, body mass index (BMI) value, estrogen receptor (ER) status, progesterone receptor (PR) status, HER-2 status, KI67 expression, P53 expression, T stage, N stage, molecular subtype, type of chemotherapeutic drugs, and pCR status. Patient information and treatment details were recorded from the beginning of the diagnosis.
Patients were divided according to age: patients ≤35 years were classified as the YWBC group. There were two surgical methods: breast-conserving surgery (BCS) and mastectomy. All patients underwent sentinel lymph node biopsy (SLNB). When axillary lymph nodes were abnormal, axillary lymph node dissection was performed. BMI values are stratified according to international standards of health: lean, BMI <18.5 kg/m2; normal, 18.5≤ BMI <24 kg/m2; overweight, 24≤ BMI <30 kg/m2, and obese BMI ≥30 kg/m2. Pathologists in our hospital evaluated the hormone receptor (HR) status by immunohistochemistry (IHC). ER and PR nuclear ≥1% were defined as positive. When one of ER or PR was positive, HR was positive. KI67 positive nuclear ≥15% was defined as high expression, and <15% as low expression. IHC staining 3+ was positive for HER-2, IHC staining 0 or 1+ was negative for HER-2, and when IHC staining was 2+, fluorescence in situ hybridization (FISH) was used to detect its status. When FISH was negative, HER-2 was considered negative; otherwise, it was positive. All patients underwent clinical and radiographic staging. T stage was determined using palpation and ancillary examination measures. N stage was defined as axillary lymph nodes or ultrasound with abnormal lymph nodes. Metastatic disease was assessed by ultrasound and computed tomography (CT). We divided the cancer molecular subtype into four types: HR(+)/HER-2(+), HR(+)/HER-2(−), HR(−)/HER-2(+), TNBC.
All patients were thoroughly assessed for pathologic response after surgery. pCR was defined as no evidence of invasive cancer in the examined breast tissue and lymph nodes [noninvasive breast residuals allowed (ypT0/is, ypN0)]. The presence of invasive residuals in either the breast or axilla was considered non-pCR.
Statistical analysis
The software SPSS 26.0 (IBM Corp., Armonk, NY, USA) was used to analyze the data presented in this paper, which were stratified by age. Categorical data were expressed as counts and percentages, basic data of patients were continued when compared and analyzed using the chi-square test, and correlations between clinical case parameters and pCR rates within each subgroup were performed using the chi-square test and the univariate logistic regression analysis. Statistically, significant variables from the univariate analysis were included in the multivariate analysis. To determine which variables are independent factors of pCR, P<0.05 was considered statistically significant. The nomogram was established based on the clinicopathologic factors of BC patients. In addition, we analyzed the overall performance of the nomogram by plotting the receiver operating characteristic (ROC) curve and then calculating the area under the curve (AUC) of the ROC curves to analyze the overall performance of the nomogram, with the AUC exceeding 0.7 being deemed as that the nomogram provided a reasonable estimation. The above statistical analyses were performed using R4.1.0 software (R Foundation for Statistical Computing, Vienna, Austria), including the car, rms, pROC, and rmda packages.
Results
Characteristics of study sample
From 1 January 2012 to 31 December 2019, at Harbin Medical University Cancer Hospital, 1,419 patients were diagnosed with BC; 540 patients were excluded (366 patients without complete data, 92 patients stopped treatment or transferred to another hospital, 13 patients diagnosed with occult BC, 1 male patient, 68 stage IV BC). A total of 879 patients were finally included in the study, with an age range of 21–72 years, and median age of 52 years. Patients were classified according to age (≤35 vs. >35 years old) with the clinical parameters and treatment shown in Table 1. Age of 35 years was defined as YWBC, with 71 (8.1%) individuals in this age group, and 808 (91.9%) patients were aged >35 years. There was a significant difference between the two age groups in terms of type of surgery, molecular subtype, clinical stage, and BMI (P<0.05). Compared with older women, YWBC patients were more likely to receive BCS. Still, the overall breast preservation rate was not high at only 4.1%, which may have related to the treatment environment and patients’ mind frame at that time. In the YWBC group, the overall BMI was relatively normal, and there were more overweight patients in the older group (P<0.001). The most common molecular subtype was HR(+)/HER-2(−) (43.1%, n=379), 116 (13.2%) patients were HR(+)/HER-2(+), 218 (24.8%) patients were HR(−)/HER-2(+), and 166 (18.9%) patients were TNBC. Patients were grouped by stage: 545 women were early BC (stage I, stage II), and 334 were locally advanced BC (stage III). Only 12 were treated with trastuzumab combined with pertuzumab due to the social environment, and 322 were treated with trastuzumab.
Table 1
| Patient characteristic | Age group | P value | ||||
|---|---|---|---|---|---|---|
| ≤35 years (n=71) | >35 years (n=808) | |||||
| N | % | N | % | |||
| Surgical methods | <0.001* | |||||
| BCS | 12 | 16.9 | 24 | 3.0 | ||
| Mastectomy | 59 | 83.1 | 784 | 97.0 | ||
| BMI (kg/m2) | <0.001* | |||||
| <18.5 | 3 | 4.2 | 16 | 2.0 | ||
| ≥18.5 and <24 | 47 | 66.2 | 351 | 43.4 | ||
| ≥24 and <30 | 21 | 29.6 | 388 | 48.0 | ||
| ≥30 | 0 | 0.0 | 53 | 6.6 | ||
| Clinical T stage | 0.75 | |||||
| 1 | 9 | 12.7 | 98 | 12.1 | ||
| 2 | 49 | 69.0 | 588 | 72.8 | ||
| 3 | 13 | 18.3 | 122 | 15.1 | ||
| Clinical N stage | 0.007* | |||||
| 0 | 12 | 16.9 | 98 | 12.1 | ||
| 1 | 32 | 45.1 | 467 | 57.8 | ||
| 2 | 16 | 22.5 | 84 | 10.4 | ||
| 3 | 11 | 15.5 | 159 | 19.7 | ||
| ER | 0.47 | |||||
| Positive | 42 | 59.2 | 442 | 54.7 | ||
| Negative | 29 | 40.8 | 366 | 45.3 | ||
| PR | 0.78 | |||||
| Positive | 32 | 45.1 | 350 | 43.3 | ||
| Negative | 39 | 54.9 | 458 | 56.7 | ||
| HER-2 | 0.20 | |||||
| Positive | 32 | 45.1 | 302 | 37.4 | ||
| Negative | 39 | 54.9 | 506 | 62.6 | ||
| KI67 | 0.13 | |||||
| ≤15% | 19 | 26.8 | 288 | 35.6 | ||
| >15% | 52 | 73.2 | 520 | 64.4 | ||
| P53 | 0.22 | |||||
| 0 | 44 | 62.1 | 417 | 51.5 | ||
| 1 | 17 | 23.9 | 192 | 23.8 | ||
| 2 | 5 | 7.0 | 91 | 11.3 | ||
| 3 | 5 | 7.0 | 108 | 13.4 | ||
| Stage | 0.13 | |||||
| I + II | 38 | 53.5 | 507 | 62.7 | ||
| III | 33 | 46.5 | 301 | 37.3 | ||
| Molecular subtype | 0.002* | |||||
| HR+/HER-2+ | 19 | 26.8 | 97 | 12.0 | ||
| HR+/HER-2− | 23 | 32.4 | 356 | 44.0 | ||
| HR−/HER-2+ | 13 | 18.3 | 205 | 25.4 | ||
| TNBC | 16 | 22.5 | 150 | 18.6 | ||
| Targeted therapy | 0.29 | |||||
| Trastuzumab + pertuzumab | 2 | 2.8 | 10 | 1.3 | ||
| Trastuzumab | 30 | 42.3 | 292 | 36.1 | ||
| Other therapy | 39 | 54.9 | 506 | 62.6 | ||
*, statistically significant at P≤0.05. BC, breast cancer; BCS, breast conserving surgery; BMI, body mass index; ER, estrogen receptor; PR, progesterone receptor; HER-2, human epidermal growth factor receptor 2; HR, hormone receptor; TNBC, triple-negative breast cancer.
Association of clinical factors with pCR in the whole cohort
In the entire cohort, 144 people (16.4%) had reached pCR, and 735 people (83.6%) had not reached pCR. The probability of achieving pCR is still high before 35 years of age and decreases slightly after age 35. The univariate analysis determined the factors affecting the pCR rate after NAC. Age, T stage, ER expression, PR expression, HER-2 expression, KI67 expression, and clinical stage were closely related to the pCR rate (P<0.05) (Table 2). However, no significant correlation existed between N stage, surgical methods, BMI, P53 expression, and pCR (P>0.05). Patients with molecular typing of HR(−)/HER-2(+) and HR(+)/HER-2(+) had a higher pCR rate of 27.1% and 21.6%, respectively and the worst sensitivity to NAC was observed in patients with HR(+)/HER-2(−) (Table S1, Figure 2). YWBC patients achieved pCR more easily than other ages (25.4% vs. 15.6%, P<0.05). Moreover, the higher the T stage, ER positive, PR positive, and HER-2 negative, the less likely the patients are to reach pCR, including the statistically significant ones in the univariate analysis into the multivariate analysis. The logistic regression analysis revealed that patients with YWBC were more likely to achieve pCR than older patients [odds ratio (OR) =2.003, 95% confidence interval (CI): 1.101–3.645, P=0.02]. When the PR expression was negative, patients were more likely to reach pCR (OR =2.003, 95% CI: 1.098–3.656, P=0.02). Besides, patients who were HER-2 positive were more likely to achieve pCR than negative patients (OR =2.003, 95% CI: 1.392–3.051, P<0.001). The pCR rate of the Ki67 >15% group was higher than that of the Ki67 ≤15% group (OR =1.773, 95% CI: 1.142–2.752, P=0.01). When the clinical stage was I + II, the probability of obtaining PCR is higher (OR =1.740, 95% CI: 1.111–2.723, P=0.02). Therefore, age, PR expression, HER-2 expression, KI67 expression, and clinical stage are closely related to whether patients can reach pCR (P<0.05), which are the independent predictors of reaching pCR (Table 3). In the group of HER-2 positive patients, the pCR rate was lower in patients treated with the combination of trastuzumab and pertuzumab than in patients treated with trastuzumab alone (16.7% vs. 25.5%, P>0.05), which was most likely due to the small number of patients (Table S2).
Table 2
| Patient characteristic | All patients | χ2 | P value | ||||
|---|---|---|---|---|---|---|---|
| pCR (n=144) | Non-pCR (n=735) | ||||||
| N | % | N | % | ||||
| Age (years) | 4.537* | 0.03* | |||||
| ≤35 | 18 | 12.5 | 53 | 7.2 | |||
| >35 | 126 | 87.5 | 682 | 92.8 | |||
| Surgical methods | 0.761 | 0.39 | |||||
| BCS | 4 | 27.8 | 32 | 4.4 | |||
| Mastectomy | 140 | 72.2 | 703 | 95.6 | |||
| BMI (kg/m2) | 3.488 | 0.32 | |||||
| <18.5 | 1 | 0.6 | 18 | 2.4 | |||
| ≥18.5 and <24 | 73 | 50.7 | 325 | 44.2 | |||
| ≥24 and <30 | 61 | 42.4 | 348 | 47.3 | |||
| ≥30 | 9 | 6.3 | 44 | 6.1 | |||
| Clinical T stage | 18.043* | <0.001* | |||||
| 1 | 31 | 21.5 | 76 | 10.3 | |||
| 2 | 101 | 70.1 | 536 | 73.0 | |||
| 3 | 12 | 8.4 | 123 | 16.7 | |||
| Clinical N stage | 5.099 | 0.17 | |||||
| 0 | 26 | 18.1 | 84 | 11.4 | |||
| 1 | 79 | 54.9 | 420 | 57.1 | |||
| 2 | 14 | 9.7 | 86 | 11.7 | |||
| 3 | 25 | 17.3 | 145 | 19.8 | |||
| ER | 28.795* | <0.001* | |||||
| Positive | 50 | 34.7 | 434 | 59.0 | |||
| Negative | 94 | 65.3 | 301 | 41.0 | |||
| PR | 31.607* | <0.001* | |||||
| Positive | 32 | 22.2 | 350 | 47.6 | |||
| Negative | 112 | 77.8 | 385 | 52.4 | |||
| HER-2 | 31.139* | <0.001* | |||||
| Positive | 84 | 58.3 | 250 | 34.0 | |||
| Negative | 60 | 41.7 | 485 | 66.0 | |||
| KI67 | 10.672* | <0.001* | |||||
| ≤15% | 32 | 22.2 | 267 | 36.3 | |||
| >15% | 112 | 77.8 | 468 | 63.7 | |||
| P53 | 6.215 | 0.10 | |||||
| 0 | 66 | 45.8 | 395 | 53.8 | |||
| 1 | 34 | 23.6 | 175 | 23.8 | |||
| 2 | 17 | 11.8 | 79 | 10.7 | |||
| 3 | 27 | 18.8 | 86 | 11.7 | |||
| Chemotherapy regimen | 2.007 | 0.16 | |||||
| Anthracycline-based | 38 | 26.4 | 238 | 32.4 | |||
| Other therapy | 106 | 73.6 | 497 | 67.6 | |||
| Stage | 6.632* | 0.01* | |||||
| I + II | 103 | 71.5 | 442 | 60.2 | |||
| III | 41 | 28.5 | 293 | 39.8 | |||
*, statistically significant at P≤0.05. pCR, pathologic complete response; BCS, breast conserving surgery; BMI, body mass index; ER, estrogen receptor; PR, progesterone receptor; HER-2, human epidermal growth factor receptor 2.

Table 3
| Patient characteristic | B | SE | Wald | OR | 95% CI | P value |
|---|---|---|---|---|---|---|
| Age (years) | ||||||
| >35 | Ref | |||||
| ≤35 | 0.695 | 0.305 | 5.174 | 2.003 | 1.101–3.645 | 0.02* |
| Clinical T stage | ||||||
| 3 | Ref | |||||
| 1+2 | 0.65 | 0.355 | 3.363 | 1.916 | 0.956–3.840 | 0.07 |
| ER | ||||||
| Positive | Ref | |||||
| Negative | 0.324 | 0.276 | 1.383 | 1.383 | 0.806–2.375 | 0.24 |
| PR | ||||||
| Positive | Ref | |||||
| Negative | 0.695 | 0.307 | 5.122 | 2.003 | 1.098–3.656 | 0.02* |
| HER-2 | ||||||
| Negative | Ref | |||||
| Positive | 0.723 | 0.2 | 13.062 | 2.003 | 1.392–3.051 | <0.001* |
| KI67 | ||||||
| ≤15% | Ref | |||||
| >15% | 0.573 | 0.224 | 6.513 | 1.773 | 1.142–2.752 | 0.01* |
| Stage | ||||||
| III | Ref | |||||
| I + II | 0.554 | 0.229 | 5.864 | 1.740 | 1.111–2.723 | 0.02* |
*, statistically significant at P≤0.05. pCR, pathologic complete response; ER, estrogen receptor; PR, progesterone receptor; HER-2, human epidermal growth factor receptor 2; Ref, reference; SE, standard error; OR, odds ratio; CI, confidence interval.
Predictive analysis of patients obtaining pCR or non-pCR
Age, PR expression, HER-2 expression, KI67 expression, and clinical stage were independent predictors of patients obtaining pCR after NAC. The ROC curve was used to analyze these clinical factors to estimate the predictive probability of patients achieving pCR (Figures 3,4). It was found that when patients had KI67 >15%, there was a 57% probability of pCR. When HER-2 expression is positive in patients, the probability of pCR is 61.7% (Table 4), which was statistically significant (P<0.05). In addition, the predictive analysis of non-pCR was conducted. The study found that the probability of patients over the age of 35 years achieving no-pCR was 52.6% (P>0.05). With PR expression positive and clinical stage III, the odds of non-pCR were also very high, at 62.8% and 55.7%, respectively, and both were statistically significant (P<0.05) (Table 5).


Table 4
| Patient characteristic | AUC | SE | 95% CI | P value |
|---|---|---|---|---|
| HER-2 | ||||
| Negative | Ref | |||
| Positive | 0.617 | 0.026 | 0.567–0.668 | <0.001* |
| KI67 | ||||
| ≤15% | Ref | |||
| >15% | 0.571 | 0.025 | 0.522–0.620 | 0.007* |
*, statistically significant at P≤0.05. HER-2, human epidermal growth factor receptor 2; pCR, pathologic complete response; Ref, reference; AUC, area under the curve; SE, standard error; CI, confidence interval.
Table 5
| Patient characteristic | AUC | SE | 95% CI | P |
|---|---|---|---|---|
| Age | ||||
| ≤35 years | Ref | |||
| >35 years | 0.526 | 0.027 | 0.473–0.579 | 0.32 |
| PR | ||||
| Negative | Ref | |||
| Positive | 0.628 | 0.024 | 0.581–0.676 | <0.001* |
| Stage | ||||
| I + II | Ref | |||
| III | 0.557 | 0.026 | 0.507–0.607 | 0.03* |
*, statistically significant at P≤0.05. PR, progesterone receptor; pCR, pathologic complete response; Ref, reference; AUC, area under the curve; SE, standard error; CI, confidence interval.
Construction of a nomogram-to predict pCR in patients with BC after receiving NAC
A nomogram based on clinicopathologic characteristics of BC patients was designed to predict the probability of reaching pCR when BC patients had undergone NAC. PR status has the most significant influence on pCR, and age also has a significant influence. YWBC are more likely to reach pCR (Figure 5). We used the ROC curve to assess the predictive ability of the nomogram graph-based prediction model to achieve pCR in BC patients. The AUC was 0.717, indicating that the prediction model had good judgment (Figure 6).


Correlation between clinical factors and pCR in the YWBC and older groups
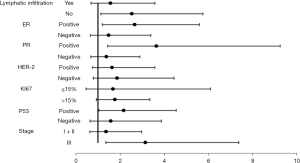
We also analyzed the pCR rate of each clinical feature according to the age group (Table S3). It was revealed that most clinical characteristics were not statistically significant according to age group and pCR rate (P>0.05). We further analyzed the interaction of age and the clinical characteristics of YWBC through a general linear model and calculated the relative risk (RR) value between the subgroups (Table 6, Figure 7).
Table 6
| Patient characteristic | YWBC | pCR (YWBC vs. other) | Relative risk (95% CI) | P for interaction |
|---|---|---|---|---|
| ER | ||||
| Positive | 42 | 9/42 vs. 41/442 | 2.667 (1.194–5.591) | <0.001* |
| Negative | 29 | 9/29 vs. 85/366 | 1.488 (0.653–3.389) | |
| PR | ||||
| Positive | 32 | 7/32 vs. 25/350 | 3.640 (1.434–9.241) | <0.001* |
| Negative | 39 | 11/39 vs. 101/428 | 1.389 (0.668–2.886) | |
| HER-2 | ||||
| Positive | 32 | 11/32 vs. 73/302 | 1.634 (0.757–3.569) | <0.001* |
| Negative | 39 | 7/39 vs. 53/506 | 1.870 (0.787–4.445) | |
| KI67 | ||||
| ≤15% | 19 | 3/19 vs. 29/288 | 1.675 (0.460–6.092) | <0.001* |
| >15% | 52 | 15/52 vs. 97/520 | 1.768 (0.933–3.350) | |
| P53 | ||||
| Positive | 44 | 11/44 vs. 55/417 | 2.170 (1.036–4.543) | <0.001* |
| Negative | 27 | 7/27 vs. 71/391 | 1.577 (0.642–3.873) | |
| Stage | ||||
| I + II | 38 | 9/38 vs. 94/507 | 1.364 (0.625–2.977) | <0.001* |
| III | 33 | 9/33 vs. 32/301 | 3.152 (1.348–7.370) |
*, statistically significant at P≤0.05. YWBC, young women with breast cancer; pCR, pathologic complete response; ER, estrogen receptor; PR, progesterone receptor; HER-2, human epidermal growth factor receptor 2; CI, confidence interval.
Comparison of the relationship between age and pCR by propensity score matching (PSM)
This study continued to analyze the relationship between age and pCR by PSM. The YWBC group had 71 people after matching and 71 people in the older group (Table S4), with no difference in most baseline characteristics (P>0.05). The pCR rate of YWBC patients was still very high (25.4% vs. 8.5%, P<0.05), and the binary logistic regression analysis of age and pCR found that age was still an independent predictor of patients achieving pCR after NAC (P=0.01) Patients with YWBC reached pCR more efficiently than older patients (Tables S5,S6).
Discussion
A total of 879 female patients with BC were selected for this study. In this cohort, we found that YWBC in our hospital was about 8.1%, similar to the epidemiological survey in China, with YWBC in developed countries such as the US even less than 7% (1). This makes it challenging to draw reasonable conclusions and to provide a detailed description when studying patients with YWBC, which was another purpose of this study. This retrospective study examining the response to NAC in BC women found a better pCR rate in the YWBC group (25.4% vs. 15.6%, P=0.03) and was more pronounced in patients molecularly typed as TNBC and HER-2(+). This study also found that age, PR expression, HER-2 expression, KI67 expression, and clinical stage were independent predictors of achieving pCR in BC patients.
Age stratification has been widely used to determine patient treatment decisions. However, there still needs to be an expert consensus on YWBC thresholds, and most studies believe that reasonable age cut-offs should be between 35 and 40 years (16). NAC is increasingly being employed in the management of YWBC. NAC can find sensitive drugs for BC patients, and the effect of treatment can be better observed through each imaging examination to provide favorable and effective treatment (17). In 1999, Braud et al. first evaluated BC patients’ NAC response and prognosis (18). They found that the outcomes of young patients were highly dependent on their response to primary chemotherapy. The pCR rate was significantly higher than that of older patients, but the 5-year disease-free survival (DFS) rate and overall survival (OS) rate were lower than those of older patients (19). Spring et al. found that pCR after NAC is strongly associated with significantly improved DFS and OS in YWBC (20). Although NAC controls the primary tumors better, young patients remain at high risk of local and metastatic recurrence. Nevertheless, age should not justify overtreatment; it is an alternative risk factor. Age does not determine treatment intensity, and all patients should be treated objectively (21,22).
BCS is currently a recognized surgical method for women with BC, which is usually preferred as a means to mitigate psychological distress after breast removal. A total of 36 people (4.1%) retained their breasts, and the breast preservation rate in the YWBC group was significantly higher than that in the older group (16.9% vs. 3.7%, P<0.001). However, no significant relationship existed between the surgical method and pCR rate (P>0.05). A prospective cohort study in Sweden found that 5-year OS reached 91.1% after receiving BCS, with a significantly better prognosis than with mastectomy with or without radiotherapy (23). Vila et al. conducted a meta-analysis in which they found that mastectomy seemed unlikely to provide better OS than BCS in BC patients aged 40 years or younger (24). Scardina et al. found that conservative mastectomy with immediate prosthetic breast reconstruction in addition to BCS after NAC was superior in the improvement of aesthetic outcomes and patient quality of life (25). However, it has also been found that breast preservation in YWBC with lymphovascular invasion, extensive intra-ductal component, and high nodal status increases the risk of local recurrence (26).
HR has been an essential factor affecting NAC efficacy and survival in BC patients, and some studies have shown that ER and PR negativity occur higher in BC patients under 30 years of age, reaching 51–53%, with more increased biological invasion and poor prognosis, but a better response to chemotherapy (27,28). In this cohort, there were 20 people with ER negative expression (40.8%) and 39 (54.9%) with PR negative expression in the YWBC group. Although there was no significant relationship with the older group (P>0.05), the chemotherapeutic effect among YWBC was generally better than that in the older group regardless of HR negativity or positivity. When performing the subgroup analysis, the pCR rate was significantly higher in the HR negative group than in the positive group. Woo et al. compared the chemotherapy response of YWBC with ER-negative and ER-positive patients in the older group and found no difference in response to NAC between younger and older patients in the ER-positive group. In contrast, younger patients in the ER-negative group responded better to NAC than did older patients (12). Most studies indicate that chemotherapy effects are significantly lower in HR positive patients than in negative patients, mainly due to the presence of cancer stem cells in HR-positive patient cells, which can escape the effects of chemotherapy through elevated P53 expression (29). Therefore, many experts believe that the impact of HR on patients has exceeded the effects of age.
In our study, 334 (38.0%) patients were HER-2 positive, and the proportion of HER-2 positivity among YWBC patients was also high (45.1% vs. 37.4%, P=0.20), similar to the Ararat statistics (30). We also concluded that HER-2 expression was an independent predictor of pCR (P<0.001). HER2-positive cancers are more aggressive than HER2-negative more readily achieve pCR via chemotherapy and endocrine therapy (31). The application of trastuzumab is also becoming increasingly popular, and the prognosis of YWBC has improved. Rouzier et al. compared the pCR rates of patients with different molecular subtypes of BC. The basal-like and HER-2-positive subtypes of BC are more sensitive to paclitaxel- and doxorubicin-containing preoperative chemotherapy than the luminal and normal-type cancers (32).
In addition to these differences in clinicopathological factors, many problems facing YWBC must also be addressed. First of all, young patients are susceptible to increased anxiety and depression due to long-term treatment, which will seriously affect the quality of life of the patients (33). Furthermore, the pressure on the family is also tremendous, not only the need to provide material security for patients but also to provide necessary emotional support. A good family environment is essential for treatment (34). In addition, some YWBC are mothers, and parenting while dealing with BC is a difficult challenge for many women. Over time, maternal happiness decreases, and they may require external support to control their fear of recurrence (35).
YWBC may differ from older patients in terms of their biological characteristics, disease progression rate, treatment response, and prognosis due to their younger age. There are relatively few studies on the effects of NAC in this specific group; therefore, this study can fill the gap in this area and provide a more comprehensive basis for clinical decision-making. In addition, by deeply studying the response of YWBC to NAC, we can further reveal the differences in the molecular mechanisms of BC in patients of different ages. This will help us better understand the heterogeneity of BC and the sensitivity of different molecular subtypes to specific therapeutic regimens, thus promoting the development of BC precision therapy.
Limitations
There are still many aspects that can be improved in this study. Firstly, this study is a single-center retrospective analysis, and each patient’s treatment was different, which may have affected the patient’s outcome and therefore lack uniformity. Second, this study did not analyze the effect of BRCA gene mutation on YWBC. Third, there needs to be more information on the quality of life of BC patients. Lifestyle is also an essential factor affecting the chemotherapeutic outcome of BC patients, and we need to analyze the relationship between the two further in the follow-up. Fourth, BC patients’ race, ethnicity, and socioeconomic status were not analyzed in this study, which need to be contextualized in subsequent studies. Fifth, the high percentage of HER-2 positive BC patients in this study may have made our results controversial, and we need to include more patients for subsequent analysis. Sixth, although patients were widely selected, survival analysis was not performed, and patient prognosis remains the most critical study endpoint. In the future, we need continuous postoperative follow-up to study DFS and OS and to explore the relationship between YWBC and elderly patients.
Conclusions
Patient age, ER expression, PR expression, HER-2 expression, KI67 expression, and clinical stage were independent predictors of patients reaching pCR in Chinese women. YWBC patients tend to be more biologically aggressive and their tumors are more likely to grow and spread rapidly, so their OS and prognosis are usually poorer. They also have a higher need for preservation of breast appearance, genetic testing, protection of ovarian function, fertility, and quality of life compared to older patients. This study found that YWBC are more likely to achieve pCR, and by studying the response of YWBC patients to NAC, it is possible to identify a group of patients who are sensitive or resistant to this treatment regimen, so that a more personalized treatment plan can be developed for them, and the relevance and efficacy of the treatment can be improved. This is not only an exploration of therapeutic strategies for this specific group, but also a boost to the entire field of BC research.
Acknowledgments
We thank the patients participating in this study and all staff at the hospital for their contributions to this study.
Funding: This study was funded by
Footnote
Reporting Checklist: The authors have completed the STROBE reporting checklist. Available at https://gs.amegroups.com/article/view/10.21037/gs-24-293/rc
Data Sharing Statement: Available at https://gs.amegroups.com/article/view/10.21037/gs-24-293/dss
Peer Review File: Available at https://gs.amegroups.com/article/view/10.21037/gs-24-293/prf
Conflicts of Interest: All authors have completed the ICMJE uniform disclosure form (available at https://gs.amegroups.com/article/view/10.21037/gs-24-293/coif). The authors have no conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved. The study was conducted in accordance with the Declaration of Helsinki (as revised in 2013). The study was approved by the Ethics Committee of Harbin Medical University (No. KY2019-17). Individual consent for this retrospective analysis was waived.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- US Preventive Services Task Force. Screening for Breast Cancer: US Preventive Services Task Force Recommendation Statement. JAMA 2024;331:1918-30. Erratum in: JAMA 2024 doi: 10.1001/jama.2024.19851. [Crossref] [PubMed]
- Santos P, Barnes J, Boe L, et al. Identifying modifiable risk factors to improve immigrant breast cancer screening in the United States. J Clin Oncol 2024;42:abstr 1530.
- Siegel RL, Giaquinto AN, Jemal A. Cancer statistics, 2024. CA Cancer J Clin 2024;74:12-49. [Crossref] [PubMed]
- Bray F, Laversanne M, Sung H, et al. Global cancer statistics 2022: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J Clin 2024;74:229-63. [Crossref] [PubMed]
- Li P, Li L, Xiu B, et al. The Prognoses of Young Women With Breast Cancer (≤35 years) With Different Surgical Options: A Propensity Score Matching Retrospective Cohort Study. Front Oncol 2022;12:795023. [Crossref] [PubMed]
- Han W, Kang SYKorean Breast Cancer Society. Relationship between age at diagnosis and outcome of premenopausal breast cancer: age less than 35 years is a reasonable cut-off for defining young age-onset breast cancer. Breast Cancer Res Treat 2010;119:193-200. [Crossref] [PubMed]
- Culha Y, Davarci SE, Ünlü B, et al. Comparison of clinicopathological and prognostic features of breast cancer patients younger than 40 years and older than 65 years. Discov Oncol 2024;15:126. [Crossref] [PubMed]
- Ehsan AN, Wu CA, Minasian A, et al. Financial Toxicity Among Patients With Breast Cancer Worldwide: A Systematic Review and Meta-analysis. JAMA Netw Open 2023;6:e2255388. [Crossref] [PubMed]
- Altherr A, Bolliger C, Kaufmann M, et al. Education, Employment, and Financial Outcomes in Adolescent and Young Adult Cancer Survivors-A Systematic Review. Curr Oncol 2023;30:8720-62. [Crossref] [PubMed]
- Wang X, Xia C, Wang Y, et al. Landscape of young breast cancer under 35 years in China over the past decades: a multicentre retrospective cohort study (YBCC-Catts study). EClinicalMedicine 2023;64:102243. [Crossref] [PubMed]
- Wang S, Wen W, Zhao H, et al. Prediction of clinical response to neoadjuvant therapy in advanced breast cancer by baseline B-mode ultrasound, shear-wave elastography, and pathological information. Front Oncol 2023;13:1096571. [Crossref] [PubMed]
- Woo J, Oh SJ, Song JY, et al. Response to neoadjuvant chemotherapy based on pathologic complete response in very young patients with ER-positive breast cancer: a large, multicenter, observational study. BMC Cancer 2021;21:647. [Crossref] [PubMed]
- Oprea AL, Gulluoglu B, Aytin YE, et al. Conventional Tools for Predicting Satisfactory Response to Neoadjuvant Chemotherapy in HR+/HER2- Breast Cancer Patients. Breast Care (Basel) 2023;18:344-53. [Crossref] [PubMed]
- Arecco L, Latocca MM, Blondeaux E, et al. Adjuvant endocrine therapy choices in premenopausal patients with hormone receptor-positive early breast cancer: Insights from the prospective GIM23-POSTER study. Breast 2024;77:103769. [Crossref] [PubMed]
- Gentile D, Sagona A, De Carlo C, et al. Pathologic response and residual tumor cellularity after neo-adjuvant chemotherapy predict prognosis in breast cancer patients. Breast 2023;69:323-9. [Crossref] [PubMed]
- Farkas AH, Nattinger AB. Breast Cancer Screening and Prevention. Ann Intern Med 2023;176:ITC161-76. [Crossref] [PubMed]
- Sbaity E, Tamim H, El-Hajj Fuleihan G, et al. Effect of young age (below 40 years) on oncologic outcomes in Lebanese patients with breast cancer: a matched cohort study. BMC Cancer 2024;24:560. [Crossref] [PubMed]
- Braud AC, Asselain B, Scholl S, et al. Neoadjuvant chemotherapy in young breast cancer patients: correlation between response and relapse? Eur J Cancer 1999;35:392-7. [Crossref] [PubMed]
- Jung JJ, Cheun JH, Kim SY, et al. Omission of Breast Surgery in Predicted Pathologic Complete Response after Neoadjuvant Systemic Therapy: A Multicenter, Single-Arm, Non-inferiority Trial. J Breast Cancer 2024;27:61-71. [Crossref] [PubMed]
- Spring L, Greenup R, Niemierko A, et al. Pathologic Complete Response After Neoadjuvant Chemotherapy and Long-Term Outcomes Among Young Women With Breast Cancer. J Natl Compr Canc Netw 2017;15:1216-23. [Crossref] [PubMed]
- Lv FY, Mo Z, Chen B, et al. Locoregional recurrence and survival of breast-conserving surgery compared to mastectomy following neoadjuvant chemotherapy in operable breast cancer. Front Oncol 2024;14:1308343. [Crossref] [PubMed]
- Emirzeoglu L, Arici S, Sahin AB, et al. The Predictive Importance of Body Mass Index on Response to Neoadjuvant Chemotherapy in Patients with Breast Cancer. Breast Care (Basel) 2023;18:42-8. [Crossref] [PubMed]
- Tamminen A, Aaltonen RI, Ristola MT. Postoperative bleeding complications in breast conserving surgery and the role of antithrombotic medications: retrospective analysis of 4712 operations. World J Surg Oncol 2024;22:234. [Crossref] [PubMed]
- Vila J, Gandini S, Gentilini O. Overall survival according to type of surgery in young (≤40 years) early breast cancer patients: A systematic meta-analysis comparing breast-conserving surgery versus mastectomy. BREAST 2015;24:175-81. [Crossref] [PubMed]
- Scardina L, Di Leone A, Biondi E, et al. Prepectoral vs. Submuscular Immediate Breast Reconstruction in Patients Undergoing Mastectomy after Neoadjuvant Chemotherapy: Our Early Experience. J Pers Med 2022;12:1533. [Crossref] [PubMed]
- Tsai HY, Kao YW, Wang JC, et al. Multitask deep learning on mammography to predict extensive intraductal component in invasive breast cancer. Eur Radiol 2024;34:2593-604. [Crossref] [PubMed]
- Eckardt NK, Ignatov A, Meinecke AM, et al. Tumor characteristics, therapy, and prognosis in young breast cancer patients ≤ 35 years. J Cancer Res Clin Oncol 2023;149:709-19. [Crossref] [PubMed]
- Li C, Du C, Wang Y, et al. Risk, molecular subtype and prognosis of second primary breast cancer: an analysis based on first primary cancers. Am J Cancer Res 2023;13:3203-20.
- Ansar M, Thu LTA, Hung CS, et al. Promoter hypomethylation and overexpression of TSTD1 mediate poor treatment response in breast cancer. Front Oncol 2022;12:1004261. [Crossref] [PubMed]
- Ararat E, Sahin I, Arslan C, et al. Clinical and pathological characteristics of very young breast cancer patients (≤ 25 years of age). J BUON 2011;16:372.
- Fang Y, Zhang Q, Wu Y, et al. HER2-positive is an independent indicator for predicting pathological complete response to neoadjuvant therapy and Ki67-changed after neoadjuvant chemotherapy predicts favorable prognosis in Chinese women with locally advanced breast cancer. Medicine (Baltimore) 2024;103:e37170. [Crossref] [PubMed]
- Rouzier R, Perou CM, Symmans WF, et al. Breast cancer molecular subtypes respond differently to preoperative chemotherapy. Clin Cancer Res 2005;11:5678-85. [Crossref] [PubMed]
- Kim KH, Kim MS, Choi S, et al. Health behaviors and psychological burden of adolescents after parental cancer diagnosis. Sci Rep 2022;12:21018. [Crossref] [PubMed]
- Petermann-Meyer A, Panse JP, Bremen R, et al. Effectiveness of a comprehensive support program for families with parental cancer (Family-SCOUT): results of a multicenter non-randomized controlled trial. ESMO Open 2024;9:103493. [Crossref] [PubMed]
- Romare Strandh M, Enebrink P, Stålberg K, et al. Parenting under pressure: a cross-sectional questionnaire study of psychological distress, parenting concerns, self-efficacy, and emotion regulation in parents with cancer. Acta Oncol 2024;63:468-76. [Crossref] [PubMed]
(English Language Editor: J. Jones)


