
Development and validation of a novel pancreaticojejunostomy strategy based on the anatomical location of the main pancreatic duct that can reduce the risk of postoperative pancreatic fistula after pancreatoduodenectomy
Highlight box
Key findings
• Based on the anatomical location of the main pancreatic duct, the novel pancreaticojejunostomy strategy effectively reduces the incidence of postoperative pancreatic fistula.
What is known and what is new?
• Pancreatoenteric anastomotic failure remains the primary etiology of pancreatic fistula following pancreaticoduodenectomy (PD), and debate continues regarding the optimal choice of anastomosis. The impact of the location of the main pancreatic duct in the pancreas on pancreatic fistula remains uncertain.
• The classification was performed for the first time based on the anatomical location of the main pancreatic duct, which exhibits a specific prognostic potential for postoperative pancreatic fistula. Based on the anatomical location of the main pancreatic duct, the novel pancreaticojejunostomy strategy effectively reduces the incidence of postoperative pancreatic fistula.
What is the implication, and what should change now?
• We posit that this pancreaticojejunostomy strategy can serve as an optional anastomotic technique for PD and merits promotion and application.
Introduction
Background
Pancreaticoduodenectomy (PD) is a well-established surgical procedure that is commonly used to manage neoplasms or inflammatory lesions in the pancreatic head (1). After surgeons have implemented cautious efforts, the perioperative mortality rate of patients undergoing PD has significantly reduced. However, the prevalence of postoperative complications remains substantial (2,3). Postoperative pancreatic fistula (POPF) is the most serious postoperative complication of pancreatic surgery (4). The activation of the leaked pancreatic fluid can lead to the destruction of pancreaticojejunostomy and the corrosion of surrounding tissues, which may result in the development of multiple postoperative complications and even death (1). At present, extensive studies on the risk factors associated with POPF, such as body mass index (BMI), pancreatic texture and duct diameter, surgical duration, intraoperative blood loss volume, and the type of pancreaticojejunostomy, have been conducted (5,6). However, among the factors affecting the development of pancreatic fistula, the type of pancreaticojejunostomy is a significantly controllable factor to prevent POPF development. Further, improvements in pancreaticojejunostomy can effectively reduce the incidence of POPF (7-11).
Currently, there are several pancreaticojejunostomy techniques available. However, none of them can completely prevent POPF development (8,12,13). The selection process for pancreaticojejunostomy among pancreatic surgeons is not standardized. Even the most commonly used duct-to-mucosa pancreaticojejunostomy techniques differ in terms of factors such as the number of anastomotic layers, injection angle of the suture, and number of stitches. Thus, whether the pancreas can autonomously determine the specific type of anastomosis has been questioned. In various clinical studies, the position of the main pancreatic duct within the cross-section of the pancreatic stump is not consistently fixed, with a portion of it located at the pancreatic periphery. When anastomosing, the proximity of the pancreatic duct to the pancreatic edge directly influences the amount of tissues traversed by the suture. Consequently, this can lead to a greater shear force exerted on the corresponding pancreatic tissue, thereby increasing susceptibility to tear and, subsequently, the risk of pancreatic fistula formation. Therefore, based on clinical experience and the three-dimensional anatomical characteristics of the pancreas, this study hypothesized that POPF development is associated with the anatomical location of the main pancreatic duct.
Objective
The current study aimed to investigate the impact of the location of the main pancreatic duct on POPF development. Moreover, it validated whether a novel pancreaticojejunostomy approach based on the anatomical location of the main pancreatic duct can reduce the incidence of POPF. This manuscript is written in accordance with the STROBE reporting checklist (14) (available at https://gs.amegroups.com/article/view/10.21037/gs-24-235/rc).
Methods
Study design
This retrospective cohort study was performed in accordance with the Declaration of Helsinki (as revised in 2013) and was approved by the institutional review boards of West China Hospital of Sichuan University (No. 2023-1880), and the requirement of individual consent for this retrospective analysis was waived. The data of 871 patients who had undergone PD at the department of pancreatic surgery in our hospital between January 2018 and December 2021 were retrospectively collected.
Inclusion and exclusion criteria
The inclusion criteria were as follows: patients aged between 18 and 85 years and those who underwent PD with pancreatic duct anastomosis to the intestinal duct. Abdominal enhanced computed tomography (CT) was conducted within 1 month prior to surgery at our hospital to ensure comprehensive data availability.
The exclusion criteria were as follows: patients who had undergone combined distal pancreatectomy, those with multiple pancreatic injuries, those with intraoperative modifications in surgical techniques, and those with concurrent resection of arteries.
Research procedure
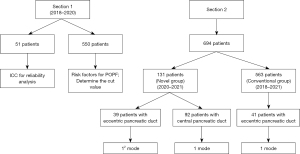
The pancreatic neck and the area in front of the confluence of the superior mesenteric and splenic veins are commonly selected as the precise transection sites in pancreatic surgery due to the anatomical characteristics of the pancreas. The consistency of the transection site position among patients can make it a suitable point for analysis. In Section 1, the intraoperative pancreatic cross-sectional data and preoperative CT cross-sectional images of 51 patients undergoing PD were analyzed. Then, a consistency test was performed (Figure 1). After the initial analysis, a retrospective study on 550 patients was conducted from January 2018 to February 2020. The univariate and multivariate analyses were performed to evaluate the association between the location of the main pancreatic duct and the development of POPF. The predictive performance of the anatomical location of the main pancreatic duct was assessed using the area under the receiver operating characteristic (ROC) curve. In Section 2, 694 patients were enrolled between September 2018 and December 2021 to investigate the efficacy of the novel pancreaticojejunostomy method based on the main pancreatic duct location in reducing POPF.
Data collection
Patient data including perioperative evaluation results, age, sex, BMI, hematocrit values, total blood bilirubin levels, amylase levels, surgical duration, pancreatic texture and duct diameter, and pathological examination results were retrospectively collected. Hemoglobin and hematocrit levels were reassessed on the 3rd–5th day after surgery. In addition, an intraoperative pancreatic duct diameter of 3 mm could be appropriate (15). The intraoperative blood loss volume was examined using the Mercuriali’s (16) method. The specific formula as follows: intraoperative blood loss volume = blood volume × (preoperative hematocrit Hct level − postoperative 5-day hematocrit Hct levels) + intraoperative blood transfusion. In this formula, the blood volume was determined using the Nadler’s (17) approach. The aforementioned calculation methods have been extensively used and validated (18).
Outcome definition
Based on the International Study Group on Pancreatic Surgery (19-21), the primary outcome was the development of clinically relevant POPF. Meanwhile, the secondary outcomes encompassed delayed gastric emptying (DGE), postpancreatectomy hemorrhage, reoperation, 30-day mortality, and other postoperative complications. The postoperative complications were categorized as mild (grades 1–2) or severe (grades ≥3) based on the Clavien-Dindo (CD) classification of surgical complications (22).
Perioperative treatment
During the perioperative period, standardized treatment measures were implemented in all patients. The amylase levels in the drain were regularly assessed on postoperative days 1, 3, and 5. Cefuroxime was administered intravenously as a prophylactic measure against infection. The recommended dose was 0.75 g every 8 h within the first 72 h after surgery. Parenteral treatment primarily involved administering medium and long-chain fat emulsion, ω-3 fish oil fat emulsion, alanyl-glutamine, and other amino acids. Enteral nutrition was initiated based on the physician’s assessment of the patient’s condition.
Surgical procedure
All patients underwent PD. In the novel group, the short distance from the center of the pancreatic duct to the edge of the pancreas (Rs) and the total thickness of the pancreas (R) were measured. The subsequent pancreaticojejunostomy mode was selected based on the Rs/R ratio (Figure 2). In the novel group, surgeries were consistently conducted by a dedicated surgical team exclusively. Meanwhile, in the conventional group, surgeries were performed by our team and other experienced teams. External stenting after PD was found to be associated with a higher incidence of clinically relevant POPF (23). Thus, the consistent implementation of internal pancreatic stenting during surgery was performed on all patients.

1-mode pancreaticojejunostomy
Patients with the central pancreatic duct underwent single-layer pancreaticojejunostomy to achieve mucosal anastomosis, commonly referred to as the 1 mode (Figure 3). 1-mode pancreaticojejunostomy is suitable for the central pancreatic duct, regardless of whether the main pancreatic duct is located near the anterior or posterior wall of the pancreas.
The specific steps of 1-mode pancreaticojejunostomy are as follows: (I) compare the pancreatic stump and the jejunum feeding loop using the end-to-side pancreaticojejunal anastomosis technique, confirm the anastomosis position, create a wound on the serosal surface of the jejunum feeding loop by appropriately destroying the serosa through electrocautery (to promote healing), and make a small incision in the central part of the anastomosis site to prepare for the end-to-side pancreaticojejunal anastomosis. (II) For pancreatic posterior wall anastomosis, starting from the upper end, 4-0 absorbable sutures are used, which enter the pancreatic side, exit the pancreatic duct, enter the jejunal mucosa, exit the serous membrane (outside-in, outside-out), and are then suspended. The middle stitches are sutured with 3–5 stitches according to the intraoperative conditions. The stitches enter the pancreatic duct, exit from the pancreatic side, and enter the jejunal serous membrane; the mucosa is exited (inside-in, inside-out), and the stitches are suspended successively. Similarly, the bottom end of the suture (outside-in, outside-out) is sewed and hanged. After the pancreatic posterior wall suture is completed, all sutures are straightened evenly, the jejunum is pushed to the remaining pancreatic stump to close it, and the knot is then uniformly tied successively. Subsequently, the pancreatic enterostomy support tube is inserted, and the suture in the middle is selected to fix the support tube. Notably, the sutures should be uniformly arranged radially with the pancreatic duct as the center. (III) For pancreatic anterior wall anastomosis, 4-0 absorbable sutures are used, which enter the pancreatic side, exit the pancreatic duct, enter the jejunal mucosa, exit the serous membrane (outside-in, outside-out), and are then suspended. Suture is performed with 3–5 stitches. (IV) Additional decompression sutures are made at the upper and lower ends of the pancreatic–intestinal anastomosis, allowing the intestine to wrap around the pancreatic stump (similar to a C-shape). (V) The anastomosis is completed.
12-mode pancreaticojejunostomy
The patients with the eccentric pancreatic duct underwent single-layer pancreaticojejunostomy with reinforcement of the anterior or posterior wall, also known as 12 mode (Figure 4). Unlike 1-mode pancreaticojejunostomy, two horizontal mattress sutures are taken from the dorsal or front side of the remaining pancreatic stump and are sutured to the serosal layer of the jejunum in the second step to strengthen the off-center anastomosis. The rest of the procedure is the same.

If the main pancreatic duct is located near the posterior wall of the pancreas, it can be divided into two cases based on whether the remaining pancreatic stump can be separated. If the remaining pancreatic stump can be separated, the pancreatic posterior wall tissue and serosal layer of the jejunum can be sutured with two horizontal mattress sutures (Figure 4A). If the remaining pancreatic stump cannot be separated, the suture is passed through the posterior sides of the pancreatic tissue from behind the pancreatic duct to the posterior wall of the pancreas and is then sutured to the serosal layer of the jejunum (two horizontal mattress sutures) (Figure 4B).
If the main pancreatic duct is located near the anterior wall of the pancreas, two horizontal mattress sutures are used to sew the remaining anterior wall tissue of the pancreatic stump and the serosal layer of the jejunum, similar to Figure 4A. The rest of the procedure remains unchanged.
Traditional pancreaticojejunostomy
The conventional group did not undergo preoperative or intraoperative measurement and pancreatic duct classification, and traditional pancreaticojejunostomy techniques, including duct-to-mucosa pancreaticojejunostomy and invagination pancreaticojejunostomy, were used. There is no significant difference in the development of POPF between these two anastomotic approaches (24-26).
Statistical analysis
Categorical data were presented as numbers with percentages and were analyzed using the χ2 test or the Fisher’s exact test, if appropriate. Continuous data were expressed as median [interquartile range (IQR)] and were compared using the Mann-Whitney U test. The interclass correlation coefficient was calculated to assess the reliability of the preoperative image measurements and the corresponding intraoperative measurements. Variables with a P value of <0.1 in the univariate regression analyses were subjected to further evaluation via multivariate regression analyses. In this study, a P value of <0.05 was considered significant. All statistical analyses were conducted using the Statistical Package for the Social Sciences software version 26 (SPSS Inc., Chicago, IL, USA).
Results
Preoperative and intraoperative measurements
The preoperative and intraoperative data of 51 patients were compared to assess their consistency (Table S1). The interclass correlation coefficient of the preoperative and intraoperative data was >0.75 (P<0.001). Thus, the level of agreement between these two measurement methods was high. Further, the preoperative pancreatic cross-sectional image measurement value can be used as an alternative to the intraoperative measurement value.
Risk factors of POPF
Patients with pancreatic fistula and those without did not significantly differ in terms of sex, age, BMI, hypertension/diabetes status, extended resection rate, preoperative total bilirubin level, American Society of Anesthesiologists score, estimated intraoperative blood loss volume, the number of the main pancreatic duct located near the anterior wall of the pancreas, and distance from the center of the pancreatic duct to the posterior edge of the pancreas (Table 1). The factors that significantly differed between the pancreatic fistula and non-pancreatic fistula groups were subjected to the multivariate analysis (Table 2). Results showed that a soft pancreas was independently associated with an increased risk of POPF. Meanwhile, a high Rs/R ratio was associated with a decreased risk of POPF [odds ratio (OR) =0.901, 95% confidence interval (CI): 0.875–0.928, P<0.001].
Table 1
| Variables | POPF (n=107) | No-POPF (n=443) | P value |
|---|---|---|---|
| Age (years) | 59 [51–66] | 58 [51–67] | 0.63 |
| Male | 62 (57.9) | 265 (59.8) | 0.72 |
| BMI (kg/m2) | 23.2 [22.64–25.06] | 21.3 [19.72–23.59] | 0.28 |
| Hypertension | 22 (20.6) | 79 (17.8) | 0.51 |
| Diabetes | 13 (12.1) | 64 (14.4) | 0.59 |
| Jaundice | 46 (43.0) | 203 (45.8) | 0.60 |
| Extended resection | 16 (15.0) | 51 (11.5) | 0.33 |
| ASA score | |||
| 1, 2 | 95 (88.8) | 387 (87.4) | 0.69 |
| 3, 4 | 12 (11.2) | 56 (12.6) | |
| Operative time (≥300 min) | 68 (63.6) | 232 (52.4) | 0.04 |
| Estimated blood loss (≥400 mL) | 50 (46.7) | 176 (39.7) | 0.19 |
| Pancreatic texture | <0.001 | ||
| Hard | 52 (48.6) | 305 (68.8) | |
| Soft | 55 (51.4) | 138 (31.2) | |
| Dilated pancreatic duct (≥3 mm) | 48 (44.9) | 299 (67.5) | <0.001 |
| Pathological outcomes | 0.001 | ||
| CP or PDAC | 49 (45.8) | 283 (63.9) | |
| Others | 58 (54.2) | 160 (36.1) | |
| The main pancreatic duct located near the anterior wall of the pancreas | 31 (28.9) | 85 (19.2) | 0.03 |
| The distance between the center of the pancreatic duct and the posterior margin of the pancreas (mm) | 6.3 [4.2–8.5] | 6.5 [5.3–7.9] | 0.31 |
| Rs/R (%) | 37.88 [29.36–43.21] | 45.13 [41.10–47.40] | <0.001 |
Data are presented as median [Q1–Q3] or n (%). POPF, postoperative pancreatic fistula; BMI, body mass index; ASA, American Society of Anesthesiologists; CP, chronic pancreatitis; PDAC, pancreatic ductal adenocarcinoma; the main pancreatic duct is located near the anterior wall of the pancreas, which means that the ratio of the distance from the center of the main pancreatic duct to the posterior wall of the pancreas to the total thickness of the pancreas is greater than or equal to 0.5; Rs/R, short distance from the center of the main pancreatic duct to the edge of the pancreas/total thickness of the pancreas.
Table 2
| Variables | Odds ratio | 95% CI | P value |
|---|---|---|---|
| Operative time (<300 min) | 0.701 | 0.433–1.136 | 0.15 |
| Pancreatic texture (soft) | 1.804 | 1.064–3.060 | 0.03 |
| Pancreatic duct (<3 mm) | 1.577 | 0.955–2.605 | 0.08 |
| Pathological outcomes (other) | 1.300 | 0.775–2.181 | 0.32 |
| The main pancreatic duct located near the anterior wall of the pancreas | 1.627 | 0.944–2.804 | 0.08 |
| Rs/R | 0.901 | 0.875–0.928 | <0.001 |
Rs/R, short distance from the center of the pancreatic duct to the edge of the pancreas/total thickness of the pancreas; CI, confidence interval.
Pancreatic duct classification
The ROC curve was plotted to identify the superior predictive accuracy of the fistula risk score (FRS) and alternative fistula risk score (a-FRS) risk assessments (Figure 5), with an area under the curve (AUC) value of 0.761 (95% CI: 0.711–0.811, P<0.001). The FRS and a-FRS yielded AUC values of 0.666 (95% CI: 0.608–0.723) and 0.703 (95% CI: 0.65–0.756), respectively. Considering that the optimal cutoff Rs/R ratio index was 0.397, the eccentric pancreatic duct was characterized by an Rs/R ratio of ≤0.397. Meanwhile, the central pancreatic duct was identified based on Rs/R ratio values of >0.397 (Figure 2). However, the incidence of POPF in the central pancreatic duct group (10.6%, n=43/407) was significantly lower than that in the eccentric pancreatic duct group (44.8%, n=64/143, P<0.001).

Characteristics of the patients
Table 3 shows the characteristics of 694 patients who underwent PD. There were no statistically significant differences between the two groups in terms of sex, age, BMI, hypertension, diabetes, jaundice, surgical duration, extended resection, intraoperative blood loss volume, pancreatic duct diameter and texture, pathological type, the number of the main pancreatic duct located near the anterior wall of the pancreas, and Rs/R ratio. Notably, the subgroups did not significantly differ in terms of baseline assessment results (Tables S2,S3).
Table 3
| Variables | Novel group (n=131) | Conventional group (n=563) | P value |
|---|---|---|---|
| Age (years) | 57 [51–66] | 58 [51–66] | 0.64 |
| Male | 80 (61.1) | 370 (65.7) | 0.32 |
| BMI (kg/m2) | 22.4 [19.9–24.7] | 21.7 [19.9–24.0] | 0.19 |
| Hypertension | 25 (19.1) | 124 (22.0) | 0.46 |
| Diabetes | 19 (14.5) | 83 (14.7) | 0.95 |
| Jaundice | 58 (44.2) | 255 (45.2) | 0.73 |
| Extended resection | 13 (10.0) | 61 (10.8) | 0.76 |
| ASA score | 0.09 | ||
| 1, 2 | 99 (75.6) | 462 (82.1) | |
| 3, 4 | 32 (24.4) | 101 (17.9) | |
| Operative time (≥300 min) | 70 (53.4) | 300 (53.2) | 0.98 |
| Estimated blood loss (≥400 mL) | 43 (32.8) | 230 (40.9) | 0.09 |
| Pancreatic texture | 0.21 | ||
| Hard | 79 (60.3) | 372 (66.1) | |
| Soft | 52 (39.7) | 191 (33.9) | |
| Dilated pancreatic duct (≥3 mm) | 95 (72.5) | 424 (75.3) | 0.51 |
| Pathological outcomes | 0.73 | ||
| CP or PDAC | 72 (55.0) | 300 (53.3) | |
| Others | 59 (45.0) | 263 (46.7) | |
| The main pancreatic duct located near the anterior wall of the pancreas | 28 (21.4) | 117 (20.8) | 0.88 |
| Rs/R (%) | 43.8 [37.0–46.9] | 43.4 [36.6–46.9] | 0.84 |
| Morbidity | 40 (30.5) | 205 (36.4) | 0.21 |
| CD <3 | 26 (19.8) | 108 (19.2) | |
| CD ≥3 | 14 (10.7) | 97 (17.2) | |
| Clinical related pancreatic fistula | 18 (13.7) | 130 (23.0) | 0.02 |
| Grade B | 14 (10.6) | 107 (19.0) | |
| Grade C | 4 (3.1) | 23 (4.1) | |
| Delayed gastric emptying | 14 (10.7) | 70 (12.4) | 0.58 |
| Grade B | 11 (8.4) | 60 (10.7) | |
| Grade C | 3 (2.3) | 10 (1.8) | |
| Postpancreatectomy hemorrhage | 7 (5.3) | 41 (7.3) | 0.43 |
| Intra-abdominal hemorrhage | 5 (3.8) | 10 (2.0) | |
| Gastrointestinal bleeding | 3 (2.3) | 35 (6.2) | |
| Abdominal infection | 18 (13.7) | 126 (22.4) | 0.03 |
| Bile leakage | 1 (0.8) | 9 (1.6) | 0.75 |
| Chylous fistula | 3 (2.3) | 9 (1.6) | 0.86 |
| Pulmonary infection | 6 (4.6) | 42 (7.5) | 0.24 |
| Wound infection | 3 (2.3) | 24 (4.3) | 0.29 |
| Reoperation | 7 (5.3) | 31 (5.5) | 0.94 |
| 30-day mortality | 2 (1.5) | 5 (0.9) | 0.51 |
Data are presented as median [Q1–Q3] or n (%). BMI, body mass index; ASA, American Society of Anesthesiologists; CP, chronic pancreatitis; PDAC, pancreatic ductal adenocarcinoma; the main pancreatic duct is located near the anterior wall of the pancreas, which means that the ratio of the distance from the center of the main pancreatic duct to the posterior wall of the pancreas to the total thickness of the pancreas is greater than or equal to 0.5; Rs/R, short distance from the center of the pancreatic duct to the edge of the pancreas/total thickness of the pancreas, CD, Clavien-Dindo.
Operative outcomes
The novel group had a lower overall incidence of postoperative complication than the conventional group (30.5% vs. 36.4%). However, the results did not significantly differ (P=0.21). Notably, the novel group had a significantly lower incidence rate of POPF than the conventional group (13.7% vs. 23.0%, P=0.02) (Table 3). In addition, the novel group exhibited a significantly lower abdominal infection rate than the conventional group (13.7% vs. 22.4%, P=0.03). Meanwhile, the incidence rates of other complications and 30-day mortality did not differ between the two groups.
Based on a subgroup analysis, the incidence rates of POPF between patients with the central pancreatic duct and those with the eccentric pancreatic duct in the novel group were similar (11.9% vs. 17.9%, P=0.36) (Table S2). However, patients with the eccentric pancreatic duct had a higher incidence of abdominal infection (23.1% vs. 9.8%, P=0.04) and wound infection (7.7% vs. 0.0%, P=0.007) than those with the central pancreatic duct. In patients with the eccentric pancreatic duct (Table S3), the incidence of POPF in patients who underwent PD using the 12 mode was significantly lower than that in patients who underwent PD using the 1 mode (17.9% vs. 41.5%, P=0.02).
Risk factors of POPF
Table S4 depicts the risk factors associated with POPF. According to the multivariate analysis, the risk factors of POPF included a soft pancreatic texture (OR =1.712, 95% CI: 1.066–2.749, P=0.03) and conventional anastomosis (OR =2.170, 95% CI: 1.230–3.826, P=0.007). The Rs/R ratio (OR =0.941, 95% CI: 0.920–0.963, P<0.001) and a surgical duration of 300 min (OR =0.655, 95% CI: 0.444–0.967, P=0.03) were protective factors against the development of POPF.
Discussion
The incidence of POPF is still high among patients even at experienced pancreatic centers. Thus, the complete elimination of POPF has been challenging (25,27). The current study aimed to compare the conventional pancreaticojejunostomy and a novel approach that involves the intraoperative assessment of the location of the main pancreatic duct within the pancreatic section. The application of this innovative technique improved the individualization and accuracy of pancreaticojejunostomy, thereby reducing the incidence of POPF.
According to the academic community, drainage techniques for reducing the risk of POPF, such as the use of pancreatic duct stents for the diversion of pancreatic fluid and enhancement of the peripancreatic drainage via modified pancreaticojejunostomy, are important (28). The mainstream techniques for pancreaticojejunostomy (29,30) include duct-to-mucosa pancreaticojejunostomy, invagination pancreaticojejunostomy, sleeve pancreaticojejunostomy, bundle pancreaticojejunostomy, and Blumgart pancreaticojejunostomy. The duct-to-mucosa pancreaticojejunostomy, which is a predominant technique, distinguishes itself by achieving mucosal anastomosis that closely resembles the normal physiological state and decreases impact on postoperative pancreatic exocrine function. Several studies have analyzed the etiology of POPF in this anastomosis technique. In particular, based on the study of Torres et al. (31) and Papalampros et al. (32), POPF may be caused by pancreatic fluid seepage via the suture eyelet and partial damage to the pancreas during suturing. Consequently, both approaches are associated with a decreased number of sutures, thereby reducing the incidence of pancreatic fistula. In addition, Lee et al. revealed that continuous suture was associated with a significantly reduced incidence of POPF compared with intermittent suture (6% vs. 11%, P=0.03) (33). Compared with intermittent sutures, continuous sutures may be associated with a more uniform distribution of stress, contributing to fewer puncture sites and lower tissue cutting damage. Based on these details, decreasing pancreatic puncture damage during anastomosis may be associated with a lower risk of POPF. Similarly, reducing anastomotic layers may result in a lower quantity of pancreatic sutures, thereby decreasing the risk of pancreatic fistula. Zhang et al. (34) showed that mucosal anastomosis with a single layer could significantly reduce the number of required anastomotic needles while ensuring optimal tightness, resulting in a significant improvement in POPF prevention (14.6% vs. 40.0%, P=0.01). Therefore, reducing the utilization of unnecessary sutures can effectively decrease the risk of pancreatic fistula, which is in accordance with the primary objective of this article. In addition, some studies with the reinforcement of anastomosis have shown that the utilization of fibrin sealants or autologous grafts did not decrease the risk of POPF (35). However, the application of polyglycolic acid mesh during distal pancreatectomy can effectively prevent POPF (36). Nevertheless, there are concerns regarding the effect of polyglycolic acid mesh on the development of infection or the delayed healing process of POPF (37). Currently, some researchers have proposed utilizing coronary stents from the field of cardiology to address pancreaticojejunostomy issues in patients with high-risk pancreatic fistulas (soft pancreatic texture and main pancreatic duct diameter ≤3 mm) and have achieved promising outcomes (38,39). However, due to the potential risks associated with these materials, it is still necessary to collect long-term complication data and further validate and compare them with the current standard of care in a prospective controlled setting.
Previous studies have investigated the impact of the pancreatic duct’s position on the incidence of POPF (40,41). Yamamoto et al. (40) incorporated the distance between the pancreatic duct and the posterior margin of the pancreas in their prediction system as a risk factor of POPF. In the validation group, this scoring system had a high predictability for POPF with an AUC of 0.834. Moreover, El Nakeeb et al. (41) revealed that a close proximity between the pancreatic duct and the posterior margin of the pancreas was considered a substantial risk factor for POPF, with a specific threshold set at 3 mm. If this distance falls below the said threshold, there is an increased risk of POPF development. However, the distance between the pancreatic duct and the posterior margin of the pancreas alone cannot be a reliable predictor of POPF. If the pancreatic duct is located near the posterior edge of the pancreas, the ratio between the distance from its center to the edge of the pancreas and the total thickness of the pancreas is lower. Conversely, if it is positioned away from this edge, it may be closer to the anterior side, resulting in a similar reduction in this ratio. In both scenarios, nonuniformity in pancreatic thickness during pancreaticojejunostomy can lead to a vulnerable anastomotic area where sutures are more at risk for cutting through the pancreatic tissues and a high postoperative risk for pancreatic fistula due to impaired healing. Regardless of whether the pancreatic duct is close to or far away from the posterior margin, the ratio of the minimum/short distance from the pancreatic duct center to the pancreatic edge to the total pancreatic thickness was more likely to decrease. The ratio provides a more accurate evaluation of the main pancreatic duct position within the pancreatic section. The univariate analysis indicated that the location of the main pancreatic duct near the anterior wall of the pancreas was associated with an increased risk of POPF (Table 1). However, this association did not remain significant after conducting multivariate analysis. Furthermore, the thickness and characteristics of the main pancreatic duct in PD may become a new cornerstone for the risk assessment of POPF. The wall of the main pancreatic duct is particularly important because it represents the anchoring element for the anastomosis (42). However, it still needs further validation and comparison analysis with current standard predictive models. Therefore, the current study aimed to determine the association of the Rs/R ratio with POPF development. With an optimal cutoff value of 0.397, the Rs/R ratio surpassed both FR and a-FRS values in predicting POPF development. In patients with the eccentric pancreatic duct, the incidence of POPF was >40%. This value was significantly higher than that in patients with the central pancreatic duct, which underscored the need for vigilant perioperative measures in those with the eccentric pancreatic duct. Our approach provided an individualized mode of pancreaticojejunostomy based on the pancreatic duct position, thereby significantly reducing unnecessary anastomosis. Compared with the traditional pancreaticojejunostomy, this novel method was associated with a significant lower incidence of POPF (13.7% vs. 23%, P=0.02) and abdominal infection (13.7% vs. 22.4%, P=0.03). In patients with an eccentric pancreatic duct, compared with pancreaticojejunostomy with 1 mode, pancreaticojejunostomy with the 12 mode of pancreaticojejunostomy was associated with a significantly reduced incidence of POPF (17.9% vs. 41.5%, P=0.02). Therefore, it has a prominent role in POPF prevention. Therefore, our novel strategy can have a significant potential in reducing suture requirements, optimizing surgical procedures, and streamlining surgical complexity. Further, considering the availability of preoperative imaging and intraoperative measurements, the anastomosis method had a remarkable reproducibility and applicability.
Based on the multivariate analysis, the pancreatic texture is a significant risk factor for POPF development, which is consistent with previous findings (5,7,43). The correlation between surgical duration and POPF may be attributed to factors such as the surgical technique used, the surgeon’s expertise, and intraoperative occult blood loss. Importantly, the procedure had a protective effect against the formation of pancreatic fistula in relation to the Rs/R ratio (OR =0.941, 95% CI: 0.920–0.963, P<0.001), and the novel anastomotic mode, which confirmed our initial hypothesis. Of course, long-term follow-up observation and further research are still needed to evaluate the durability and recurrence rates of POPF as long-term outcomes for this pancreatic duct anastomosis strategy. However, in our data, the incidence of POPF within 30 days during surgery demonstrates the reliability of our new anastomosis method.
The current study had several limitations. Firstly, this was a single-center retrospective study with inevitable selection bias, which might have weakened the persuasiveness of our findings. However, we have designed and conducted a randomized controlled trial (registered on Clinical Trials as NCT05475275) to understand the impact of anatomical location of the pancreatic duct on development of POPF. Secondly, the potential biases introduced by variations in surgical techniques among different surgeons, especially in anastomosis patterns within the conventional group. However, in our department, several lead surgeons have undergone consistency testing before operation, especially in PD, and the difference is not significant basing previous studies in our center. Thirdly, in the group with the eccentric pancreatic duct, although it has been confirmed that the main pancreatic duct is located near the anterior or posterior wall of the pancreas has no effect on POPF. The incidence of POPF might be lower in those with the pancreatic duct adjacent to the anterior margin than in those with the pancreatic duct close to the posterior margin. The reason may be positioning the drainage at the anterior margin facilitate convenience, thereby inhibiting the risk of high-grade pancreatic fistula. Currently, there is a limited number of patients with eccentric pancreatic ducts, and it remains uncertain whether there are any pertinent distinctions within this cohort that impact the occurrence of POPF. Further investigation is warranted.
Conclusions
The Rs/R ratio and anastomosis mode are significantly associated with POPF. Moreover, the innovative pancreaticojejunostomy strategy based on the main pancreatic duct position is effective in reducing the risk of POPF. Before recommending widespread clinical adoption of the novel pancreaticojejunostomy strategy, further multicentric series are needed to validate its utility and actual incorporation in the clinical practice.
Acknowledgments
We would like to thank Professor Rongxiang Li for his invaluable suggestions on the illustration techniques employed in this article.
Funding: This work was supported by
Footnote
Reporting Checklist: The authors have completed the STROBE reporting checklist. Available at https://gs.amegroups.com/article/view/10.21037/gs-24-235/rc
Data Sharing Statement: Available at https://gs.amegroups.com/article/view/10.21037/gs-24-235/dss
Peer Review File: Available at https://gs.amegroups.com/article/view/10.21037/gs-24-235/prf
Conflicts of Interest: All authors have completed the ICMJE uniform disclosure form (available at https://gs.amegroups.com/article/view/10.21037/gs-24-235/coif). The authors have no conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved. The study was conducted in accordance with the Declaration of Helsinki (as revised in 2013). The study was approved by the institutional review boards of West China Hospital of Sichuan University (No. 2023-1880), and the requirement of individual consent for this retrospective analysis was waived.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Wang M, Li D, Chen R, et al. Laparoscopic versus open pancreatoduodenectomy for pancreatic or periampullary tumours: a multicentre, open-label, randomised controlled trial. Lancet Gastroenterol Hepatol 2021;6:438-47. [Crossref] [PubMed]
- D'Angelica MI, Ellis RJ, Liu JB, et al. Piperacillin-Tazobactam Compared With Cefoxitin as Antimicrobial Prophylaxis for Pancreatoduodenectomy: A Randomized Clinical Trial. JAMA 2023;329:1579-88. [Crossref] [PubMed]
- Wang M, Pan S, Qin T, et al. Short-Term Outcomes Following Laparoscopic vs Open Pancreaticoduodenectomy in Patients With Pancreatic Ductal Adenocarcinoma: A Randomized Clinical Trial. JAMA Surg 2023;158:1245-53. [Crossref] [PubMed]
- Schuh F, Mihaljevic AL, Probst P, et al. A Simple Classification of Pancreatic Duct Size and Texture Predicts Postoperative Pancreatic Fistula: A classification of the International Study Group of Pancreatic Surgery. Ann Surg 2023;277:e597-608. [Crossref] [PubMed]
- Søreide K, Labori KJ. Risk factors and preventive strategies for post-operative pancreatic fistula after pancreatic surgery: a comprehensive review. Scand J Gastroenterol 2016;51:1147-54. [Crossref] [PubMed]
- Schouten TJ, Henry AC, Smits FJ, et al. Risk Models for Developing Pancreatic Fistula After Pancreatoduodenectomy: Validation in a Nationwide Prospective Cohort. Ann Surg 2023;278:1001-8. [Crossref] [PubMed]
- Kawaida H, Kono H, Hosomura N, et al. Surgical techniques and postoperative management to prevent postoperative pancreatic fistula after pancreatic surgery. World J Gastroenterol 2019;25:3722-37. [Crossref] [PubMed]
- Andrianello S, Marchegiani G, Malleo G, et al. Pancreaticojejunostomy With Externalized Stent vs Pancreaticogastrostomy With Externalized Stent for Patients With High-Risk Pancreatic Anastomosis: A Single-Center, Phase 3, Randomized Clinical Trial. JAMA Surg 2020;155:313-21. [Crossref] [PubMed]
- Zhang L, Zhu X, Zhu Y, et al. Chen's penetrating-suture technique for pancreaticojejunostomy following pancreaticoduodenectomy. BMC Surg 2023;23:146. [Crossref] [PubMed]
- Zhang B, Li L, Liu H, et al. A modified single-needle continuous suture of duct-to-mucosa pancreaticojejunostomy in pancreaticoduodenectomy. Gland Surg 2023;12:1642-53. [Crossref] [PubMed]
- Hao X, Li Y, Liu L, et al. Is duct-to-mucosa pancreaticojejunostomy necessary after pancreaticoduodenectomy: A meta-analysis of randomized controlled trials. Heliyon 2024;10:e33156. [Crossref] [PubMed]
- Sun Y, Yu XF, Yao H, et al. Safety and feasibility of modified duct-to-mucosa pancreaticojejunostomy during pancreatoduodenectomy: A retrospective cohort study. World J Gastrointest Surg 2023;15:1901-9. [Crossref] [PubMed]
- Hai H, Li Z, Zhang Z, et al. Duct-to-mucosa versus other types of pancreaticojejunostomy for the prevention of postoperative pancreatic fistula following pancreaticoduodenectomy. Cochrane Database Syst Rev 2022;3:CD013462. [Crossref] [PubMed]
- von Elm E, Altman DG, Egger M, et al. The Strengthening the Reporting of Observational Studies in Epidemiology (STROBE) Statement: guidelines for reporting observational studies. Int J Surg 2014;12:1495-9. [Crossref] [PubMed]
- Xingjun G, Feng Z, Meiwen Y, et al. A score model based on pancreatic steatosis and fibrosis and pancreatic duct diameter to predict postoperative pancreatic fistula after Pancreatoduodenectomy. BMC Surg 2019;19:75. [Crossref] [PubMed]
- Mercuriali F, Inghilleri G. Proposal of an algorithm to help the choice of the best transfusion strategy. Curr Med Res Opin 1996;13:465-78. [Crossref] [PubMed]
- Nadler SB, Hidalgo JH, Bloch T. Prediction of blood volume in normal human adults. Surgery 1962;51:224-32.
- Ayçiçek SG, Akhoundova D, Bacher U, et al. Determinants of Interpatient Variability in Treosulfan Pharmacokinetics in AML Patients Undergoing Autologous Stem Cell Transplantation. Int J Mol Sci 2024;25:8215. [Crossref] [PubMed]
- Wente MN, Veit JA, Bassi C, et al. Postpancreatectomy hemorrhage (PPH): an International Study Group of Pancreatic Surgery (ISGPS) definition. Surgery 2007;142:20-5. [Crossref] [PubMed]
- Wente MN, Bassi C, Dervenis C, et al. Delayed gastric emptying (DGE) after pancreatic surgery: a suggested definition by the International Study Group of Pancreatic Surgery (ISGPS). Surgery 2007;142:761-8. [Crossref] [PubMed]
- Bassi C, Marchegiani G, Dervenis C, et al. The 2016 update of the International Study Group (ISGPS) definition and grading of postoperative pancreatic fistula: 11 Years After. Surgery 2017;161:584-91. [Crossref] [PubMed]
- Clavien PA, Barkun J, de Oliveira ML, et al. The Clavien-Dindo classification of surgical complications: five-year experience. Ann Surg 2009;250:187-96. [Crossref] [PubMed]
- Jang JY, Chang YR, Kim SW, et al. Randomized multicentre trial comparing external and internal pancreatic stenting during pancreaticoduodenectomy. Br J Surg 2016;103:668-75. [Crossref] [PubMed]
- Yin T, Qin T, Wei K, et al. Comparison of safety and effectiveness between laparoscopic and open pancreatoduodenectomy: A systematic review and meta-analysis. Int J Surg 2022;105:106799. [Crossref] [PubMed]
- Zhang S, Lan Z, Zhang J, et al. Duct-to-mucosa versus invagination pancreaticojejunostomy after pancreaticoduodenectomy: a meta-analysis. Oncotarget 2017;8:46449-60. [Crossref] [PubMed]
- Popp FC, Bruns CJ. Range of variation of pancreaticojejunostomy in pancreatic head resection. Chirurg 2017;88:3-10. [Crossref] [PubMed]
- Wang W, Zhang Z, Gu C, et al. The optimal choice for pancreatic anastomosis after pancreaticoduodenectomy: A network meta-analysis of randomized control trials. Int J Surg 2018;57:111-6. [Crossref] [PubMed]
- Ecker BL, McMillan MT, Asbun HJ, et al. Characterization and Optimal Management of High-risk Pancreatic Anastomoses During Pancreatoduodenectomy. Ann Surg 2018;267:608-16. [Crossref] [PubMed]
- Olakowski M, Grudzińska E, Mrowiec S. Pancreaticojejunostomy-a review of modern techniques. Langenbecks Arch Surg 2020;405:13-22. [Crossref] [PubMed]
- Gai YW, Wang HT, Tan XD. Pancreaticojejunostomy Conducive to Biological Healing in Minimally Invasive Pancreaticoduodenectomy. J Gastrointest Surg 2022;26:1967-81. [Crossref] [PubMed]
- Torres OJM, Costa RCNDC, Costa FFM, et al. Modified heidelberg technique for pancreatic anastomosis. Arq Bras Cir Dig 2017;30:260-3. [Crossref] [PubMed]
- Papalampros A, Niehaus K, Moris D, et al. A safe and feasible "clock-face" duct-to-mucosa pancreaticojejunostomy with a very low incidence of anastomotic failure: A single center experience of 248 patients. J Visc Surg 2016;153:425-31. [Crossref] [PubMed]
- Lee SE, Yang SH, Jang JY, et al. Pancreatic fistula after pancreaticoduodenectomy: a comparison between the two pancreaticojejunostomy methods for approximating the pancreatic parenchyma to the jejunal seromuscular layer: interrupted vs continuous stitches. World J Gastroenterol 2007;13:5351-6. [Crossref] [PubMed]
- Zhang T, Wang X, Huo Z, et al. Shen's Whole-Layer Tightly Appressed Anastomosis Technique for Duct-to-Mucosa Pancreaticojejunostomy in Pancreaticoduodenectomy. Med Sci Monit 2016;22:540-8. [Crossref] [PubMed]
- Schindl M, Függer R, Götzinger P, et al. Randomized clinical trial of the effect of a fibrin sealant patch on pancreatic fistula formation after pancreatoduodenectomy. Br J Surg 2018;105:811-9. [Crossref] [PubMed]
- Jang JY, Shin YC, Han Y, et al. Effect of Polyglycolic Acid Mesh for Prevention of Pancreatic Fistula Following Distal Pancreatectomy: A Randomized Clinical Trial. JAMA Surg 2017;152:150-5. [Crossref] [PubMed]
- Zhang W, Wei Z, Che X. Effect of polyglycolic acid mesh for prevention of pancreatic fistula after pancreatectomy: A systematic review and meta-analysis. Medicine (Baltimore) 2020;99:e21456. [Crossref] [PubMed]
- Huscher CGS, Lazzarin G. Coronary artery stent for securing pancreatico-jejunal anastomosis after PD: The "Huscher technique". Pancreatology 2022;22:1057-8. [Crossref] [PubMed]
- Huscher C, Perri G, Lazzarin G, et al. Coronary Artery Stent for Securing High-risk Pancreatico-jejunal Anastomosis After Pancreatoduodenectomy: A Pilot Series. Ann Surg 2022;275:e665-8. [Crossref] [PubMed]
- Yamamoto Y, Sakamoto Y, Nara S, et al. A preoperative predictive scoring system for postoperative pancreatic fistula after pancreaticoduodenectomy. World J Surg 2011;35:2747-55. [Crossref] [PubMed]
- El Nakeeb A, Salah T, Sultan A, et al. Pancreatic anastomotic leakage after pancreaticoduodenectomy. Risk factors, clinical predictors, and management (single center experience). World J Surg 2013;37:1405-18. [Crossref] [PubMed]
- Huscher CGS, Lazzarin G, Marchegiani G, et al. Intraoperative intraductal ultrasonography of the main pancreatic duct during pancreatoduodenectomy: technical description of a pilot series. Updates Surg 2024; Epub ahead of print. [Crossref]
- Gu Z, Du Y, Wang P, et al. Development and validation of a novel nomogram to predict postoperative pancreatic fistula after pancreatoduodenectomy using lasso-logistic regression: an international multi-institutional observational study. Int J Surg 2023;109:4027-40. [Crossref] [PubMed]



