Microwave ablation for thyroid nodules: a new string to the bow for percutaneous treatments?
Introduction
Nodular thyroid disease is detected in 3–75% of the general population, and in 20–76% at ultrasound (US) scan, especially in the context of multinodular goiter (1). Even if most of the thyroid nodules are benign, a treatment can be however necessary, in particular for subjective symptoms, cosmetic concerns, patient’s fear for malignant transformation (2). If a therapy is necessary, total/partial thyroidectomy is actually the standard of care, while iodine therapy has been used with poor results (3). Many studies suggest total thyroidectomy in case of multinodular goiter, because of the risk of nodular recurrence in the thyroid remnant, with the need of reintervention, which carries out a higher risk of complications (4). Surgery has in fact some limitations, like a 2–10% complication rate, hiatrogenic hypothyroidism, hospitalization, general anesthesia and scar formation (5). In the last years, non surgical minimally invasive techniques have been developed to treat this pathology, starting from percutaneous ethanol injection (PEI), to laser ablation (LA) and radiofrequency ablation (RFA) (6-8). Most recently, microwave ablation (MWA) has been proposed to treat thyroid nodules, taking experience from its use in other organs like liver, kidney and lung in particular (9). MWA has the following advantages respect to RFA: reduction in treatment time, larger ablation zone, less heat sink effect (10). Aim of this review article was to evaluate all the studies concerning thyroid MWA, with a particular focus on safety and efficacy of the procedure and on results compared to RFA.
Methods
We reviewed all medical literature searching in pubmed.gov the terms “microwave” & “thyroid”. We found three original studies concerning MWA treatment, for a total of 263 patients (mean age 51.0 years; range, 15–80 years; male to female ratio 2.55) and 522 nodules (11-13).
Preablation assessment
Nodule size and composition were recorded with US scan: nodule size was obtained by multiplying three orthogonal measures by 0.525 (resulted from ellipsoid volume equation); composition of the nodules was divided in: mainly solid (>80% solid), mainly cystic (>80% liquid) or mixed type. Laboratory tests were: thyroid function [thyroid stimulating hormone (TSH), triiodothyronine (fT3), free thyroxine (fT4)], thyroid antibodies, blood count, coagulation tests. Inclusion and exclusion criteria were quite similar among the studies. Inclusion criteria were: benign thyroid nodule proven with at least one recent fine needle aspiration biopsy (FNAB); subjective symptoms (compressive symptoms, neck discomfort, foreign body sensation); cosmetic concerns; refusal or contraindications to undergo surgery. Exclusion criterium was only FNAB evidence of malignancy, but caution should be exercised for retrosternal growth and for nodules with US aspect of malignancy.
Procedure
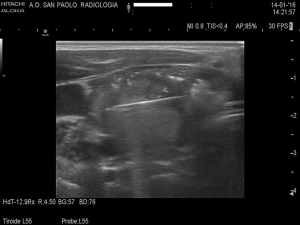
Feng et al. and Yue et al. performed MWA on an inpatient basis, while Heck et al. on an outpatient basis. Patients were put in a supine position with mild hyperextended neck, in aseptic conditions. Feng et al. and Heck et al. performed a 1–2 mm incision after local anesthesia. Only Feng et al. spoke about unconscious i.v. anesthesia by an anesthesiologist but, according to literature on RFA, a bland systemic sedation is routinely performed and perhaps not described in the other two studies. A fine needle aspiration was conducted in mainly cystic nodules before ablation. Then, an internally cooled antenna with various diameters (from 16 to 14 Gauge) was positioned in the thyroid nodules (Figure 1). All the authors used the “moving-shot” technique with trans-isthmic approach, as possible (14). This approach allows to see the entire length of the antenna on US view; furthermore, the danger triangle, which includes recurrent laryngeal nerve, trachea and esophagus, is more easily avoided. Multiple areas of the nodule are treated unit-by-unit by moving the electrode tip, until an echoic area in the unit is developed (Figure 2). The procedure is terminated when hyperechoic changes are present in the entire nodule, even if undertreated areas are recommended if the nodule is nearby the danger triangle. Mean power and treatment time were recorded by Heck et al. and Feng et al. Yue et al. injected a mixture of 0.9% lidocaine and physiological saline under the thyroid capsule to achieve a “liquid isolating region” protecting the surrounding structures of the neck (carotid artery, nerves, trachea, esophagus) from thermal injury.
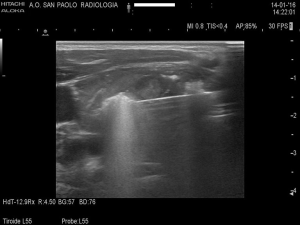
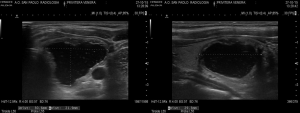
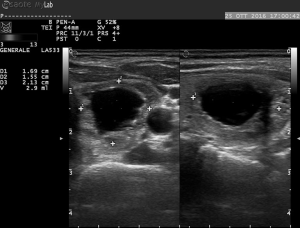
Yue et al. and Feng et al. kept patients under observation, with compression of the neck lasting for 15–30 minutes. Follow up was quite different among the authors. However, all performed a 3- and 6-month US control of nodule volume (Figures 3,4), assessing also symptom score (visual analog scale of subjective symptoms, from 0 to 10) and cosmetic score (1: no palpable mass; 2: invisible but palpable mass; 3: mass visible only swallowing; 4: easily visible mass). Thyroid laboratory tests were also examined by all.
Results
Nodules treated were 338 solid, 22 cystic, 162 mixed. Feng et al. and Heck et al. recorded both mean power ablation and mean time of ablation (see Table 1). Heck et al. did not found significant changes in laboratory parameters at 3 and 6 months follow-up, except for one case of persistent suppressed TSH. Thyreoglobulin levels increased 1 day after MWA but were normal at 3 and 6 months follow up. Yue et al. did not report any new case of hypothyroidism after the treatment. Feng et al. found cases of decrease in TSH and increase in fT4, but only in the day after MWA.
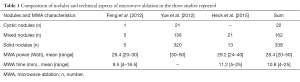
Full table
Reduction analysis
Volume reductions of the three studies and of other three studies concerning RFA, matched in number, were reported in the Table 2 (2,15,16). The study conducted by Feng et al. demonstrated a mean reduction of nodule volume of 45.9% at the least follow up, with a volume decrease from 5.30 to 2.40 mL. Yue et al., in a large series study, confirmed these results showing a mean reduction of nodule volume of 65% at the 6 months follow up, with a volume decrease from 2.13 to 0.45 mL. Only 254 of 477 nodules reached a 6-month follow up: as a consequence, a high number of patients did not reach an adequate follow up, resulting in a poor volume reduction at last control (38%). Heck et al. in their study demonstrated a mean reduction of nodule volume of 51.4% at 3 months follow-up, which was attended by all 30 patients, and a mean reduction of 58.8% at 6 months follow-up, reached only by 11 patients. Yue et al. and Feng et al. observed that cystic nodules decrease more respect to mixed nodules, and that mixed nodules show better results than solid nodules. Furthermore, Yue et al. found an inverse correlation between baseline volume and volume reduction after ablation. Feng et al. recorded symptom score and cosmetic score: symptom score was reduced in all symptomatic patients (4/4); cosmetic score was reduced in 8/11 patients.
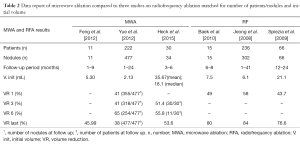
Full table
Side effects
After MWA, all studies reported that most patients complained of a sensation of heat in the neck and/or slight pain in the ablated site, whereas no one claimed the procedure to stop. Feng et al. and Yue et al. described choking and coughing at the end of treatment in some patients, which disappeared without any treatment or after corticosteroids physical therapy, water training, and vocal exercises. Only Heck et al. reported that two patients developed first-degree burns alongside the puncture channel, which did not require any therapy. In the study conducted by Heck et al. was described the formation of a cervical hematoma without active bleeding, with development of a mild Horner’s syndrome that disappeared without any treatment. There were no major complications such as esophageal perforation and tracheal injury.
Discussion
Thyroid nodules have a very high prevalence in the general population. Even if benign, they tend to increase over the years, in some cases causing symptoms or cosmetic concerns. The standard option has been so far surgical thyroidectomy, while other treatments (radioiodine therapy or levothyroxine) failed. Since the first study on PEI in 1990 (17), other mini-invasive treatments have been developed for this condition: LA, RFA and then MWA. They all have some advantages on surgery: no hiatrogenic hypothyroidism, no scar formation, lesser costs. Adverse events are comparable: the most common major adverse effect, recurrent laryngeal nerve palsy, is 11.8% (9.8% transient, 2% permanent) in surgery (18) and 0–8% (only transient) in RFA and LA (6-8,17,19-23). PEI has revealed to be useful for cystic nodules: it can be used with fine needles (21G), and is quite inexpensive and shows good results (53–80% reduction in volume) (24). Because of this, PEI is still the percutaneous technique of choice in cystic/predominantly cystic lesions (25). LA consists on photons emission by excited atoms toward a target lesion. Energy is delivered with a thin needle (21G), through which a spherical area of necrosis is created. However, maximum diameter of coagulative necrosis is never bigger than 2 cm in diameter, so that multiple needles or multiple treatments are necessary to treat large nodules, thus increasing the risk of adverse effects (26). Mean volume reduction of both solid and cystic lesions is 44–73% (20). RFA is the most promising percutaneous technique for both solid and cystic lesions (27). Thermal injury induced by an 18G needle induces a coagulative necrosis of the nodule. Transisthmic approach and the moving-shot technique have improved efficacy and reduced adverse effects (14). Mean volume reduction is the higher among percutaneous techniques: 51–85% (2,15-28). One of the major limits is the heat sink effect, which impacts a lot on high vascularized structures like thyroid parenchyma and thyroid nodules (29). MWA is the more recent percutaneous technique developed. Original articles in literature show that, respect to RFA, MWA has a similar complication rate and a slightly lower efficacy (30). Rate of recurrent laryngeal nerve palsy was 3.4% in the three studies examinated. Hematoma developed in more than one half of the patients, a data in harmony with the large needle of MWA (14–16G). Fortunately, there was only one deep and large hematoma (47×22), not requiring treatments. Other adverse events were rare and did not differ from those recorded in RFA studies. The mean reduction volume at 6 months, or at last follow-up, was 45.9–65%, which still overlaps with RFA results. The results may depend from the limited knowledge of this new technique, which is from learning curve effect. According to experimental studies and studies on other organs, MWA could be a further step in percutaneous techniques for thyroid nodules, giving the advantage to reduce treatment time, reduce the heat sink effect, create an ellipsoidal ablated zone which best fits with the shaping of thyroid nodules. In our opinion, these advantages are poor in thyroid ablation: treatment time depends much more from repositioning of the needle that from effective ablation time; heat sink effect could reduce the effective ablation volume, but in a benign condition like thyroid nodules the target is only the volume reduction, and not the ablation of the entire lesion; trans-isthmic approach does not allow to treat thyroid nodules through their main axes, which is the craniocaudal. On the other side, the use of a larger needle carries out a higher complication rate in MWA respect to RFA, in particular the development of neck hematomas, as seen before. In conclusion, MWA is a new, promising technique among the minimally invasive treatments of thyroid nodules. Actually, the larger diameter of MW antenna seems to be the major limiting factor in the use of this technique. More studies are necessary to evaluate feasibility, safety and efficacy of the procedure. In particular, randomized studies between MWA and RFA are necessary to define which should be the ablative treatment of choice for thyroid nodules.
Acknowledgements
None.
Footnote
Conflicts of Interest: The authors have no conflicts of interest to declare.
References
- Gharib H, Papini E, Paschke R, et al. American Association of Clinical Endocrinologists, Associazione Medici Endocrinologi, and European Thyroid Association medical guidelines for clinical practice for the diagnosis and management of thyroid nodules. J Endocrinol Invest 2010;33:1-50. [Crossref] [PubMed]
- Jeong WK, Baek JH, Rhim H, et al. Radiofrequency ablation of benign thyroid nodules: safety and imaging follow-up in 236 patients. Eur Radiol 2008;18:1244-50. [Crossref] [PubMed]
- Levine RA. Current guidelines for the management of thyroid nodules. Endocr Pract 2012;18:596-9. [Crossref] [PubMed]
- Sancho JJ, Prieto R, Dueñas JP, et al. A randomized trial of hemithyroidectomy versus Dunhill for the surgical management of asymmetrical multinodular goiter. Ann Surg 2012;256:846-51; discussion 851-2. [Crossref] [PubMed]
- Rosato L, Avenia N, Bernante P, et al. Complications of thyroid surgery: analysis of a multicentric study on 14,934 patients operated on in Italy over 5 years. World J Surg 2004;28:271-6. [Crossref] [PubMed]
- Jang SW, Baek JH, Kim JK, et al. How to manage the patients with unsatisfactory results after ethanol ablation for thyroid nodules: role of radiofrequency ablation. Eur J Radiol 2012;81:905-10. [Crossref] [PubMed]
- Tarantino L, Giorgio A, Mariniello N, et al. Percutaneous ethanol injection of large autonomous hyperfunctioning thyroid nodules. Radiology 2000;214:143-8. [Crossref] [PubMed]
- Valcavi R, Riganti F, Bertani A, et al. Percutaneous laser ablation of cold benign thyroid nodules: a 3-year follow-up study in 122 patients. Thyroid 2010;20:1253-61. [Crossref] [PubMed]
- Liang P, Wang Y, Yu X, et al. Malignant liver tumors: treatment with percutaneous microwave ablation--complications among cohort of 1136 patients. Radiology 2009;251:933-40. [Crossref] [PubMed]
- Wright AS, Sampson LA, Warner TF, et al. Radiofrequency versus microwave ablation in a hepatic porcine model. Radiology 2005;236:132-9. [Crossref] [PubMed]
- Feng B, Liang P, Cheng Z, et al. Ultrasound-guided percutaneous microwave ablation of benign thyroid nodules: experimental and clinical studies. Eur J Endocrinol 2012;166:1031-7. [Crossref] [PubMed]
- Yue W, Wang S, Wang B, et al. Ultrasound guided percutaneous microwave ablation of benign thyroid nodules: safety and imaging follow-up in 222 patients. Eur J Radiol 2013;82:e11-6. [Crossref] [PubMed]
- Heck K, Happel C, Grünwald F, et al. Percutaneous microwave ablation of thyroid nodules: effects on thyroid function and antibodies. Int J Hyperthermia 2015;31:560-7. [Crossref] [PubMed]
- Ha EJ, Baek JH, Lee JH. Moving-shot versus fixed electrode techniques for radiofrequency ablation: comparison in an ex-vivo bovine liver tissue model. Korean J Radiol 2014;15:836-43. [Crossref] [PubMed]
- Baek JH, Kim YS, Lee D, et al. Benign predominantly solid thyroid nodules: prospective study of efficacy of sonographically guided radiofrequency ablation versus control condition. AJR Am J Roentgenol 2010;194:1137-42. [Crossref] [PubMed]
- Spiezia S, Garberoglio R, Milone F, et al. Thyroid nodules and related symptoms are stably controlled two years after radiofrequency thermal ablation. Thyroid 2009;19:219-25. [Crossref] [PubMed]
- Livraghi T, Paracchi A, Ferrari C, et al. Treatment of autonomous thyroid nodules with percutaneous ethanol injection: preliminary results. Work in progress. Radiology 1990;175:827-9. [Crossref] [PubMed]
- Jeannon JP, Orabi AA, Bruch GA, et al. Diagnosis of recurrent laryngeal nerve palsy after thyroidectomy: a systematic review. Int J Clin Pract 2009;63:624-9. [Crossref] [PubMed]
- Kim JH, Lee HK, Lee JH, et al. Efficacy of sonographically guided percutaneous ethanol injection for treatment of thyroid cysts versus solid thyroid nodules. AJR Am J Roentgenol 2003;180:1723-6. [Crossref]
- Døssing H, Bennedbaek FN, Hegedüs L. Effect of ultrasound-guided interstitial laser photocoagulation on benign solitary solid cold thyroid nodules - a randomised study. Eur J Endocrinol 2005;152:341-5. [Crossref] [PubMed]
- Pacella CM, Bizzarri G, Spiezia S, et al. Thyroid tissue: US-guided percutaneous laser thermal ablation. Radiology 2004;232:272-80. [Crossref] [PubMed]
- Kim YS, Rhim H, Tae K, et al. Radiofrequency ablation of benign cold thyroid nodules: initial clinical experience. Thyroid 2006;16:361-7. [Crossref] [PubMed]
- Duka E, De Marchi G, Giacchero R, et al. Image-guided thyroid nodule ablation: technical notes and critical appraisal. Surg Technol Int 2014;25:103-9. [PubMed]
- Bennedbaek FN, Hegedüs L. Percutaneous ethanol injection therapy in benign solitary solid cold thyroid nodules: a randomized trial comparing one injection with three injections. Thyroid 1999;9:225-33. [Crossref] [PubMed]
- Sung JY, Kim YS, Choi H, et al. Optimum first-line treatment technique for benign cystic thyroid nodules: ethanol ablation or radiofrequency ablation? AJR Am J Roentgenol 2011;196:W210-4. [Crossref] [PubMed]
- Stafford RJ, Fuentes D, Elliott AA, et al. Laser-induced thermal therapy for tumor ablation. Crit Rev Biomed Eng 2010;38:79-100. [Crossref] [PubMed]
- De Bernardi IC, Floridi C, Muollo A, et al. Vascular and interventional radiology radiofrequency ablation of benign thyroid nodules and recurrent thyroid cancers: literature review. Radiol Med 2014;119:512-20. [Crossref] [PubMed]
- Baek JH, Lee JH, Valcavi R, et al. Thermal ablation for benign thyroid nodules: radiofrequency and laser. Korean J Radiol 2011;12:525-40. [Crossref] [PubMed]
- Pillai K, Akhter J, Chua TC, et al. Heat sink effect on tumor ablation characteristics as observed in monopolar radiofrequency, bipolar radiofrequency, and microwave, using ex vivo calf liver model. Medicine (Baltimore) 2015;94:e580. [Crossref] [PubMed]
- Gharib H, Hegedüs L, Pacella CM, et al. Clinical review: Nonsurgical, image-guided, minimally invasive therapy for thyroid nodules. J Clin Endocrinol Metab 2013;98:3949-57. [Crossref] [PubMed]