
Current status of prepectoral breast reconstruction in Argentina
Introduction
Breast cancer is among the most common cancers diagnosed in women, affecting one in eight women per year. Immediate implant-based breast reconstruction has emerged as the predominant approach for postmastectomy reconstruction, with a growing preference for the direct-to-implant (DTI) method over the traditional tissue expander technique. While conventionally, implants were typically positioned beneath the pectoralis major muscle, recent advancements have paved the way for implant placement above the muscle, in the prepectoral plane (1).
The landscape of breast cancer surgery has undergone a profound transformation over the past five decades. It has transitioned from aggressive interventions like radical mastectomy, to minimally invasive approaches. Furthermore, this shift has involved a transition from a primarily anatomical understanding of cancer progression to a more nuanced biological perspective. Advances in implantable biological and synthetic products over the last decade have enabled surgeons to replace traditional submuscular implant-based breast reconstruction techniques with a prepectoral or muscle-sparing technique. The availability of a range of biological and synthetic meshes helps the surgeon to secure the device and minimize the pressure on mastectomy flaps. Various methods involving wrapping and anchoring techniques have been employed to secure these implants with the assistance of these meshes. The rising popularity of prepectoral breast reconstruction among both surgeons and patients can be attributed to its ability to preserve the normal anatomy of the chest wall. Additionally, this approach offers advantages such as enhanced restoration of body image, reduced morbidity, and quicker recovery times compared to alternative techniques (2).
Nipple-sparing mastectomy (NSM) and skin-sparing mastectomy (SSM) techniques can be combined with prepectoral breast reconstruction. Two common methods of prepectoral implant reconstruction are the one-stage DTI method and the two-stage tissue expander/implant method. One-stage immediate prepectoral breast reconstruction using implants has gained growing popularity as an effective treatment option for carefully selected patients diagnosed with breast carcinoma. NSM, SSM, and skin-reducing mastectomy (SRM) techniques can be combined with immediate one-step single-stage breast reconstruction for risk reduction and treatment of breast cancer (3).
NSM mitigates postoperative deformity while facilitating one-stage, immediate breast reconstruction with implants for women with medium-sized breasts, resulting in exceptional cosmetic outcomes. NSM, in conjunction with SSM and SRM, enables the removal of glandular tissue while preserving the integrity of the native breast skin envelope, thereby supporting immediate implant reconstruction with remarkable aesthetic results. The integration of advanced implant technologies, particularly the use of highly cohesive silicone implants, significantly expands the possibilities for prepectoral implant reconstruction, ensuring a superior, high-quality immediate reconstruction option for these patients. Conservative mastectomies combine the benefits of tumor and complete glandular removal, as seen in traditional total mastectomy, with an enhancement in aesthetic outcomes achieved through the preservation of both the skin envelope and the nipple-areola complex (NAC). NAC ischemia and mastectomy flap necrosis are feared complications. There are several ways of assessing the risk for potential postoperative complications and reconstruction failure, and selecting the appropriate patients for DTI approach. The most common approach in many parts of the world is the surgeon’s clinical assessment of flap perfusion during surgery, like the Breast Reconstruction Assessment (BRA) Score (4) whiles other objective methods such as indocyanine green (ICG) fluorescence imaging systems, are more objective and accurate methods of assessment of flap perfusion perioperatively (5).
Digital mammography provides a clear differentiation between the density of glandular tissue and the non-glandular breast tissue that overlies it, which corresponds to the existing tissue, such as dermis and subcutaneous fat, between the Cooper’s ligaments surrounding the gland and the skin. Preoperative digital imaging assesses the breast tissue thickness, aiding in the planning of the most suitable surgical technique to reduce the incidence of necrotic complications following DTI reconstruction in NSM and SSM. The possibility of preoperatively identifying high-risk patients for NSM, SSM, and SRM using breast imaging can help in the selection of the correct breast reconstruction techniques for these patients (5,6).
The purpose of this manuscript is to describe our experience with the use of breast imaging preoperatively to identifying the best reconstruction techniques in breast cancer patients.
Oncoplastic management of breast cancer
Oncoplastic management of breast cancer is a comprehensive approach that integrates oncological and reconstructive surgical techniques. Conservative mastectomy techniques, encompassing SSM, NSM, and SRM, have established as validated and widely used methods for the treatment of breast cancer (5-9).
In an ideal scenario, oncoplastic surgery aims to deliver both aesthetically pleasing outcomes and ensure adequate oncological safety (5). Nevertheless, a potential risk is an insufficient blood supply to the remaining flaps and the NAC (8,10). Post-procedural rates of nipple and skin necrosis have been reported as high as 38% (11). Patients with large cup size, a history of prior surgery or radiation treatment, and active smokers are deemed at elevated risk for NSMs and SSMs, as these factors are associated with even higher complication rates (8).
Numerous recommendations have been suggested to prevent mastectomy flap and/or NAC necrosis (12-14). Maintaining a sufficient flap thickness during mastectomy plays a critical role in reducing the incidence of skin necrosis. Nevertheless, achieving this goal may not always be feasible, as it relies on the unique anatomical attributes of each patient (15). To ensure an oncologically safe mastectomy, it is essential to perform dissection beneath the superficial layer of the fascia superficialis (16). The distance between the skin and the glandular tissue determines the thickness of the subcutaneous tissue or the “glandular envelope” thickness.
Standard film mammograms lack the capability to provide a distinct identification and precise measurement of non-glandular breast tissue coverage. In contrast, digital mammography and magnetic resonance imaging (MRI) effectively differentiate glandular tissue density from the skin and adipose tissue coverage. Therefore, this preoperative imaging modality can accurately determine the thickness of this coverage, the distance between the breast skin and the Cooper’s ligaments surrounding the gland (Figures 1-3) (11-13).

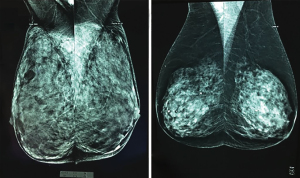

Usually, the decisions related to incision planning, treatment selection, surgical techniques, and reconstructive procedures are influenced by factors such as breast volume, tumor attributes, as well as the preferences of both surgeons and patients. Nevertheless, preoperative insights into the thickness of breast tissue coverage could potentially offer valuable insights into the risk of postmastectomy flap complications and contribute to the planning process. This information can be particularly useful as it provides an additional dimension to consider alongside breast volume, rather than relying solely on volume as the guiding factor (8,9).
The presence of sufficient fatty tissue coverage is considered one of the foremost independent factors influencing the success of immediate breast reconstruction and flap viability (17-21). Anatomically, the vascular network that ensures the flap and NAC survival runs within the space between the Cooper’s ligaments and the skin (20). Factors such as compression of this vascular network due to implant insertion, surgical trauma, tension during tissue closure, or exceptionally thin flaps may endanger vascularization. Studies have revealed that such occurrences can lead to tissue damage in the distal portions of the flaps. Consequently, it is imperative to recognize the significance of preoperative assessment of breast tissue coverage as a pivotal element in immediate reconstruction considerations (21-23).
Indications for prepectoral DTI
The indications for prepectoral DTI are the same as those for subpectoral DTI. Patients should possess healthy skin quality, robust mastectomy flap blood flow, and ideally exhibit mild to moderate ptosis and size, ideally with small to medium breast dimensions. In specific cases where patients have larger, ptotic breasts, SRM techniques may be thoughtfully employed for prepectoral DTI reconstruction (23,24).
Breast tissue coverage classification (BTCC)
Assessing the gland coverage preoperatively can serve as a valuable tool for predicting the viability of the remaining flaps in the context of conservative mastectomies. This assessment aids in the selection of the most suitable immediate reconstructive approach, with the goal of minimizing postoperative coverage-related complications. The preservation of skin perforators and flap thickness ranks among the foremost considerations for ensuring adequate vascularization of the postmastectomy flaps (20). The thickness of the remaining skin flap after the removal of the gland during conservative mastectomy significantly impacts both flap integrity and the vitality of the NAC. Cooper’s ligaments serve as the anatomical demarcation separating the mammary gland from the superficial layers of fat and skin tissue, which house the vascular plexus forming the mastectomy flaps. Hence, preoperative information regarding this tissue coverage assumes importance in averting complications associated with immediate reconstruction procedures (17-19,24,25).
Larson et al. (26) described variations in the thickness of subcutaneous breast tissue not related to breast volume. Thus, it becomes beneficial to ascertain the thickness of the subcutaneous breast tissue as a preliminary step in contemplating a DTI reconstruction following an NSM.
To comprehensively assess the appropriateness of a reconstructive approach, it is prudent to consider preoperative data in relation to the potential resulting flap thickness post-mastectomy. In this regard, we propose a BTCC based on digital mammography findings (Table 1) (27).
Table 1
| BTCC | Size | Coverage |
|---|---|---|
| Type 1 | <1 cm | Poor |
| Type 2 | 1–2 cm | Medium |
| Type 3 | >2 cm | Good |
BTCC, breast tissue coverage classification.
We confirmed that the selection of patients with breast subcutaneous tissue coverage above 2 cm, as an evidence of preoperative digital mammogram evaluation, determines an adequate flap for NSM, directly representing the distance between the Cooper’s ligaments (the “safe” mastectomy surgical plane) and the skin (26). This classification may also allow a rational use of materials for individual patients (27).
The option of selecting cases for this procedure based on the preoperative digital mammogram, revealing a superficial tissue thickness exceeding 2 cm, holds promise in mitigating the risk of immediate ischemic complications. Furthermore, the choice of surgical materials may be influenced by this coverage measurement.
In accordance with our classification, it would be prudent to consider additional coverage strategies for reconstruction, such as acellular dermal matrix (ADM), meshes, retropectoral implant placement, and delayed fat grafting, for patients in the category with insufficient coverage (Type 1).
For those in the category with moderate coverage (Type 2), we recommend a two-stage expander-implant reconstruction approach to circumvent tension during flap closure.
In the good coverage group (Type 3), single-stage DTI prepectoral reconstruction with implants could be performed without any mesh.
The decision-making process for mastectomy and reconstructive procedures is best achieved through collaborative efforts between the oncologic and plastic surgeon, or by a specialized oncoplastic breast surgeon. This decision should be grounded in objective preoperative data (19,22,28,29).
We observed that breast tissue coverage and breast volume are two distinct factors, irrespective of whether they exist in larger breasts with inadequate coverage or smaller breasts with ample coverage. This underscores the importance of measuring breast tissue coverage thickness preoperatively, as it is a critical consideration in surgical decisions, independent of breast volume (7,30-32).
Irrespective of breast volume, the preoperative assessment of tissue coverage holds vital significance in surgical planning for both oncologic and plastic surgeons, as it directly correlates with the risk of flap and NAC ischemia or necrosis (33,34). Consequently, prior communication between the reconstructive and oncologic surgeons regarding the selection of incision and integumentary preservation based on digital mammogram findings has the potential to yield enhanced outcomes and reduce the incidence of complications, as illustrated in Figure 4.

In cases with thin flaps, the potential for ischemic complications following mastectomy and reconstructive procedures is heightened (17,24). Aesthetic complications as rippling can occur if prepectoral implants are placed in Type 1 patients (Figure 5).
In light of these findings, preoperative digital mammography or MRI emerges as a valuable tool, not only for tumor detection but also as an objective means of predicting the resultant flap thickness. This information substantially contributes to enhancing patient safety (20,21,35-38).
Surgical technique
A meticulous surgical technique is essential to preserve the vascular network that guarantees the survival of the skin flap and NAC. Skin flaps are dissected with or without prior infiltration using either blunt or sharp techniques.
The NSM is performed with blunt scissors to dissect the breast gland from the skin flap in the plane of the Scarpa fascia as a first step, and previous infiltration with Klein solution of the whole breast between the gland and the cutaneous coverage is performed with 250 cc per breast. Electrocautery is then used to dissect the gland from the pectoralis major muscle in the second step. With this technique, thermal injury of the skin can be avoided, the best blood supply of the skin flaps can be preserved, and skin perfusion can be protected (39). Then, the complete breast gland is dissected peripherally with sufficient exposure of the axillary tail. After glandular resection ablation, the volume and weight of the breast gland are measured, and the final implant size is determined according to this information. As most of our patients want their reconstructed breasts to look natural and similar to their original breasts, anatomical implants and implant sizes resembling the removed breast volumes are used.
To improve oncologic safety, reduce ischemic risk, and assure the dissection of the subareolar and periareolar tissue, we perform specific hydrodissection under the NAC, which we find very helpful at this point of practice to remove maximum breasts and ducts. Hydrodissection with Klein solution creates a subdermal plane facilitating NAC dissection and permits a more complete removal of breast tissue in NSM. Such could prove important in the treatment of breast cancer and in breast cancer 1/2 (BRCA 1/2) mutation carriers because of its potential to reduce the risk of relapse (40-45).
Retroareolar nipple biopsy NAC tissue biopsy is also essential to ensure oncological safety. Partial necrosis of the nipple or NAC and loss of sensation are the most frequent complications of this surgery. The balance between oncological safety and preservation of vascularity is vital for obtaining optimal surgical outcomes (12,13,39).
Another option to go prepectoral in risky patients as smokers, is to completely delay the reconstruction until wound healing is complete.
With appropriate block by local anesthesia, most of these patient’s single stage direct to implant mastectomies with prepectoral reconstruction can be performed on an outpatient basis.
In our series, there has been no use of meshes, biological meshes like ADM are not available in Argentina.
Conclusions
DTI is a safe approach for prepectoral implant-based reconstruction with a number of advantages. However, careful patient selection and judicious assessment of flap perfusion help identify an appropriate subset of patients for prepectoral DTI reconstruction. Proposed BTCC and rigorous perfusion assessment techniques will aid to minimize postoperative complications and reconstruction failure.
Mastectomy flap thickness can have a dramatic effect on blood supply and is highly dependent on the anatomical basis and surgical oncologist’s technique.
Prepectoral DTI reconstruction provides good results with complication rates similar to those of subpectoral techniques, eliminating breast animation, with significantly lower rates of capsular contracture, prosthesis failure (46). Aesthetic complications as rippling can occur if prepectoral implants are placed in Type 1 patients. Preoperative planning for prepectoral placement should not depend on breast volume, but on breast tissue coverage. Flap evaluation based on preoperative imaging measurements may be helpful when planning a conservative mastectomy. Patient selection, preoperative and intraoperative mastectomy flap evaluation, and modifications in implant technology play a critical role in this new and rapidly growing method for implant-based breast reconstruction.
Acknowledgments
Funding: None.
Footnote
Provenance and Peer Review: This article was commissioned by the Guest Editors (Tine Engberg Damsgaard, C. Andrew Salzberg and Jørn Bo Thomsen) for the series “Hot Topics in Breast Reconstruction World Wide” published in Gland Surgery. The article has undergone external peer review.
Peer Review File: Available at https://gs.amegroups.com/article/view/10.21037/gs-23-291/prf
Conflicts of Interest: All authors have completed the ICMJE uniform disclosure form (available at https://gs.amegroups.com/article/view/10.21037/gs-23-291/coif). The series “Hot Topics in Breast Reconstruction World Wide” was commissioned by the editorial office without any funding or sponsorship. The authors have no other conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved. All clinical procedures described in this study were performed in accordance with the ethical standards of the institutional and/or national research committee(s) and with the Helsinki Declaration (as revised in 2013). Written informed consent was obtained from the patient for the publication of this article and accompanying images.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Tasoulis MK, Iqbal FM, Cawthorn S, et al. Subcutaneous implant breast reconstruction: Time to reconsider? Eur J Surg Oncol 2017;43:1636-46. [Crossref] [PubMed]
- Vidya R, Iqbal FM. A Guide to Prepectoral Breast Reconstruction: A New Dimension to Implant-based Breast Reconstruction. Clin Breast Cancer 2017;17:266-71. [Crossref] [PubMed]
- Sbitany H. Important Considerations for Performing Prepectoral Breast Reconstruction. Plast Reconstr Surg 2017;140:7S-13S. [Crossref] [PubMed]
- Casella D, Kaciulyte J, Lo Torto F, et al. "To Pre or Not to Pre": Introduction of a Prepectoral Breast Reconstruction Assessment Score to Help Surgeons Solving the Decision-Making Dilemma. Retrospective Results of a Multicenter Experience. Plast Reconstr Surg 2021;147:1278-86. [Crossref] [PubMed]
- Vidya R, Iqbal FM. Breast anatomy: Time to classify the subpectoral and prepectoral spaces. Clin Anat 2017;30:434-5. [Crossref] [PubMed]
- Rehnke RD, Groening RM, Van Buskirk ER, et al. Anatomy of the Superficial Fascia System of the Breast: A Comprehensive Theory of Breast Fascial Anatomy. Plast Reconstr Surg 2018;142:1135-44. [Crossref] [PubMed]
- Phillips BT, Lanier ST, Conkling N, et al. Intraoperative perfusion techniques can accurately predict mastectomy skin flap necrosis in breast reconstruction: results of a prospective trial. Plast Reconstr Surg 2012;129:778e-88e. [Crossref] [PubMed]
- Nava MB, Cortinovis U, Ottolenghi J, et al. Skin-reducing mastectomy. Plast Reconstr Surg 2006;118:603-10; discussion 611-3. [Crossref] [PubMed]
- Zenn MR. Staged immediate breast reconstruction. Plast Reconstr Surg 2015;135:976-9. [Crossref] [PubMed]
- Staub G, Fitoussi A, Falcou MC, et al. Breast cancer surgery: use of mammaplasty. Results. Series of 298 cases. Annales de Chirurgie Plastique Esthétique 2008;53:124-34. [Crossref] [PubMed]
- della Rovere GQ, Nava M, Bonomi R, et al. Skin-reducing mastectomy with breast reconstruction and sub-pectoral implants. J Plast Reconstr Aesthet Surg 2008;61:1303-8. [Crossref] [PubMed]
- Longo B, Farcomeni A, Ferri G, et al. The BREAST-V: a unifying predictive formula for volume assessment in small, medium, and large breasts. Plast Reconstr Surg 2013;132:1e-7e. [Crossref] [PubMed]
- Kuhl C. The current status of breast MR imaging. Part I. Choice of technique, image interpretation, diagnostic accuracy, and transfer to clinical practice. Radiology 2007;244:356-78. [Crossref] [PubMed]
- Kuhl CK. Current status of breast MR imaging. Part 2. Clinical applications. Radiology 2007;244:672-91. [Crossref] [PubMed]
- Lalardie JP, Jouglard P. Chirurgie Plastique du Sein. Paris: Masson; 1974.
- van Dongen JA, Voogd AC, Fentiman IS, et al. Long-term results of a randomized trial comparing breast-conserving therapy with mastectomy: European Organization for Research and Treatment of Cancer 10801 trial. J Natl Cancer Inst 2000;92:1143-50. [Crossref] [PubMed]
- R Development Core Team. R: a language and environment for statistical computing. Vienna: R Foundation for Statistical Computing. Available online: http://www.R-project.org/
- Nahabedian MY. Overview of perforator imaging and flap perfusion technologies. Clin Plast Surg 2011;38:165-74. [Crossref] [PubMed]
- Seitz IA, Nixon AT, Friedewald SM, et al. "NACsomes": A new classification system of the blood supply to the nipple areola complex (NAC) based on diagnostic breast MRI exams. J Plast Reconstr Aesthet Surg 2015;68:792-9. [Crossref] [PubMed]
- Spear SL, Rottman SJ, Seiboth LA, et al. Breast reconstruction using a staged nipple-sparing mastectomy following mastopexy or reduction. Plast Reconstr Surg 2012;129:572-81. [Crossref] [PubMed]
- Nahabedian MY, Tsangaris TN. Breast reconstruction following subcutaneous mastectomy for cancer: a critical appraisal of the nipple-areola complex. Plast Reconstr Surg 2006;117:1083-90. [Crossref] [PubMed]
- Colwell AS, Tessler O, Lin AM, et al. Breast reconstruction following nipple-sparing mastectomy: predictors of complications, reconstruction outcomes, and 5-year trends. Plast Reconstr Surg 2014;133:496-506. [Crossref] [PubMed]
- Vidya R, Berna G, Sbitany H, et al. Prepectoral implant-based breast reconstruction: a joint consensus guide from UK, European and USA breast and plastic reconstructive surgeons. Ecancermedicalscience 2019;13:927. [Crossref] [PubMed]
- Onesti MG, Di Taranto G, Ribuffo D, et al. ADM-assisted prepectoral breast reconstruction and skin reduction mastectomy: Expanding the indications for subcutaneous reconstruction. J Plast Reconstr Aesthet Surg 2020;73:673-80. [Crossref] [PubMed]
- Wapnir I, Dua M, Kieryn A, et al. Intraoperative imaging of nipple perfusion patterns and ischemic complications in nipple-sparing mastectomies. Ann Surg Oncol 2014;21:100-6. [Crossref] [PubMed]
- Larson DL, Basir Z, Bruce T. Is oncologic safety compatible with a predictably viable mastectomy skin flap? Plast Reconstr Surg 2011;127:27-33. [Crossref] [PubMed]
- Rancati A, Angrigiani C, Hammond D, et al. Preoperative digital mammography imaging in conservative mastectomy and immediate reconstruction. Gland Surg 2016;5:9-14. [Crossref] [PubMed]
- Rancati AO, Angrigiani CH, Hammond DC, et al. Direct to Implant Reconstruction in Nipple Sparing Mastectomy: Patient Selection by Preoperative Digital Mammogram. Plast Reconstr Surg Glob Open 2017;5:e1369. [Crossref] [PubMed]
- Heywang-Köbrunner SH, Hacker A, Sedlacek S. Magnetic resonance imaging: the evolution of breast imaging. Breast 2013;22:S77-82. [Crossref] [PubMed]
- Cunningham L. The anatomy of the arteries and veins of the breast. J Surg Oncol 1977;9:71-85. [Crossref] [PubMed]
- Longo B, Campanale A, Santanelli di Pompeo F. Nipple-areola complex cutaneous sensitivity: a systematic approach to classification and breast volume. J Plast Reconstr Aesthet Surg 2014;67:1630-6. [Crossref] [PubMed]
- Murray JD, Jones GE, Elwood ET, et al. Laser angiography as a predictor of mastectomy flap necrosis after breast reconstruction. Plast Reconstr Surg 2012;129:1017e-8e. [Crossref] [PubMed]
- Gerber B, Krause A, Dieterich M, et al. The oncological safety of skin sparing mastectomy with conservation of the nipple-areola complex and autologous reconstruction: an extended follow-up study. Ann Surg 2009;249:461-8. [Crossref] [PubMed]
- Salgarello M, Visconti G, Barone-Adesi L. Nipple-sparing mastectomy with immediate implant reconstruction: cosmetic outcomes and technical refinements. Plast Reconstr Surg 2010;126:1460-71. [Crossref] [PubMed]
- Spear SL, Willey SC, Feldman ED, et al. Nipple-sparing mastectomy for prophylactic and therapeutic indications. Plast Reconstr Surg 2011;128:1005-14. [Crossref] [PubMed]
- Cordeiro PG, Pusic AL, Disa JJ, et al. Irradiation after immediate tissue expander/implant breast reconstruction: outcomes, complications, aesthetic results, and satisfaction among 156 patients. Plast Reconstr Surg 2004;113:877-81. [Crossref] [PubMed]
- Chu CK, Carlson GW. Techniques and Outcomes of Nipple Sparing Mastectomy in the Surgical Management of Breast Cancer. Current Breast Cancer Reports 2013;5:118-24.
- Catanuto G, Khan A, Ursino V, et al. De-escalation of complexity in oncoplastic breast surgery: Case series from a specialized breast center. Breast 2019;46:12-8. [Crossref] [PubMed]
- Rancati AO, Nahabedian MY, Angrigiani C, et al. Revascularization of the Nipple-Areola Complex following Nipple-Sparing Mastectomy. Plast Reconstr Surg 2023;151:254-62. [Crossref] [PubMed]
- Folli S, Curcio A, Buggi F, et al. Improved sub-areolar breast tissue removal in nipple-sparing mastectomy using hydrodissection. Breast 2012;21:190-3. [Crossref] [PubMed]
- Rawlani V, Fiuk J, Johnson SA, et al. The effect of incision choice on outcomes of nipple-sparing mastectomy reconstruction. Can J Plast Surg 2011;19:129-33. [Crossref] [PubMed]
- Vidya R, Masià J, Cawthorn S, et al. Evaluation of the effectiveness of the prepectoral breast reconstruction with Braxon dermal matrix: First multicenter European report on 100 cases. Breast J 2017;23:670-6. [Crossref] [PubMed]
- Masià J. iBAG Working Group. The largest multicentre data collection on prepectoral breast reconstruction: The iBAG study. J Surg Oncol 2020;122:848-60. [Crossref] [PubMed]
- Abbate O, Rosado N, Sobti N, et al. Meta-analysis of prepectoral implant-based breast reconstruction: guide to patient selection and current outcomes. Breast Cancer Res Treat 2020;182:543-54. [Crossref] [PubMed]
- Li Y, Xu G, Yu N, et al. Prepectoral Versus Subpectoral Implant-Based Breast Reconstruction: A Meta-analysis. Ann Plast Surg 2020;85:437-47. [Crossref] [PubMed]
- Ostapenko E, Nixdorf L, Devyatko Y, et al. Prepectoral Versus Subpectoral Implant-Based Breast Reconstruction: A Systemic Review and Meta-analysis. Ann Surg Oncol 2023;30:126-36. [Crossref] [PubMed]



