
The effect of total thyroidectomy and radioactive iodine on long-term survival in unilateral T3/T4 follicular thyroid carcinoma: insights from a propensity-matched retrospective analysis
Highlight box
Key findings
• For patients with T3/T4 stage follicular thyroid carcinoma (FTC), no significant differences in overall survival or cancer-specific survival (CSS) were observed between patients who underwent total thyroidectomy (TT) and those who did not, in the short to medium term.
• Patients receiving radioactive iodine therapy (RAIT) combined with unilateral lobectomy showed better CSS than those receiving unilateral lobectomy alone.
What is known and what is new?
• TT is a common treatment for advanced FTC. RAIT is typically used after TT to target residual and metastatic disease.
• Evidence suggests that TT does not enhance survival rates in stage T3/T4 FTC, challenging conventional treatment approaches. RAIT combined with unilateral lobectomy might be better for CSS than unilateral lobectomy alone.
What is the implication, and what should change now?
• It is suggested to reevaluate the necessity of TT in clinical guidelines for stage T3/T4 FTC, especially when RAIT combined with unilateral lobectomy is considered.
• Oncologists should consider the benefits of RAIT with unilateral lobectomy versus the risks and costs of TT.
• Further research is needed to assess the long-term outcomes of RAIT with unilateral lobectomy to validate these findings and improve FTC treatment strategies.
Introduction
Follicular thyroid carcinoma (FTC) is a malignant tumor derived from the epithelial cells of the thyroid gland and accounts for about 9% of all thyroid cancers (1). The incidence of this type of cancer exhibits significant variations among different countries. In female patients, the incidence rate ranges from 0.53 to 2.52 per 100,000, whereas in male patients, the incidence rate oscillates between 0.3 and 1.12 per 100,000 individuals (2). Unlike papillary thyroid cancer, FTC is difficult to definitively diagnose through fine needle aspiration biopsy (FNAB) or intraoperative rapid pathology.
FNAB is a cornerstone technique in the evaluation of thyroid nodules, but its efficacy is limited in the early detection of FTC (3,4). The intricate attributes of FTC cells often mimic those of benign follicular lesions or nonneoplastic follicular alterations when scrutinized microscopically. This resemblance hampers the capacity of cytological evaluation to unequivocally differentiate malignant from benign follicular entities (5). Consequently, even with FNAB, achieving an accurate preoperative diagnosis of FTC remains a challenging endeavor.
Intraoperative rapid pathology diagnosis, also known as frozen section diagnosis, is a method of histopathological examination performed swiftly during surgery to immediately provide information on the nature of a tumor. However, diagnosing FTC poses numerous challenges in intraoperative rapid pathology diagnosis. The diagnostic criteria for FTC include capsular invasion or vascular invasion (6), which are often difficult to reliably observe in frozen sections. The sample processing and slice quality of frozen sections are generally not as good as those of conventional paraffin-embedded sections, possibly making it difficult for pathologists to make accurate judgments (7,8). Given these limitations, even with intraoperative rapid pathology diagnosis, the final diagnosis may still rely on detailed pathological analysis after surgery. Consequently, a substantial number of patients diagnosed with FTC are more frequently subjected to hemithyroidectomy as opposed to total thyroidectomy (TT) (4).
In the treatment of patients with locally advanced FTC, surgeons face a multitude of challenges. The tumor might have encroached upon the recurrent laryngeal nerve on one side, or preservation of the parathyroid glands on the affected side may be unfeasible. In such instances, performing a TT could risk bilateral recurrent laryngeal nerve injury, which may result in breathing difficulties, or potentially leads to permanent hypoparathyroidism, among other serious complications (9). To mitigate surgical risks and preserve the patient’s quality of life, surgeons may consider adopting a more conservative surgical strategy that eschews total or subtotal thyroidectomy.
For patients diagnosed with FTC at stages T3 and T4, as classified by the American Joint Committee on Cancer (AJCC) staging system, most treatment guidelines advocate for TT as the primary treatment strategy (3,10). However, considering the previously discussed challenges, some patients opt against undergoing complete thyroid removal, accepting a heightened risk of recurrence to ensure a superior quality of life. The debate over whether TT confers survival advantages for those with locally advanced thyroid cancer remains contentious.
In this context, we initiated a retrospective investigation utilizing propensity score matching (PSM) (11), drawing data from the Surveillance, Epidemiology, and End Results (SEER) program. The objective was to meticulously evaluate the differential impact of TT versus partial thyroidectomy on the survival rates of patients afflicted with locally advanced FTC. The methodology was designed to curtail the selection bias and confounding variables that are often inherent in observational studies. By aligning patients with analogous propensity scores, we facilitated a more accurate comparison of the outcomes associated with distinct surgical approaches, thereby emulating the research design of randomized controlled trials (RCTs) to the greatest extent feasible. In addition, for patients with locally advanced FTC who have foregone a TT, the implications and results pertaining to radioactive iodine therapy (RAIT) could diverge (12,13). Therefore, our research further investigated the effect of RAIT on both the overall survival (OS) and cancer-specific survival (CSS) rates in this subset of patients. We present this article in accordance with the STROBE reporting checklist (available at https://gs.amegroups.com/article/view/10.21037/gs-24-231/rc).
Methods
Data source and ethics statement
The SEER database established in 1973, serves as a public database and research resource developed by the National Cancer Institute (NCI) of the United States. It encompasses data covering approximately 30% of the U.S. population. The dataset for this retrospective study was obtained from the SEER Research Data, encompassing information from 17 registries as of November 2022, which includes cases recorded between the years 2000 and 2020. Data extraction was performed using the SEER*Stat software, version 8.4.3. The data used in this study are publicly available, having secured access to the SEER database via a signed SEER Data-Use Agreement. This study has been registered on the ClinicalTrials.gov website with the identifier NCT06437873. The study was conducted in accordance with the Declaration of Helsinki (as revised in 2013) and was approved by the Institutional Ethics Committee of Qilu Hospital of Shandong University Dezhou Hospital (No. 2024053). As this was a retrospective cohort study using anonymized data from the SEER database, the requirement for informed consent was waived.
Patient selection
The following parameters for data extraction were selected: the primary site code C73.9, denoting the thyroid gland; and the International Classification of Diseases for Oncology, third edition (ICD-O-3) histology types comprising 8330 [follicular adenocarcinoma, not otherwise specified (NOS)], 8331 (follicular adenocarcinoma well differentiated), 8332 (follicular adenocarcinoma trabecular), 8335 (follicular carcinoma, minimally invasive), and 8339 (FTC, encapsulated angioinvasive). The exclusion criteria were as follows: (I) absence of T stage information or classified as T1 or T2; (II) missing surgery codes; (III) cases not verified by histopathological analysis; (IV) FTC not being the initial malignancy diagnosed in the patient; (V) unknown survival duration or survival less than 1 month; and (VI) presence of bilateral tumors. It is important to highlight that this study incorporated data from various versions of the AJCC tumor-node-metastasis (TNM) staging system, namely AJCC TNM-6, AJCC TNM-7, and AJCC TNM-8, due to minor discrepancies in the classification criteria across these editions.
Variables
In this study, we selected the following 18 variables for analysis: sex, age at diagnosis, marital status at diagnosis, year of diagnosis, race, median household income, histologic type, combined summary stage, AJCC T stage, AJCC N stage, AJCC M stage, radiation recode, chemotherapy recode, tumor size, vital status, sequence number, cause of death, and survival months.
We re-categorized “age at diagnosis” into two groups, namely <55 and ≥55 years. Marital status at diagnosis was classified into three categories, namely married, single, or divorced/separated/widowed/unknown. Year of diagnosis was grouped into three intervals, namely 2004–2010, 2011–2015, and 2016–2020. Race was divided into three categories, namely Black, White, and other. Median household income was segmented into three ranges as follows: less than $50,000, $50,000–$74,999, and $75,000 or higher. Histologic type was categorized based on five ICD-O-3 codes i.e., 8330, 8331, 8332, 8335, and 8339. Combined summary stage was divided into localized, regional, and distant stages. AJCC T stage was split into two groups, namely T3 and T4. AJCC N stage was classified into N0, N1, and Nx. AJCC M stage was categorized into M0, M1, and Mx. Radiation recode was grouped into none/unknown, radioisotopes, and beam radiation/radioactive implants/other. Chemotherapy recode was differentiated into yes and no. Tumor size in mm was divided into the following four ranges: ≤10, >10 and ≤20, >20 and ≤40, and >40 mm. Vital status was categorized as alive or dead. For sequence number, we distinguished whether the FTC was the only primary tumor with yes or no.
PSM
The PSM analysis was conducted to mitigate baseline confounding factors between two patient cohorts, namely those who underwent a TT and those who did not undergo TT. For this purpose, we utilized the “MatchIt” package in R Studio to facilitate the matching of propensity scores between the groups. The matching procedure was executed using the nearest neighbor algorithm, adhering to a 1:1 matching ratio and employing a caliper width of 0.02 to ensure close matches between individuals from each cohort. To validate the effectiveness of the PSM process, we conducted a comparative analysis of both the TT group and the non-TT group across all observed variables, before and after the application of PSM.
Patients who did not undergo TT we further classified into two distinct groups based on their treatment, namely those who received RAIT and those who did not. Subsequently, we employed a PSM analysis using the previously described methodology.
Statistical analysis
Categorical variables were assessed using Pearson’s Chi-squared test or Fisher’s exact test. To analyze OS and CSS curves before and after PSM between the TT group and the non-TT group, as well as between the RAIT group and the non-RAIT group, the Kaplan-Meier method was employed. The log-rank test was subsequently utilized to evaluate statistical significance between these groups.
Univariate and multivariate Cox regression analyses were conducted to estimate the hazard ratios (HRs) and 95% confidence intervals (CIs) for OS and CSS. A two-sided P value of less than 0.05 was considered statistically significant. Variables that demonstrated significant P values in the univariate Cox analysis, along with those of clear clinical significance, were incorporated into the multivariate Cox regression analysis for further evaluation. The selection of variables for the final multivariate Cox regression model was based on achieving the minimum Akaike information criterion (AIC) value (14), ensuring an optimal balance between model complexity and fit.
These analyses were executed using R software (version 4.3.3), employing the “survminer”, “survival”, and “coxph” functions for the Cox regression. The Kaplan-Meier survival curves, along with landmark analysis survival curves, were constructed with the aid of the “jskm”, “ggplot2”, and “ggpubr” functions, providing both visual and statistical insights into the survival data.
Results
Section 1: comparison between the TT group and the non-TT group
Selection of the study population, clinical pathological characteristics, and PSM analysis
A cohort of 2,957 patients was selected from the SEER database for inclusion in this study. The detailed flow diagram for patient selection, including inclusion and exclusion criteria utilized within the SEER database, is depicted in Figure 1. Out of the total patient population, 2,271 individuals (representing 76.8% of the sample) underwent a TT, while the remaining 686 patients (23.2%) received alternative treatments. Among these, 2,769 patients were classified as stage T3, constituting 93.6% of the sample, and 188 were classified as stage T4, making up the remaining 6.4%.
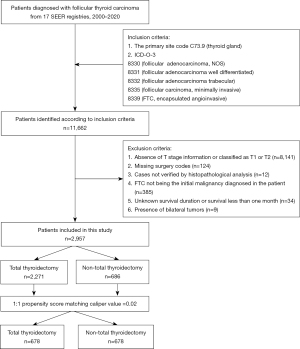
Figure 2 shows a virtually identical distribution between the two patient groups after matching, indicating the effectiveness of the PSM process. Table 1 delineates the demographic and clinical characteristics of the patients in both groups, with comparisons drawn both before and after PSM. Before PSM, significant disparities in baseline characteristics across several covariates were noted between the groups (P<0.05). Among the 2,271 patients who underwent TT, 61.4% received RAIT, 5.1% underwent other forms of radiation therapy, and the remaining 33.5% either did not receive RAIT or had unknown RAIT status.
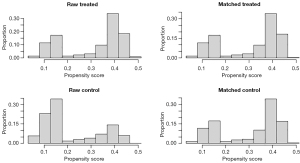
Table 1
| Variables | Pre-PSM | Post-PSM | |||||||
|---|---|---|---|---|---|---|---|---|---|
| Total (n=2,957), n (%) | TT (n=2,271), n (%) | Non-TT (n=686), n (%) | P value | Total (n=1,356), n (%) | TT (n=678), n (%) | Non-TT (n=678), n (%) | P value | ||
| Sex | 0.22 | 0.82 | |||||||
| Female | 1,749 (59.1) | 1,329 (58.5) | 420 (61.2) | 819 (60.4) | 407 (60.0) | 412 (60.8) | |||
| Male | 1,208 (40.9) | 942 (41.5) | 266 (38.8) | 537 (39.6) | 271 (40.0) | 266 (39.2) | |||
| Age (years) | >0.99 | 0.96 | |||||||
| <55 | 1,440 (48.7) | 1,106 (48.7) | 334 (48.7) | 660 (48.7) | 331 (48.8) | 329 (48.5) | |||
| ≥55 | 1,517 (51.3) | 1,165 (51.3) | 352 (51.3) | 696 (51.3) | 347 (51.2) | 349 (51.5) | |||
| Marital status at diagnosis | 0.40 | 0.99 | |||||||
| Married | 1,617 (54.7) | 1,235 (54.4) | 382 (55.7) | 754 (55.6) | 378 (55.7) | 376 (55.5) | |||
| Single | 726 (24.5) | 552 (24.3) | 174 (25.4) | 342 (25.2) | 170 (25.1) | 172 (25.3) | |||
| Divorced/separated/widowed/unknown | 614 (20.8) | 484 (21.3) | 130 (18.9) | 260 (19.2) | 130 (19.2) | 130 (19.2) | |||
| Year of diagnosis | 0.64 | 0.41 | |||||||
| 2004–2010 | 1,060 (35.8) | 804 (35.4) | 256 (37.3) | 515 (38.0) | 266 (39.2) | 249 (36.7) | |||
| 2011–2015 | 869 (29.4) | 670 (29.5) | 199 (29.0) | 377 (27.8) | 178 (26.3) | 199 (29.4) | |||
| 2016–2020 | 1,028 (34.8) | 797 (35.1) | 231 (33.7) | 464 (34.2) | 234 (34.5) | 230 (33.9) | |||
| Race | 0.81 | 0.27 | |||||||
| Black | 423 (14.3) | 320 (14.1) | 103 (15.0) | 189 (14.0) | 87 (12.8) | 102 (15.1) | |||
| White | 2,181 (73.8) | 1,681 (74.0) | 500 (72.9) | 1,012 (74.6) | 519 (76.6) | 493 (72.7) | |||
| Other | 353 (11.9) | 270 (11.9) | 83 (12.1) | 155 (11.4) | 72 (10.6) | 83 (12.2) | |||
| Median household income | <0.05 | 0.08 | |||||||
| <$50,000 | 256 (8.7) | 185 (8.2) | 71 (10.3) | 119 (8.8) | 49 (7.2) | 70 (10.3) | |||
| $50,000–$74,999 | 1,441 (48.7) | 1,134 (49.9) | 307 (44.8) | 639 (47.1) | 334 (49.3) | 305 (45.0) | |||
| $75,000+ | 1,260 (42.6) | 952 (41.9) | 308 (44.9) | 598 (44.1) | 295 (43.5) | 303 (44.7) | |||
| Histological type | 0.20 | 0.22 | |||||||
| 8330 (follicular adenocarcinoma, NOS) | 1,880 (63.6) | 1,447 (63.7) | 433 (63.1) | 887 (65.4) | 462 (68.1) | 425 (62.7) | |||
| 8331 (follicular adenocarcinoma well differentiated) | 118 (4.0) | 87 (3.8) | 31 (4.5) | 57 (4.2) | 26 (3.8) | 31 (4.6) | |||
| 8332 (follicular adenocarcinoma trabecular) | 28 (0.9) | 18 (0.8) | 10 (1.5) | 15 (1.1) | 5 (0.7) | 10 (1.5) | |||
| 8335 (follicular carcinoma, minimally invasive) | 817 (27.6) | 638 (28.1) | 179 (26.1) | 338 (24.9) | 159 (23.5) | 179 (26.4) | |||
| 8339 (FTC, encapsulated angioinvasive) | 114 (3.9) | 81 (3.6) | 33 (4.8) | 59 (4.4) | 26 (3.8) | 33 (4.8) | |||
| Combined summary stage | 0.06 | 0.92 | |||||||
| Localized | 2,185 (73.9) | 1,665 (73.3) | 520 (75.8) | 1,035 (76.3) | 520 (76.7) | 515 (76.0) | |||
| Regional | 541 (18.3) | 414 (18.2) | 127 (18.5) | 246 (18.1) | 122 (18.0) | 124 (18.3) | |||
| Distant | 231 (7.8) | 192 (8.5) | 39 (5.7) | 75 (5.5) | 36 (5.3) | 39 (5.7) | |||
| T stage | 0.49 | 0.82 | |||||||
| T3 | 2,769 (93.6) | 2,131 (93.8) | 638 (93.0) | 1,275 (94.0) | 639 (94.2) | 636 (93.8) | |||
| T4 | 188 (6.4) | 140 (6.2) | 48 (7.0) | 81 (6.0) | 39 (5.8) | 42 (6.2) | |||
| N stage | <0.05 | 0.31 | |||||||
| N0 | 2,619 (88.5) | 1,991 (87.7) | 628 (91.6) | 1,254 (92.5) | 634 (93.5) | 620 (91.5) | |||
| N1 | 153 (5.2) | 117 (5.1) | 36 (5.2) | 61 (4.5) | 25 (3.7) | 36 (5.3) | |||
| NX | 185 (6.3) | 163 (7.2) | 22 (3.2) | 41 (3.0) | 19 (2.8) | 22 (3.2) | |||
| M stage | <0.05 | 0.88 | |||||||
| M0 | 2,744 (92.8) | 2,091 (92.1) | 653 (95.2) | 1,292 (95.3) | 647 (95.4) | 645 (95.1) | |||
| M1 | 184 (6.2) | 154 (6.8) | 30 (4.4) | 57 (4.2) | 27 (4.0) | 30 (4.4) | |||
| MX | 29 (1.0) | 26 (1.1) | 3 (0.4) | 7 (0.5) | 4 (0.6) | 3 (0.5) | |||
| Radiation recode | <0.05 | 0.19 | |||||||
| None/unknown | 1,235 (41.8) | 761 (33.5) | 474 (69.1) | 915 (67.5) | 449 (66.2) | 466 (68.7) | |||
| Radioisotopes | 1,591 (53.8) | 1,394 (61.4) | 197 (28.7) | 400 (29.5) | 203 (30.0) | 197 (29.1) | |||
| Beam radiation/radioactive implants/other | 131 (4.4) | 116 (5.1) | 15 (2.2) | 41 (3.0) | 26 (3.8) | 15 (2.2) | |||
| Chemotherapy recode | 0.32 | >0.99 | |||||||
| No | 2,933 (99.2) | 2,250 (99.1) | 683 (99.6) | 1,350 (99.6) | 675 (99.6) | 675 (99.6) | |||
| Yes | 24 (0.8) | 21 (0.9) | 3 (0.4) | 6 (0.4) | 3 (0.4) | 3 (0.4) | |||
| Tumor size (mm) | 0.36 | 0.84 | |||||||
| ≤10 | 20 (0.7) | 15 (0.6) | 5 (0.7) | 8 (0.6) | 3 (0.4) | 5 (0.7) | |||
| >10, ≤20 | 53 (1.8) | 45 (2.0) | 8 (1.2) | 16 (1.2) | 8 (1.2) | 8 (1.2) | |||
| >20, ≤40 | 213 (7.2) | 170 (7.5) | 43 (6.3) | 79 (5.8) | 37 (5.5) | 42 (6.2) | |||
| >40 | 2,671 (90.3) | 2,041 (89.9) | 630 (91.8) | 1,253 (92.4) | 630 (92.9) | 623 (91.9) | |||
| Vital status | 0.26 | 0.81 | |||||||
| Alive | 2,532 (85.6) | 1,935 (85.2) | 597 (87.0) | 1,176 (86.7) | 586 (86.4) | 590 (87.0) | |||
| Dead | 425 (14.4) | 336 (14.8) | 89 (13.0) | 180 (13.3) | 92 (13.6) | 88 (13.0) | |||
| FTC as first primary tumor | 0.09 | 0.92 | |||||||
| Yes | 2,654 (89.8) | 2,026 (89.2) | 628 (91.5) | 1,242 (91.6) | 622 (91.7) | 620 (91.4) | |||
| No | 303 (10.2) | 245 (10.8) | 58 (8.5) | 114 (8.4) | 56 (8.3) | 58 (8.6) | |||
This table compares the demographic and clinical characteristics of patients who underwent TT group and non-TT group, both before and after applying PSM to balance the groups for statistical analysis. TT, total thyroidectomy; PSM, propensity score matching; NOS, not otherwise specified; FTC, follicular thyroid carcinoma.
Following PSM adjustment, the sample size was refined to include 1,356 patients, evenly divided with 678 patients in each treatment group. Post-PSM comparisons revealed no significant differences in the covariates between the two groups (P>0.05), implying that PSM effectively mitigated potential confounding factors, thereby enhancing the comparability of the two groups.
Survival analysis results
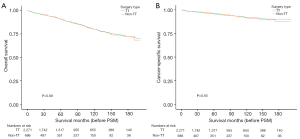
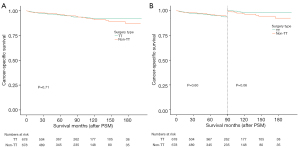
Prior to PSM, the 2-, 5-, and 10-year OS rates were 95.6%, 90.8%, and 79.7%, respectively (TT group) and 96.0%, 90.4%, and 79.6% respectively (non-TT group). The log-rank P value was >0.99 (Figure 3A). The 2-, 5-, and 10-year CSS rates were 97.7%, 95.6%, and 92.0%, respectively (TT group), and 98.1%, 96.3%, and 91.2%, respectively (non-TT group), with a log-rank P value of 0.93 (Figure 3B). There were no statistically significant differences in either OS or CSS between the two groups prior to PSM.
Following PSM, the 2-, 5-, and 10-year OS rates were 95.5%, 90.1%, and 80.4%, respectively (TT group), and 96.0%, 90.5%, and 79.6%, respectively (non-TT group), with a log-rank P value of 0.82 (Figure 4A). Beyond the 90-month mark, the survival curves for both groups began to diverge, prompting further landmark survival analysis (Figure 4B). Although the OS in the TT group was marginally superior to that in the non-TT group, the P value was 0.31, suggesting no statistically significant difference.
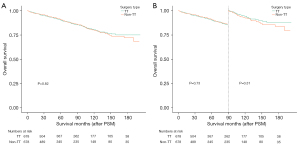
The 2-, 5-, and 10-year CSS rates after PSM were 97.4%, 95.6%, and 92.0 %, respectively (TT group), and 98.1%, 96.2%, and 91.1%, respectively (non-TT group), with a log-rank P value of 0.71 (Figure 5A). Similar to the OS, the CSS survival curves for the two groups began to diverge after the 90-month mark, necessitating further landmark survival analysis (Figure 5B). This analysis revealed that, after 90 months, the CSS in the TT group was superior to that in the non-TT group; however, the P value was 0.06, indicating that there was no significant statistical difference.
Cox proportional-hazards regression analysis
Following the application of PSM, a comprehensive analysis of OS was undertaken. Adhering to the criterion of selecting variables that contribute to the lowest AIC value, three factors were identified for inclusion in the Cox proportional-hazards regression multivariate analysis, namely marital status at the time of diagnosis, N stage, and the presence of only a primary tumor. The analysis revealed that the presence of only a primary tumor was an independent risk factor adversely affecting OS, with an HR of 2.9698 (95% CI: 2.0982–4.203), and a statistically significant P value of less than 0.05 (Table 2).
Table 2
| Variables | Univariate analysis | Multivariate analysis | |||
|---|---|---|---|---|---|
| HR (95% CI) | P value | HR (95% CI) | P value | ||
| OS | |||||
| Marital status at diagnosis | |||||
| Married | Reference | Reference | |||
| Single | 0.8268 (0.5811–1.176) | 0.29 | 0.8040 (0.5647–1.145) | 0.23 | |
| Divorced/separated/widowed/unknown | 0.7244 (0.4755–1.104) | 0.13 | 0.6723 (0.4406–1.026) | 0.07 | |
| N stage | |||||
| N0 | Reference | Reference | |||
| N1 | 0.6505 (0.288–1.469) | 0.30 | 0.6895 (0.3051–1.558) | 0.37 | |
| Nx | 2.0630 (1.089–3.907) | <0.05 | 2.1871 (1.1535–4.147) | <0.05 | |
| FTC as first primary tumor | |||||
| Yes | Reference | Reference | |||
| No | 2.843 (2.012–4.017) | <0.05 | 2.9698 (2.0982–4.203) | <0.05 | |
| CSS | |||||
| Race | |||||
| Black | Reference | Reference | |||
| White | 1.7852 (0.7708–4.135) | 0.18 | 1.6659 (0.7177–3.867) | 0.23 | |
| Other | 0.8561 (0.2415–3.035) | 0.81 | 0.7478 (0.2100–2.664) | 0.65 | |
| Histological type | |||||
| 8330 (follicular adenocarcinoma, NOS) | Reference | Reference | |||
| 8331 (follicular adenocarcinoma well differentiated) | 1.784 (0.6305–5.045) | 0.28 | 1.8833 (0.6655–5.329) | 0.23 | |
| 8332 (follicular adenocarcinoma trabecular) | 4.460 (1.0679–18.625) | <0.05 | 4.7041 (1.1218–19.727) | <0.05 | |
| 8335 (follicular carcinoma, minimally invasive) | 2.105 (1.2673–3.497) | <0.05 | 2.0202 (1.2140–3.362) | <0.05 | |
| 8339 (FTC, encapsulated angioinvasive) | 1.023 (0.3128–3.348) | 0.97 | 0.9300 (0.2833–3.053) | 0.90 | |
| N stage | |||||
| N0 | Reference | Reference | |||
| N1 | 0.5888 (0.144–2.407) | 0.46 | 0.5847 (0.1425–2.398) | 0.46 | |
| Nx | 2.7214 (1.094–6.770) | <0.05 | 2.6589 (1.0636–6.647) | <0.05 | |
This table presents the results from Cox proportional-hazards regression analyses assessing the impacts on OS and CSS following PSM of the study cohorts. OS, overall survival; CSS, cancer-specific survival; PSM, propensity score matching; HR, hazard ratio; CI, confidence interval; FTC, follicular thyroid carcinoma; NOS, not otherwise specified.
In a similar vein, an examination of factors influencing CSS was conducted, once again guided by the principle of minimizing the AIC value. This analysis considered variables such as race, histologic type, and N stage for inclusion in the Cox proportional-hazards regression multivariate analysis. The findings underscored that histological types 8332 and 8335 were independent risk factors affecting CSS. The specific results were as follows: for histological type 8332 (Follicular adenocarcinoma trabecular), HR was 4.7041 (95% CI: 1.1218–19.727), with a P value lower than 0.05; for histological type 8335 (follicular carcinoma, minimally invasive), HR was 2.0202 (95% CI: 1.2140–3.362), also with a P value lower than 0.05 (Table 2).
Section 2: comparison between the non-RAIT group and the RAIT group
Basic clinical characteristics
Given the significance of RAIT in the management of differentiated thyroid cancer (DTC), we analyzed the cohort that did not undergo TT. From the initial cohort of 686 individuals who did not receive TT, 15 individuals underwent alternative treatments such as external beam radiation, radioactive implants, or had unspecified treatment modalities. These cases were subsequently excluded from the analysis, yielding a final sample size of 671 individuals for inclusion in the study. Among these, 197 patients (29.4%) received supplementary RAIT, while the remaining 474 patients (70.6%) did not. Figure S1 demonstrates a highly similar distribution of propensity scores between the non-RAIT and RAIT groups after PSM was applied. Table 3 presents the demographic and clinical characteristics of the two groups of patients before and after PSM.
Table 3
| Variables | Pre-PSM | Post-PSM | |||||||
|---|---|---|---|---|---|---|---|---|---|
| Total (n=671), n (%) | Non-RAIT (n=474), n (%) | RAIT (n=197), n (%) | P value | Total (n=340), n (%) | Non-RAIT (n=170), n (%) | RAIT (n=170), n (%) | P value | ||
| Sex | 0.71 | >0.99 | |||||||
| Female | 411 (61.3) | 293 (61.8) | 118 (59.9) | 207 (60.9) | 104 (61.2) | 103 (60.6) | |||
| Male | 260 (38.7) | 181 (38.2) | 79 (40.1) | 133 (39.1) | 66 (38.8) | 67 (39.4) | |||
| Age (years) | 0.20 | 0.51 | |||||||
| <55 | 327 (48.7) | 223 (47.0) | 104 (52.8) | 179 (52.6) | 93 (54.7) | 86 (50.6) | |||
| ≥55 | 344 (51.3) | 251 (53.0) | 93 (47.2) | 161 (47.4) | 77 (45.3) | 84 (49.4) | |||
| Marital status at diagnosis | 0.45 | 0.50 | |||||||
| Married | 377 (56.2) | 271 (57.2) | 106 (53.8) | 202 (59.4) | 106 (62.4) | 96 (56.5) | |||
| Single | 164 (24.4) | 117 (24.7) | 47 (23.9) | 76 (22.4) | 34 (20.0) | 42 (24.7) | |||
| Divorced/separated/widowed/unknown | 130 (19.4) | 86 (18.1) | 44 (22.3) | 62 (18.2) | 30 (17.6) | 32 (18.8) | |||
| Year of diagnosis | 0.17 | 0.19 | |||||||
| 2004–2010 | 251 (37.4) | 176 (37.1) | 75 (38.1) | 133 (39.1) | 62 (36.4) | 71 (41.8) | |||
| 2011–2015 | 195 (29.1) | 147 (31.0) | 48 (24.3) | 93 (27.4) | 54 (31.8) | 39 (22.9) | |||
| 2016–2020 | 225 (33.5) | 151 (31.9) | 74 (37.6) | 114 (33.5) | 54 (31.8) | 60 (35.3) | |||
| Race | 0.87 | 0.99 | |||||||
| Black | 101 (15.1) | 71 (15.0) | 30 (15.2) | 43 (12.6) | 21 (12.4) | 22 (12.9) | |||
| White | 488 (72.7) | 343 (72.4) | 145 (73.6) | 255 (75.0) | 128 (75.3) | 127 (74.7) | |||
| Other | 82 (12.2) | 60 (12.6) | 22 (11.2) | 42 (12.4) | 21 (12.4) | 21 (12.4) | |||
| Median household income | 0.61 | 0.85 | |||||||
| <$50,000 | 71 (10.6) | 53 (11.2) | 18 (9.1) | 36 (10.6) | 19 (11.2) | 17 (10.0) | |||
| $50,000–$74,999 | 297 (44.3) | 205 (43.2) | 92 (46.7) | 155 (45.6) | 75 (44.1) | 80 (47.1) | |||
| $75,000+ | 303 (45.1) | 216 (45.6) | 87 (44.2) | 149 (43.8) | 76 (44.7) | 73 (42.9) | |||
| Histological type | 0.39 | 0.14 | |||||||
| 8330 (follicular adenocarcinoma, NOS) | 423 (63.0) | 298 (62.9) | 125 (63.4) | 216 (63.5) | 106 (62.4) | 110 (64.7) | |||
| 8331 (follicular adenocarcinoma well differentiated) | 30 (4.5) | 18 (3.8) | 12 (6.1) | 16 (4.7) | 5 (2.9) | 11 (6.5) | |||
| 8332 (follicular adenocarcinoma trabecular) | 10 (1.5) | 9 (1.9) | 1 (0.5) | 6 (1.8) | 5 (2.9) | 1 (0.6) | |||
| 8335 (follicular carcinoma, minimally invasive) | 176 (26.2) | 128 (27.0) | 48 (24.4) | 86 (25.3) | 48 (28.2) | 38 (22.3) | |||
| 8339 (FTC, encapsulated angioinvasive) | 32 (4.8) | 21 (4.4) | 11 (5.6) | 16 (4.7) | 6 (3.5) | 10 (5.9) | |||
| Combined summary stage | >0.99 | 0.76 | |||||||
| Localized | 509 (75.9) | 360 (75.9) | 149 (75.6) | 262 (77.0) | 133 (78.2) | 129 (75.9) | |||
| Regional | 125 (18.6) | 88 (18.6) | 37 (18.8) | 59 (17.4) | 27 (15.9) | 32 (18.8) | |||
| Distant | 37 (5.5) | 26 (5.5) | 11 (5.6) | 19 (5.6) | 10 (5.9) | 9 (5.3) | |||
| T stage | 0.81 | 0.60 | |||||||
| T3 | 626 (93.3) | 441 (93.0) | 185 (93.9) | 325 (95.6) | 164 (96.5) | 161 (94.7) | |||
| T4 | 45 (6.7) | 33 (7.0) | 12 (6.1) | 15 (4.4) | 6 (3.5) | 9 (5.3) | |||
| N stage | 0.64 | 0.95 | |||||||
| N0 | 615 (91.7) | 437 (92.2) | 178 (90.3) | 310 (91.2) | 154 (90.6) | 156 (91.8) | |||
| N1 | 35 (5.2) | 24 (5.1) | 11 (5.6) | 22 (6.5) | 12 (7.1) | 10 (5.9) | |||
| Nx | 21 (3.1) | 13 (2.7) | 8 (4.1) | 8 (2.3) | 4 (2.3) | 4 (2.3) | |||
| M stage | 0.73 | 0.80 | |||||||
| M0 | 640 (95.4) | 452 (95.4) | 188 (95.4) | 324 (95.3) | 161 (94.7) | 163 (95.9) | |||
| M1 | 28 (4.2) | 19 (4.0) | 9 (4.6) | 15 (4.4) | 8 (4.7) | 7 (4.1) | |||
| Mx | 3 (0.4) | 3 (0.6) | 0 | 1 (0.3) | 1 (0.6) | 0 | |||
| Chemotherapy recode | <0.05 | – | |||||||
| No | 670 (99.9) | 473 (99.8) | 197 (100.0) | 340 (100.0) | 170 (100.0) | 170 (100.0) | |||
| Yes | 1 (0.1) | 1 (0.2) | 0 | 0 | 0 | 0 | |||
| Tumor size (mm) | 0.15 | 0.055 | |||||||
| ≤10 | 5 (0.7) | 2 (0.4) | 3 (1.5) | 1 (0.3) | 0 | 1 (0.6) | |||
| >10, ≤20 | 7 (1.0) | 3 (0.6) | 4 (2.0) | 3 (0.9) | 0 | 3 (1.8) | |||
| > 20, ≤40 | 42 (6.3) | 31 (6.5) | 11 (5.6) | 18 (5.3) | 13 (7.6) | 5 (2.9) | |||
| >40 | 617 (92.0) | 438 (92.4) | 179 (90.9) | 318 (93.5) | 157 (92.4) | 161 (94.7) | |||
| Vital status | 0.52 | 0.87 | |||||||
| Alive | 586 (87.3) | 417 (88.0) | 169 (85.8) | 298 (87.6) | 148 (87.1) | 150 (88.2) | |||
| Dead | 85 (12.7) | 57 (12.0) | 28 (14.2) | 42 (12.4) | 22 (12.9) | 20 (11.8) | |||
| FTC as first primary tumor | 0.77 | 0.83 | |||||||
| Yes | 615 (91.7) | 433 (91.4) | 182 (92.4) | 316 (92.9) | 159 (93.5) | 157 (92.4) | |||
| No | 56 (8.3) | 41 (8.6) | 15 (7.6) | 24 (7.1) | 11 (6.5) | 13 (7.6) | |||
This table displays the baseline and post-matching characteristics of patients grouped according to whether they received RAIT or non-RAIT. The comparison was made to evaluate the homogeneity and balance between the two groups before and after applying PSM. RAIT, radioactive iodine therapy; PSM, propensity score matching; NOS, not otherwise specified; FTC, follicular thyroid carcinoma.
Survival analysis
Before PSM, the 2-, 5-, and 10-year OS rates were 96.1%, 91.3%, and 79.5%, respectively (non-RAIT group), and 96.7%, 89.2%, and 80.3%, respectively (RAIT group), with a log-rank P value of 0.64 (Figure 6A). The 2-, 5-, and 10-year CSS rates were 97.7%, 95.6%, and 90.3%, respectively (non-RAIT group), and 98.9%, 97.0%, and 93.1%, respectively (RAIT group), with a log-rank P value of 0.60 (Figure 6B). These results indicated no significant differences in either OS or CSS between the two groups before PSM.
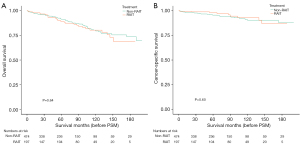
Following PSM, the 5- and 10-year OS rates were 91.1% and 76.4%, respectively (non-RAIT group), and 90.5% and 83.7%, respectively (RAIT group), with a log-rank P value of 0.72 (Figure 7A).
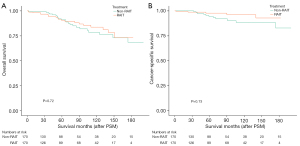
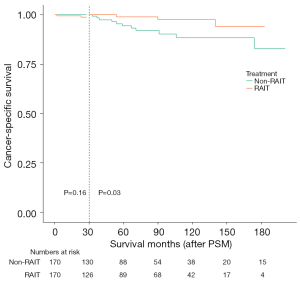
Further analysis revealed that the 5- and 10-year CSS rates in the non-RAIT group were 94.4% and 88.3%, respectively, while the 2-year and 140-month CSS rates in the RAIT group were 97.7% and 92.8%, respectively, with a log-rank P value of 0.13 (Figure 7B). After the 30th month, the CSS survival curves for the two groups began to diverge, prompting a landmark survival analysis (Figure 8). This analysis demonstrated that after 30 months, the CSS rate in the RAIT group was superior to that in the non-RAIT group. These findings suggest that for patients who have not undergone TT, supplementary RAIT may enhance long-term survival.
Discussion
This study comprehensively analyzed the long-term survival outcomes associated with TT versus non-TT in patients diagnosed with stage T3 and T4 FTC. It included an assessment of OS and CSS among patients who did not receive a TT, utilizing landmark survival analysis to evaluate temporal divergences in survival curves. Despite the theoretical advantage of TT in facilitating RAIT, our study revealed no statistically significant differences in OS or CSS between the TT and non-TT groups, even after PSM. While a trend towards improved CSS emerged in the TT group beyond 90 months post-treatment, it did not reach statistical significance (P=0.06). Notably, patients undergoing TT often presented with more advanced disease stages and severe conditions, whereas those avoiding this procedure typically exhibited less pronounced local invasion and earlier clinical stages. In addition, for those patients who did not undergo TT, supplementary RAIT demonstrated a beneficial effect on prolonged CSS.
Guidelines typically advocate for TT in cases of FTC where the primary lesion exceeds 4 cm in diameter or invades adjacent anatomical structures, conditions often corresponding to T3 or T4 staging according to the AJCC classification (3,10,15). Nevertheless, due to the diagnostic limitations of FNAB and intraoperative frozen section examination in conclusively identifying FTC, a substantial number of patients initially receive a lobectomy or partial thyroidectomy (16). To avoid severe complications associated with a second surgery, many patients refuse further surgical intervention. For locally advanced FTC patients who can be definitively diagnosed, TT is often not performed to avoid serious complications such as bilateral recurrent laryngeal nerve injury or permanent hypoparathyroidism.
To the best of our knowledge, only a limited number of studies have compared surgical approaches (TT versus non-TT) for patients with FTC (17-21). This study distinguished itself by specifically focusing on patients at T3 and T4 stages of FTC. In addition, it encompassed a larger patient cohort and utilized PSM to reduce bias to the greatest extent feasible. Kethidi et al. conducted a retrospective analysis of the National Cancer Database (NCDB), focusing on FTC patients with tumor diameters between 1 and 4 cm. A total of 6,871 patients were included, with 1,507 undergoing lobectomy and 5,364 undergoing TT. The final results showed no significant survival differences between the two groups (17). Park et al. conducted a study involving 87 patients with FTC. According to their analysis, tumor diameter of 2 cm served as a critical threshold for recommending TT in cases of minimally invasive FTC (MI-FTC) and encapsulated angioinvasive FTC (EA-FTC), both characterized by mutant telomerase reverse transcriptase (TERT). The status of the TERT promoter mutation plays a crucial role in selecting candidates for this procedure. However, further clinical studies are necessary to validate these findings (18).
For patients with T3 or T4 stage FTC who have not undergone TT, RAIT becomes a viable alternative. This contributes to the increasing prevalence of RAIT use in thyroid cancer treatment (22). However, the role of radioactive iodine (RAI) ablation after thyroid lobectomy (TL) remains unclear. Several small retrospective studies have described RAI lobe ablation after TL as a safe and effective alternative to TT in DTC (12,13,23-25). Barbesino and colleagues conducted a comparative analysis between patients who underwent partial thyroidectomy with subsequent RAIT and those who received TT. Their findings indicated that thyroglobulin levels were more readily detectable in the blood of patients who had undergone partial thyroidectomy followed by RAIT. However, that study did not compare the effects of different treatment methods on OS and CSS (12). Kiernan and colleagues conducted a comparative study on survival differences in patients who did not undergo TT, specifically examining those treated with RAIT versus those who were not. They found that RAIT could improve OS. However, the study population included patients with DTC, encompassing both papillary and follicular carcinomas, and included patients with T stages ranging from 1 to 4 (24).
The findings from this study have several practical implications for the management of FTC with T3 or T4 stage. For these patients, the decision to undergo TT should be carefully considered. In the short term, TT does not significantly alter OS or CSS compared with less extensive surgical options. Therefore, deciding on TT should account for patient-specific factors such as comorbid conditions, age, personal preferences, and potential surgical risks. In the long term, the potential for improved outcomes with TT, as suggested by our study, should be discussed with patients, particularly those with a longer life expectancy. This discussion should highlight the potential for marginal long-term survival benefits, despite the absence of significant differences in short- to medium-term survival. In addition, in the analysis of independent risk factors affecting survival, we found that FTC was not the only malignant tumor significantly affecting OS, a finding consistent with clinical patterns. Histological subtypes, such as follicular adenocarcinoma trabecular and minimally invasive follicular carcinoma, were independent risk factors affecting CSS. This finding suggests the need for further detailed research based on different histological classifications to provide a basis for more precise treatment.
For patients who did not undergo TT, RAIT showed a potential long-term CSS benefit. Clinicians should consider RAIT as a standard part of the treatment protocol for these patients, emphasizing its role in reducing long-term recurrence and improving survival. Further research is needed to optimize the timing and dosage of RAIT. However, current evidence supports its use, particularly after the 30-month mark after treatment, where CSS benefits become more pronounced.
The study utilized a large, well-defined cohort from the SEER database, enhancing the robustness and generalizability of findings within the context of T3 and T4 FTC patients. The use of PSM effectively reduced confounding by creating balanced treatment groups, thereby providing a more accurate comparison of survival outcomes. In addition, the study employed comprehensive analytical approaches, including landmark analysis and Cox proportional hazards regression, to offer detailed insights into the long-term benefits of TT and RAIT. The focus on T3 and T4 stages of FTC is particularly relevant, as these stages represent more advanced disease where treatment decisions are critical.
However, there are several limitations in this study. The retrospective study design poses challenges, but conducting prospective studies or RCTs for similar research is difficult due to ethical and practical considerations. The SEER database also lacks comprehensive information, such as distinguishing between patients who did not undergo iodine-131 (I-131) radionuclide therapy from those for whom it is unknown. Furthermore, the database does not provide detailed data on local tumor infiltration or complications such as hoarseness and hypocalcemia, which could impact the depth of the study. Notably, the radiation recode section of the SEER database provides no information on I-131 dosage or treatment frequency, further constraining the analysis.
Conclusions
This study indicates that TT does not enhance survival rates in patients with stage T3/T4 FTC. No significant differences were observed in OS and CSS in the short to medium term. However, patients who did not undergo TT but received RAIT demonstrated improved CSS. These findings highlight the necessity for personalized surgical decision-making for patients with T3/T4 stage FTC, taking into account both immediate risks and potential long-term benefits.
Acknowledgments
We extend our sincere gratitude to the SEER program for providing the data necessary for this study. We also acknowledge the dedicated research teams for their invaluable contributions. A special note of thanks is owed to the patients who consented for their data to be used, thereby making this research possible.
Funding: None.
Footnote
Reporting Checklist: The authors have completed the STROBE reporting checklist. Available at https://gs.amegroups.com/article/view/10.21037/gs-24-231/rc
Peer Review File: Available at https://gs.amegroups.com/article/view/10.21037/gs-24-231/prf
Conflicts of Interest: All authors have completed the ICMJE uniform disclosure form (available at https://gs.amegroups.com/article/view/10.21037/gs-24-231/coif). The authors have no conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved. The study was conducted in accordance with the Declaration of Helsinki (as revised in 2013) and was approved by the Institutional Ethics Committee of Qilu Hospital of Shandong University Dezhou Hospital (No. 2024053). As this was a retrospective cohort study using anonymized data from the SEER database, the requirement for informed consent was waived.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Boucai L, Zafereo M, Cabanillas ME. Thyroid Cancer: A Review. JAMA 2024;331:425-35. [Crossref] [PubMed]
- Miranda-Filho A, Lortet-Tieulent J, Bray F, et al. Thyroid cancer incidence trends by histology in 25 countries: a population-based study. Lancet Diabetes Endocrinol 2021;9:225-34. [Crossref] [PubMed]
- Haugen BR, Alexander EK, Bible KC, et al. 2015 American Thyroid Association Management Guidelines for Adult Patients with Thyroid Nodules and Differentiated Thyroid Cancer: The American Thyroid Association Guidelines Task Force on Thyroid Nodules and Differentiated Thyroid Cancer. Thyroid 2016;26:1-133. [Crossref] [PubMed]
- Daniels GH. Follicular Thyroid Carcinoma: A Perspective. Thyroid 2018;28:1229-42. [Crossref] [PubMed]
- Ali SZ, Baloch ZW, Cochand-Priollet B, et al. The 2023 Bethesda System for Reporting Thyroid Cytopathology. Thyroid 2023;33:1039-44. [Crossref] [PubMed]
- Baloch ZW, Asa SL, Barletta JA, et al. Overview of the 2022 WHO Classification of Thyroid Neoplasms. Endocr Pathol 2022;33:27-63. [Crossref] [PubMed]
- LiVolsi VA. Baloch ZW. Use and abuse of frozen section in the diagnosis of follicular thyroid lesions. Endocr Pathol 2005;16:285-93. [Crossref] [PubMed]
- Leteurtre E, Leroy X, Pattou F, et al. Why do frozen sections have limited value in encapsulated or minimally invasive follicular carcinoma of the thyroid? Am J Clin Pathol 2001;115:370-4. [Crossref] [PubMed]
- Hauch A, Al-Qurayshi Z, Randolph G, et al. Total thyroidectomy is associated with increased risk of complications for low- and high-volume surgeons. Ann Surg Oncol 2014;21:3844-52. [Crossref] [PubMed]
- Patel KN, Yip L, Lubitz CC, et al. The American Association of Endocrine Surgeons Guidelines for the Definitive Surgical Management of Thyroid Disease in Adults. Ann Surg 2020;271:e21-93. [Crossref] [PubMed]
- Austin PC. An Introduction to Propensity Score Methods for Reducing the Effects of Confounding in Observational Studies. Multivariate Behav Res 2011;46:399-424. [Crossref] [PubMed]
- Barbesino G, Goldfarb M, Parangi S, et al. Thyroid lobe ablation with radioactive iodine as an alternative to completion thyroidectomy after hemithyroidectomy in patients with follicular thyroid carcinoma: long-term follow-up. Thyroid 2012;22:369-76. [Crossref] [PubMed]
- Bal C, Satapathy S, Tupalli A, et al. Propensity Score Matched Outcome Analysis of Lobar Ablation Versus Completion Thyroidectomy in Low-Risk Differentiated Thyroid Cancer Patients: Median Follow-Up of 11 Years. Thyroid 2022;32:1220-8. [Crossref] [PubMed]
- Ikemoto K, Takahashi K, Ozawa T, et al. Akaike's Information Criterion for Stoichiometry Inference of Supramolecular Complexes. Angew Chem Int Ed Engl 2023;62:e202219059. [Crossref] [PubMed]
- Schlumberger M, Leboulleux S. Current practice in patients with differentiated thyroid cancer. Nat Rev Endocrinol 2021;17:176-88. [Crossref] [PubMed]
- Jonklaas J, Sarlis NJ, Litofsky D, et al. Outcomes of patients with differentiated thyroid carcinoma following initial therapy. Thyroid 2006;16:1229-42. [Crossref] [PubMed]
- Kethidi N, Vedula S, Shihora D, et al. Extent of Surgery for Follicular Thyroid Carcinoma. Laryngoscope 2023;133:993-9. [Crossref] [PubMed]
- Park H, Heo J, Ki CS, et al. Selection Criteria for Completion Thyroidectomy in Follicular Thyroid Carcinoma Using Primary Tumor Size and TERT Promoter Mutational Status. Ann Surg Oncol 2023;30:2916-25. [Crossref] [PubMed]
- Choi JB, Lee SG, Kim MJ, et al. Oncologic outcomes in patients with 1-cm to 4-cm differentiated thyroid carcinoma according to extent of thyroidectomy. Head Neck 2019;41:56-63. [Crossref] [PubMed]
- Sugino K, Nagahama M, Kitagawa W, et al. Risk Stratification of Pediatric Patients with Differentiated Thyroid Cancer: Is Total Thyroidectomy Necessary for Patients at Any Risk? Thyroid 2020;30:548-56. [Crossref] [PubMed]
- Spinelli C, Rallo L, Morganti R, et al. Surgical management of follicular thyroid carcinoma in children and adolescents: A study of 30 cases. J Pediatr Surg 2019;54:521-6. [Crossref] [PubMed]
- Haymart MR, Banerjee M, Stewart AK, et al. Use of radioactive iodine for thyroid cancer. JAMA 2011;306:721-8. [Crossref] [PubMed]
- Randolph GW, Daniels GH. Radioactive iodine lobe ablation as an alternative to completion thyroidectomy for follicular carcinoma of the thyroid. Thyroid 2002;12:989-96. [Crossref] [PubMed]
- Kiernan CM, Parikh AA, Parks LL, et al. Use of radioiodine after thyroid lobectomy in patients with differentiated thyroid cancer: does it change outcomes? J Am Coll Surg 2015;220:617-25. [Crossref] [PubMed]
- Bal CS, Kumar A, Pant GS. Radioiodine lobar ablation as an alternative to completion thyroidectomy in patients with differentiated thyroid cancer. Nucl Med Commun 2003;24:203-8. [Crossref] [PubMed]


