
Successful treatment of enormous locally advanced breast cancer through neoadjuvant chemotherapy and surgical intervention: a case report
Highlight box
Key findings
• This study has revealed that for patients with locally advanced, human epidermal growth factor receptor 2 (HER2)-overexpressing breast cancer, strict adherence to guidelines and completion of the recommended number of neoadjuvant chemotherapy (NACT) cycles is not essential to achieve a pathological complete response.
What is known and what is new?
• The standard treatment for patients with HER2-positive breast cancer involves NACT in combination with HER2 targeted drugs, such as trastuzumab and pertuzumab. According to the guidelines, it is recommended that patients should undergo 6 cycles of NACT prior to proceeding with further treatment.
• This article deviated from strict adherence to the guidelines, opting for flexible timing of surgery based on the patient’s evolving condition after the patient had completed 4 cycles of NACT.
What is the implication, and what should change now?
• Imaging tests may lead to misinterpretation of both diagnosis and treatment plan. Clinicians should conduct a comprehensive assessment of the patient’s condition and customize individualized treatment plans, selecting the appropriate timing for surgery, rather than solely relying on standardized guidelines.
Introduction
According to the latest epidemiological research, breast cancer has emerged as the most prevalent malignancy among women, with an average of one new diagnosis made every 1.8 s. Furthermore, in 2020, breast cancer surpassed lung cancer to claim its position as the world’s most common form of cancer (1). Spontaneously, breast cancer has become the foremost cause of mortality among women, and the majority of patients initially present with stage I–III diagnoses, in accordance with surgical indications (2). However, for patients with locally advanced disease, delayed diagnosis and large tumor sizes pose challenges to successful surgical intervention. The administration of neoadjuvant chemotherapy (NACT) may be considered for these patients in order to diminish tumor volume and burden, thereby enhancing the feasibility of surgical resection, optimizing prognosis, and extending overall survival (3). Meanwhile, the use of NACT in breast cancer treatment is continuously expanding, with medication-selection strategies based on biomarker information obtained from needle biopsies (4).
In this study, we present a case of locally advanced breast cancer wherein the effectiveness of NACT was observed, leading to successful radical mastectomy. Subsequent postoperative pathological examination confirmed the achievement of a pathological complete response (pCR). We present this article in accordance with the CARE reporting checklist (available at https://gs.amegroups.com/article/view/10.21037/gs-24-92/rc).
Case presentation
Ethical consideration and consent to participate
All procedures performed in this study were in accordance with the ethical standards of the institutional and/or national research committee(s) and with the Helsinki Declaration (as revised in 2013). Written informed consent was obtained from the patient for publication of this case report and accompanying images. A copy of the written consent is available for review by the editorial office of this journal.
Present illness history
A 54-year-old female patient visited the hospital due to a palpable mass in her right breast. It had been accidentally discovered 2 years ago, a lesion measuring approximately 3 cm × 3 cm was found in the upper quadrant. The patient experienced pain but did not present with abnormal skin discharge nor ulceration, and she did not pursue further examination or treatment. Her past medical history and family history were unremarkable.
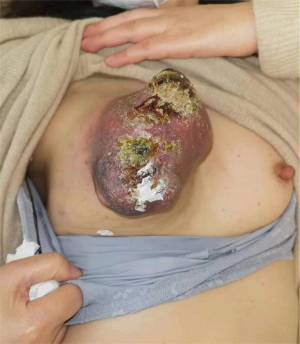
Subsequently, the mass exhibited progressive enlargement and a significant acceleration from December 2022, accompanied by the development of ulceration. Consequently, in January 2023, the patient was admitted to the hospital for further diagnosis and treatment. The mass protruded from the skin surface, measuring approximately 12 cm × 10 cm, exhibited multiple visible ulcerations (Figure 1).
A breast ultrasound examination revealed that the right breast of this patient was predominantly occupied by a substantial mass, with involvement of the skin layer. The protrusion from the skin measured approximately 7.2 cm in diameter (Figure 2A). The right axillary region exhibited the presence of multiple lymph nodes with cortical thickening, with the largest node measuring approximately 2.3 cm in diameter, suggestive of metastasis (Figure 2B). In the meantime, enhanced computed tomography (CT) was performed (Figure 2C), and a large, irregular soft tissue mass with a maximum radius of approximately 12 cm was identified, exhibiting distinct protrusion and possible involvement of the pectoralis major muscle. The biological characteristics indicated similarities to those typically observed in a malignant neoplasm.

A positron emission tomography-CT (PET-CT) scan was also conducted, revealing resemblances to the former CT findings. An unevenly increased radioactive uptake was observed in the extensive mass located in the right breast, with a maximum standardized uptake value of 12.7. The right pectoralis major of the patient exhibited thickening, with a slightly increased radioactive uptake and a standard uptake value maximum (SUVmax) value of 2.4. The presence of increased radioactive uptakes in multiple lymph nodes was observed in the right axilla, with a SUVmax value of 3.0 concurrently (Figure 2D).
In conclusion, the patient’s enormous tumor was diagnosed as a malignant lesion with invasion of the right pectoralis major and metastasis to multiple lymph nodes in the right axillary area. Fortunately, no distal metastasis was observed.
Core needle biopsy performed in February 2023 confirmed the mass as invasive breast cancer with moderate differentiation, involving the dermis of the skin. Immunohistochemical findings revealed weakly positive expression of estrogen receptors (25%) and negative expression of progesterone receptors (−). There was human epidermal growth factor receptor 2 (HER2) overexpression (3+), the Ki-67 index was 90%, and cytokeratin 5/6 expression was negative (Figure 3). The positive preoperative pathology finding of HER2 staining further corroborated the diagnosis of HER2-overexpressing breast cancer in the patient (Figure 4).


Considering the extensive size of the mass and metastasis to the axillary lymph nodes, surgical intervention was not a viable option at this particular juncture. Consequently, the patient was prescribed NACT every 3 weeks, which included a medication regimen consisting of docetaxel (75 mg/m2; total dose, 120 mg), carboplatin (area under the curve =5 mg/mL/min; total dose, 450 mg), trastuzumab (loading dose, 440 mg; maintenance dose, 330 mg), and pertuzumab (loading dose, 840 mg; maintenance dose, 420 mg). The neoadjuvant therapy commenced in mid-March 2023 and encompassed 4 cycles, with treatments administered every three weeks. The patient received all these treatments punctually and no significant adverse effects were observed during the courses.
The size of the mass was closely monitored using PET/CT, CT, and breast ultrasound imaging. Prior to treatment, the maximum tumor diameter was approximately 12 cm, as determined by CT measurement (Figure 2C). Substantial progress was achieved through advancements in therapy, with consistent shrinkage observed after each intervention. Following the second course of treatment, the tumor size was reduced to 6.4 cm × 5.8 cm × 4.0 cm, accompanied by abundant streak bloodstream signals internally (Figure 5A), the cortical thickening of the right axillary lymph nodes remains evident (Figure 5B). The dimensions of the mass decreased to 5.5 cm × 5.2 cm after completion of the third course of treatment (Figure 6A), the thickening which mentioned above was also alleviated (Figure 6B). A similar trend was observed in the size difference of lymph nodes, with the largest radius decreasing from 2.3 to 1.8 cm (Figure 6B). Multiple ulcerations were resolved.


The rate of tumor volume reduction, however, demonstrated a significant deceleration following the completion of the fourth course of treatment compared to previous responses. Therefore, assuming that there have been no significant further changes in the volume of the mass, we inferred that the local conditions were favorable for implementing radical surgical procedures. To prevent recurrence and progression, which might be caused by drug resistance, the patient was admitted to the hospital for additional surgical treatment.
Physical examination
The patient presented with nipple inversion in the right breast. Her original ulcerations had healed, and the pigmented area had reduced to a size of 5 cm × 5 cm (Figure 7). A firm, poorly mobile area with a diameter of approximately 5 cm was palpable in the upper quadrant of the right breast, exhibiting indistinct margins and tenderness. Enlarged and fused lymph nodes were palpable in the right axilla, demonstrating moderate tenderness and mobility.

Treatment
After admission, preoperative examinations were conducted, including electrocardiography (ECG), chest radiography, and breast ultrasonography. The ultrasound revealed the presence of a large mass contains multiple hypoechoic areas in the right breast, with a maximum radius of approximately 4.8 cm in the mass and 1.7 cm in the right axillary lymph nodes (Figure 8). The tumor size demonstrated a distinct reduction following the initiation of neoadjuvant therapy, with no significant difference observed in tumor size between subsequent treatments. The diagnosis of right breast cancer and right armpit lymph node metastasis was clearly established by integrating the patient’s medical history and examination findings.

A modified radical mastectomy of the right breast was performed after 4 cycles of chemotherapies (Figure 9). The patient was discharged 3 days postoperatively following removal of the drainage tube. The sutures were subsequently extracted 2 weeks after the surgical procedure, and a favorable wound-healing process without any complications was observed. The excised breast and lymph node tissue were subjected to paraffin pathological examination. The pathological test results revealed no residual cancer tissue in the excised breast or lymph node tissue. Abundant foam cells were observed in the breast tissue, along with clusters of cholesterol crystal formation, which is consistent with typical findings following neoadjuvant therapies. Additionally, only chronic lymphadenitis was detected in the dissected lymph nodes, with a total of 45 axillary lymph nodes and nine station 3 lymph nodes examined, without any showing malignant cells (Figure 10). The tumor was graded with Miller-Payne 5, indicating that the tumor site sections exhibited an absence of malignant cells, with the presence of vascular fibroelastotic stroma containing macrophages being frequently observed (5). The absence of tumor cells was further confirmed through negative cytokeratin 7 staining in the postoperative pathology (Figure 11), indicating a pCR, with the absence of residual invasive breast cancer cells as well as the absence of ductal carcinoma in situ (DCIS).


Throughout the NACT, the patients demonstrated a positive and optimistic attitude regarding the progressive tumor shrinkage. Subsequently, upon receiving postoperative pathological complete response (PCR) pathology results, she expressed appreciation for the decision to undergo surgery prior to completing the standard therapy courses.
The patient was relieved and underwent postoperative administration of chemotherapy and then 1 year of targeted therapy combined with trastuzumab and pertuzumab and another year of intensive neratinib treatment subsequently.
We conducted short-term postoperative ultrasonographic reassessment and long-term follow-up in the outpatient clinic and via telephone for the patient. The ultrasound follow-up at 2 months post-operation revealed the absence of any evident cystic or solid lesions in the right chest wall, and no significant enlargement of lymph nodes was observed in the bilateral axillary regions. Following the surgical procedure, the patient has been undergoing monthly follow-up appointments at our clinic, with no discernible indications of tumor recurrence. As of May 2024, the patient received regular semi-annual outpatient clinic follow-ups and monthly telephone follow-ups, with no definitive evidence of tumor recurrence identified
Discussion
The National Comprehensive Cancer Network guidelines recommend neoadjuvant therapy for patients with invasive breast cancer, aiming to reduce the clinical stage, enhance the breast retention rate, and effectively eliminate potential micro-metastases (2,6).
The overexpression of HER2 in breast cancer is commonly associated with aggressive biological behavior and a poor survival rate (7). Constituting approximately 15–20% of all breast cancer cases, this subset represents a significant proportion of the disease burden (8). The anti-HER2 monoclonal antibody drug, trastuzumab, has exhibited promising efficacy in the treatment of all stages of HER2+ breast cancers (9). Currently, adjuvant therapy has emerged as a standard treatment modality for patients diagnosed with HER2+ breast cancer. In the case of locally advanced disease, the implementation of neoadjuvant systemic and targeted therapies can potentially render inoperable patients operable and enhance the feasibility of local resection.
In this case, based on the patient’s medical history, imaging findings, and pathological reports, it was determined that she initially presented with stage IIIB breast cancer (T4cN2aM0) characterized by HER2 overexpression. Her enormous tumor demonstrated extensive invasion of the pectoralis major and skin, along with multiple lymph node metastases. Fortunately, this patient achieved a successful treatment response following NACT and targeted therapy, leading to radical mastectomy. Subsequent postoperative pathological analysis confirmed a complete pathological response in this patient.
The total pCR refers to the thorough elimination of invasive cancerous tissue not only in the primary breast lesion or carcinoma in situ but also in the metastatic regional lymph nodes following NACT. This outcome is strongly associated with higher overall survival and event-free survival rates, indicating a more favorable prognostic outcome compared to that in non-pCR patients (10-12).
As per the guidelines provided by the National Comprehensive Cancer Network (Version 2. 2022), both the TCbH-P regimen and AC-THP regimen are considered as optional treatment options for prescription. The TCbH-P regimen includes taxane, carboplatin, trastuzumab, and pertuzumab, while the AC-THP regimen consists of anthracycline, cyclophosphamide, taxane, trastuzumab, and pertuzumab. The TCbH-P regimen demonstrates comparable adverse effects while exhibiting more promising outcomes in terms of further reducing the risk of recurrence for high-risk patients (13). The therapeutic efficacy and safety of TCbHP and AC-THP in the treatment of HER2+ early breast cancer have been compared in multiple studies that demonstrated a significantly higher pCR rate in the TCbH-P regimen compared to anthracycline-containing regimens (14). Nevertheless, the TCbHP regimen demonstrated a shorter duration of cardiotoxicity compared to the AC-THP regimen, which may be attributed to the adverse effects of anthracycline-induced damage on cardiomyocytes, leading to diastolic and systolic dysfunction of the heart. This eventually results in a decrease in left ventricular ejection fraction and potential complications like cardiac failure and cardiomyopathy (15). Therefore, in this case, the selection of a TCbHP regimen was based not only on its mild cardiac-related adverse effects but also on our belief that early double-targeted therapy should be administered promptly to this patient with a high histological grade, large tumor size, high Ki-67 index, low estrogen receptor (ER) expression, and negative progesterone receptor (PR) expression in the initial stage of treatment. The timing of surgery plays a critical role in the management of highly advanced patients. We routinely performed imaging evaluations to assess the efficacy of preoperative treatment. Once the tumor reached a certain degree of shrinkage, without any further significant reduction, and the level of reduction was adequate for radical surgery, we tended to promptly proceed with radical surgery rather than attempting to convert to an alternative regimen. This approach aimed to mitigate drug resistance, disease progression, and missed surgical opportunities. By decisively seizing this critical moment, we successfully executed the operation. Moreover, postoperative pathological findings confirmed a complete pathological response, validating our well-timed surgical intervention and achievement of therapeutic objectives. These results also underscore the adaptability in determining the optimal timing for surgical intervention based on comprehensive patient assessment.
Despite the presence of a cancer-like manifestation in the previous preoperative ultrasound report, the subsequent postoperative pathological analysis in this study revealed a divergent outcome, highlighting the potential for misleading imaging findings in patients who have undergone NACT. As tumors exhibit expansive and invasive characteristics, they tend to exert pressure on and erode surrounding normal tissue. The administration of NACT also results in significant tumor cell death, leading to a reduction in its space-occupying properties. The reversion of former cancer cells occurs through phagocyte-mediated breakdown and digestion, leading to the restoration of adjacent tissue to its original state and subsequent tumor shrinkage or replacement with connective tissue enriched in cellular components.
Shrinkage patterns have been described differently among studies, being primarily categorized into the two types of concentric and non-concentric. In this particular case, a significant concentric reduction in mass diameter was observed, decreasing from 9.8 to 5.5 cm with a concentric pattern. Notably, some researchers have posited that the shrinkage pattern exhibits superior sensitivity and specificity as a predictor of response to HER2-targeted therapy compared to other tests, thereby aiding in prognostication for patients. Patients who have undergone NACT and present with a single focal residual disease exhibit a higher 4-year ipsilateral tumor relapse-free survival rate compared to those with multiple foci, owing to the occurrence of false-negative pathological findings in the remaining peri-cancerous tissue postoperatively (16).
Conclusions
This case report details a rare instance of huge locally advanced HER2-overexpression breast cancer with extensive axillary lymph node metastases, wherein preoperative chemotherapy and targeted therapy demonstrated efficacy, ultimately enabling the patient to undergo radical mastectomy. The postoperative pathological findings also indicated a complete pathological response. These findings suggest that patients with locally advanced disease can still achieve a radical surgical resection following appropriate comprehensive treatment, underscoring the importance for surgeons to carefully consider the optimal timing for surgical intervention. This case report aims to provide novel insights into the management of similar patients in future clinical practice.
Acknowledgments
Funding: This research was funded by
Footnote
Reporting Checklist: The authors have completed the CARE reporting checklist. Available at https://gs.amegroups.com/article/view/10.21037/gs-24-92/rc
Peer Review File: Available at https://gs.amegroups.com/article/view/10.21037/gs-24-92/prf
Conflicts of Interest: All authors have completed the ICMJE uniform disclosure form (available at https://gs.amegroups.com/article/view/10.21037/gs-24-92/coif). The authors have no conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved. All procedures performed in this study were in accordance with the ethical standards of the institutional and/or national research committee(s) and with the Helsinki Declaration (as revised in 2013). Written informed consent was obtained from the patient for publication of this case report and accompanying images. A copy of the written consent is available for review by the editorial office of this journal.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Sung H, Ferlay J, Siegel RL, et al. Global Cancer Statistics 2020: GLOBOCAN Estimates of Incidence and Mortality Worldwide for 36 Cancers in 185 Countries. CA Cancer J Clin 2021;71:209-49. [Crossref] [PubMed]
- Wang L, Jiang Q, He MY, et al. HER2 changes to positive after neoadjuvant chemotherapy in breast cancer: A case report and literature review. World J Clin Cases 2022;10:260-7. [Crossref] [PubMed]
- Li F, Yang Y, Wei Y, et al. Predicting neoadjuvant chemotherapy benefit using deep learning from stromal histology in breast cancer. NPJ Breast Cancer 2022;8:124. [Crossref] [PubMed]
- Maimaitiaili A, Fan Z, Zhang J, et al. Prognostic value of pathological nodal burden after neoadjuvant chemotherapy in initially cN0-1 breast cancer patients: a dual-center, 10-year survival analysis. Ther Adv Med Oncol 2024;16:17588359241248318. [Crossref] [PubMed]
- Xu X, Zhao W, Liu C, et al. The residual cancer burden index as a valid prognostic indicator in breast cancer after neoadjuvant chemotherapy. BMC Cancer 2024;24:13. [Crossref] [PubMed]
- Pathak M, Dwivedi SN, Deo SVS, et al. Effectiveness of Added Targeted Therapies to Neoadjuvant Chemotherapy for Breast Cancer: A Systematic Review and Meta-analysis. Clin Breast Cancer 2019;19:e690-700. [Crossref] [PubMed]
- Fernandes CL, Silva DJ, Mesquita A. Novel HER-2 Targeted Therapies in Breast Cancer. Cancers (Basel) 2023;16:87. [Crossref] [PubMed]
- Nader-Marta G, Martins-Branco D, de Azambuja E. How we treat patients with metastatic HER2-positive breast cancer. ESMO Open 2022;7:100343. [Crossref] [PubMed]
- Long-term outcomes for neoadjuvant versus adjuvant chemotherapy in early breast cancer: meta-analysis of individual patient data from ten randomised trials. Lancet Oncol 2018;19:27-39. [Crossref] [PubMed]
- Spring LM, Fell G, Arfe A, et al. Pathologic Complete Response after Neoadjuvant Chemotherapy and Impact on Breast Cancer Recurrence and Survival: A Comprehensive Meta-analysis. Clin Cancer Res 2020;26:2838-48. [Crossref] [PubMed]
- Stebbing J, Baranau Y, Manikhas A, et al. Total pathological complete response versus breast pathological complete response in clinical trials of reference and biosimilar trastuzumab in the neoadjuvant treatment of breast cancer. Expert Rev Anticancer Ther 2018;18:531-41. [Crossref] [PubMed]
- Schneeweiss A, Chia S, Hickish T, et al. Long-term efficacy analysis of the randomised, phase II TRYPHAENA cardiac safety study: Evaluating pertuzumab and trastuzumab plus standard neoadjuvant anthracycline-containing and anthracycline-free chemotherapy regimens in patients with HER2-positive early breast cancer. Eur J Cancer 2018;89:27-35. [Crossref] [PubMed]
- Lu H, Yan H, Liao S, et al. Efficacy, cardiotoxicity and factors affecting pathologic complete response of neoadjuvant chemotherapy with anthracycline-containing verses anthracycline-free regimens plus dual HER2 blockade for HER2-positive early-stage breast cancer: a retrospective study. Transl Cancer Res 2023;12:1490-502. [Crossref] [PubMed]
- Gao HF, Wu Z, Lin Y, et al. Anthracycline-containing versus carboplatin-containing neoadjuvant chemotherapy in combination with trastuzumab for HER2-positive breast cancer: the neoCARH phase II randomized clinical trial. Ther Adv Med Oncol 2021;13:17588359211009003. [Crossref] [PubMed]
- Mo Z, Deng Y, Bao Y, et al. Evaluation of cardiotoxicity of anthracycline-containing chemotherapy regimens in patients with bone and soft tissue sarcomas: A study of the FDA adverse event reporting system joint single-center real-world experience. Cancer Med 2023;12:21709-24. [Crossref] [PubMed]
- Zou J, Zhang L, Chen Y, et al. Neoadjuvant Chemotherapy and Neoadjuvant Chemotherapy With Immunotherapy Result in Different Tumor Shrinkage Patterns in Triple-Negative Breast Cancer. J Breast Cancer 2024;27:27-36. [Crossref] [PubMed]



