
A comprehensive analysis of imaging features and clinical characteristics to differentiate malignant from non-malignant mammographic architectural distortion
Highlight box
Key findings
• The comprehensive multimodal analysis of mammographic and sonographic features and clinical characteristics can improve the efficacy of differential diagnosis for mammography-detected architectural distortion (AD) lesions and could be instrumental in refining clinical management strategies.
What is known, and what is new?
• Both benign and malignant breast lesions can appear as ADs on mammograms. ADs with a corresponding abnormal appearance on ultrasound were more likely to indicate malignancy, regardless of whether they were initially detected by mammography or digital breast tomosynthesis. Presently, there is no reliable imaging standard to differentiate between malignant and benign ADs.
• The application of mammogram-guided “second look” ultrasound can enhance the detection of sonographic findings corresponding to mammographic ADs, particularly for non-mass-like findings. Moreover, the space-occupying effect scores of the correlative manifestation on ultrasound proved beneficial in distinguishing between benign and malignant ADs. The machine learning model based on comprehensive multimodal analysis of detailed mammographic and sonographic features and clinical characteristics is beneficial for diagnosing mammography-detected ADs.
What is the implication, and what should change now?
• The comprehensive multimodal analysis of mammographic and sonographic features and clinical characteristics could be implemented in clinical practice to guide management for mammography-detected ADs. Multicenter studies with larger sample sizes need to be conducted to validate this study’s findings.
Introduction
Architectural distortion (AD) represents a common finding in mammography, particularly in cases of non-palpable breast cancer and retrospective reviews of false-negative mammograms (1). As per the Breast Imaging Reporting and Data System (BI-RADS) lexicon, AD is characterized by a distortion of the normal breast architecture without the presence of a distinctly visible mass and often manifests as spiculations radiating from a point or focal retraction and distortion at the parenchymal edges (2). Both benign and malignant breast lesions, including radial scars/complex sclerotic lesions, adenosis, and carcinomas, can induce disarray and distortion in breast structure, resulting in the appearance of AD on imaging (1,3-8). AD can also be a secondary manifestation associated with primary surgical procedures, biopsies, or aspirations (1,9,10).
As AD is usually subtle and has variable presentations, it can be challenging for radiologists to make an accurate diagnosis based solely on mammographic features. The advent of digital breast tomosynthesis (DBT), which reduces the impact of superimposed breast tissue compared with conventional two-dimensional (2D) digital mammography (DM), has led to the increased detection of AD (3,5,9,11-13). Meanwhile, three-dimensional DBT imaging features of AD can help to distinguish nonmalignant types in which symmetric or spoke-wheel spiculation morphology with central lucency are more often seen (11). However, studies have indicated that DBT-detected suspicious AD carried a lower risk of malignancy than 2D mammography-detected suspicious AD but the risk remained significant enough to necessitate biopsies (11,14). Consequently, DBT has increased the number of unnecessary biopsies for ADs (8,12-14). Various modalities, such as ultrasonography (US), magnetic resonance imaging (MRI) and contrasted-enhanced mammography have been proposed to assist in distinguishing malignant AD from benign AD (3,7,8,15-21). Several studies indicate that mammographic AD having an ultrasound correlate exhibits a more likelihood of malignancy, irrespective of its detection method-whether through mammography or DBT (3-8), but the literature on examining the specific sonographic manifestations corresponding to mammographic AD and their predictive significance in diagnosing malignancy is limited. And published data suggest that the diagnostic utility and widespread application of contrasted-enhanced MRI and mammography for AD lesions remain limited (16-21).
Presently, there is no reliable imaging standard to differentiate between malignant and benign ADs. In addition, there is no consensus regarding which cases of AD necessitate biopsy or surgical excision and which cases can be managed through regular follow-up. Meticulous and thorough evaluation of the multimodal imaging findings may be beneficial in enhancing diagnostic confidence for breast radiologist encountering with AD lesions. This study sought to predict the likelihood of malignancy of AD detected on mammography through a comprehensive analysis of detailed mammographic and ultrasonographic features and clinical characteristics. The findings of this study have the potential to boost the confidence of breast radiologists in the differentiation of benign and malignant AD lesions and could be instrumental in refining clinical management strategies for AD. We present this article in accordance with the STARD reporting checklist (available at https://gs.amegroups.com/article/view/10.21037/gs-24-110/rc).
Methods
The study was conducted in accordance with the Declaration of Helsinki (as revised in 2013). The study was approved by the Ethics Committee of Tianjin Medical University Cancer Institute and Hospital (No. bc2021093), and individual consent for this retrospective analysis was waived.
Patients
We conducted a retrospective analysis of consecutive female patients who had AD on either screening or diagnostic mammograms at Tianjin Medical University Cancer Institute and Hospital from January 6, 2015, to December 28, 2018. To identify eligible cases, we scrutinized the Picture Archiving and Communication System (PACS) database for patients whose mammography reports included the term “AD” and were initially categorized under BI-RADS with codes 0, 3, 4, or 5.
Patients with AD identified on screening mammography were recalled for additional imaging evaluation, including additional mammographic views (e.g., spot compression magnification views or DBT) and often US. The standard protocol for outpatients typically involves both mammography and US examinations at our institution. However, in cases in which mammography views were inconclusive for AD (e.g., AD was only observed in one projection view, or there was a clinically palpable abnormality with a negative 2D mammography manifestation in dense breast tissue), these patients were recalled for supplementary imaging assessments, such as spot compression or DBT. The patients were included in our study if the AD persisted and was discernible on subsequent mammographic views.
Notably, over the course of this study, the automated breast volume scanner (ABVS) was not yet available at our institution, and all the ultrasound examinations were conducted using a handheld scanner. Therefore, despite the retrospective nature of this study, we systematically employed a mammogram-guided “second look” ultrasound approach in the management of pertinent cases in preparation for this investigation. Specifically, when the initial US results were negative or did not correspond to the mammographic ADs, we promptly conducted a “second look” ultrasound examination. The quadrant, location, size of the mammographic AD, and obvious adjacent anatomical structure were used as guidance to conduct targeted handheld ultrasound scanning again. The mammogram-guided “second look” ultrasound, which focused on the affected breast, was often performed by the same dedicated breast imaging radiologist who had initially interpreted the mammogram, or, if necessary, by a more experienced senior radiologist.
As a result, the majority of cases involved comprehensive sets of mammographic and sonographic images, which were classified as BI-RADS categories 4 or 5. After completing full assessment, patients with lesions that were finally diagnosed as BI-RADS category 3 by both mammography and US were generally enrolled in the follow-up group (distinct from the study cohort). Furthermore, as tomosynthesis-guided biopsy were introduced into our breast imaging department only at the end of 2018, any cases in which AD was labeled as BI-RADS category 4 or 5 by either mammography, US, or both, underwent conventional core needle biopsy (CNB) using ultrasound or mammography imaging guidance, or DM/DBT-guided wire localization followed by excision biopsy as necessary. Surgery was employed for cases where malignancy or high-risk features were identified during the CNB. For cases that yielded non-malignant results after CNB or excision, patients were recommended for imaging follow-up, typically by US and/or mammography conducted every 6–12 months, to monitor changes or the stability of the mammographic and ultrasonic findings associated with the AD.
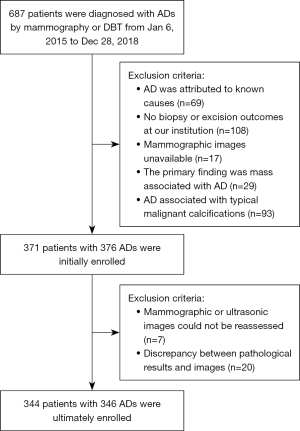
This study included cases exhibiting AD on either 2D mammograms or DBT mammograms. Cases were excluded from the study if any of the following exclusion criteria were met: (I) the mammograms were unavailable; (II) the ADs could be attributed to known causes such as post-intervention changes or cancers following neoadjuvant treatment; (III) the pathology results were unavailable; (IV) the dominant finding was a mass, and the AD was described as a secondary finding in any position view or tomography. Further, a specific exclusion criterion was applied to AD lesions with a high probability of malignant calcification, as calcification was the primary diagnostic basis in such cases. According to the mammography BI-RADS lexicon, calcifications that were classified as having a high probability of malignancy were typically characterized as fine pleomorphic, fine linear, or fine-linear branching shapes with a linear or segmental distribution type. Considering the value of this study in practical work, ADs associated with typical benign or intermediate-concern calcifications were included in this study, as in such cases, the ADs were analyzed as the primary lesion. Figure 1 shows the flow of patient selection for this study.
Imaging technique and interpretation
Mammographic examinations were conducted using the Selenia Dimensions system (Hologic, Bedford, MA, USA). These comprehensive examinations included craniocaudal and mediolateral oblique views of both breasts, with additional views obtained as necessary. Ultrasound examinations were carried out using the GE LOGIQ E9 Ultrasound System equipped with a 6–15 MHz linear transducer (GE Medical Systems, Wauwatosa, WI, USA).
DBT equipment were introduced into our unit in 2017, So, before that, for patients whose mammography was limited to 2D views, we meticulously analyzed their 2D mammograms. For patients who had both 2D and DBT images available, the DBT images were analysed preferentially. If the initial ultrasound examination revealed abnormalities corresponding to the mammographic ADs, the detailed sonographic findings were meticulously analyzed. Conversely, if the initial US results were negative or did not correspond to the mammographic ADs, the results were analyzed with “second look” ultrasound.
All the mammographic and sonographic images were meticulously reviewed separately by two dedicated breast radiologists with 10 and 13 years of experience, respectively. If a diagnosis was inconsistent, a final diagnosis was made by another senior radiologist with more than 25 years of experience in breast imaging. These experienced radiologists, who may have been involved in the prior interpretation process, were not necessarily the same professionals who initially evaluated the mammography and US. All these readers were informed that the primary aim of the study was to evaluate AD lesions detected through mammography but were blinded to the pathological outcomes.
According to Magny et al. (2) and the Japanese guidelines on non-mass abnormalities of the breast (22), the dedicated breast radiologists classified mammographic findings as either pure AD or AD associated with other findings, such as asymmetry and/or calcification. Additionally, any calcifications present were categorized as typical benign (e.g., a round calcification, rim calcification, dystrophic calcification, or punctate calcification) or an intermediate-concern calcification (e.g., a coarse heterogeneous or amorphous calcification). The US correlates were categorized into the following four groups: mass, non-mass-like hypoechoic area (comprising a patchy or mottled hypoechoic area, a geographic hypoechoic area, or an indistinct hypoechoic area, with or without associated posterior acoustic shadowing), AD (defined as a vague area with altered echotexture and associated distorted parenchymal architecture), and other abnormalities (such as duct dilatation with or without internal echoes, a ductal hypoechoic area, an irregularity of ductal caliber, clustered microcysts, or other abnormalities). Further, the readers assigned the following scores to indicate the degree of the space-occupying effect of the lesions on US: 1, which indicated that the space-occupying effect was not prominent; 2, which indicated that the space-occupying effect was localized or confined to a specific scan section; and 3, which indicated a substantial space-occupying effect. The readers also provided detailed descriptions of the lesion location and any associated findings, and the BI-RADS final assessment categories.
To compile comprehensive data, we reviewed the patient’s medical records, extracting information from the database of our hospital information system, such as patient age, personal or family history, clinical presentation and pathology results.
Machine learning model
The eXtreme Gradient Boosting (XGBoost) model is a machine learning technology that can efficiently and flexibly process missing data and build accurate prediction models with weak prediction models (23). All the AD cases were randomly divided into training (70%) and validation (30%) datasets. Univariate and multivariate logistic regression analyses were applied to examine the potential risk factors associated with pathologically malignant ADs. Machine learning model based on multimodal clinical and imaging features was constructed using R software (version 6.1, R Foundation for Statistical Computing, Vienna, Austria). The training set was applied to construct ML model and the validation cohort was used to validate the predictive performance of the XGBoost with optimization. In order to avoid over-fitting and improve the prediction ability of the model, the hold-out method was applied.
Statistical analysis
The statistical analysis was conducted using SPSS 17.0 software (IBM, Armonk, NY, USA). The independent-samples t-test was applied to investigate the continuous variables, and the Chi-square test or two-tailed Fisher’s exact test was employed to analyze the categorical variables. A receiver operating characteristic (ROC) analysis was conducted to assess the diagnostic efficacy of mammography and US. A P value <0.05 was considered statistically significant, and all P values were computed using a two-tailed test.
Results
Initially, a total of 371 patients with 376 ADs were enrolled in this study. Subsequently, seven cases were excluded due to the unavailability of mammographic or ultrasonographic images for reassessment, and 20 patients were excluded because it was not possible to definitively confirm whether the pathological findings corresponded to the imaging observations. As a result, the final analysis comprised 344 patients with 346 AD lesions, with two patients presenting with simultaneous lesions in both breasts. All the patients were females, aged 19 to 84 years (with a mean age of 47.40±10.07 years).
Of the 344 patients, 67 (19.48%) presented for screening mammography and 277 (80.52%) for diagnostic mammography. In the diagnostic cohort, 176 (63.54%, 176/277) patients had breast symptoms (a palpable mass or asymmetry, breast pain, and nipple discharge), while the other 101 patients with 103 ADs were detected through mammography follow-up because of other benign breast lesions or preoperative imaging examinations because of ipsilateral or contralateral breast cancer unrelated to the AD we studied. Twenty-four patients (6.98%, 24/344) had a personal or family history of breast cancer.
The final pathological outcomes of the 346 ADs are set out in Table 1. All high-risk lesions identified by CNB, as well as the cases in which the CNB results were inconsistent or disputed by the imaging findings, underwent surgical excision. The diagnoses of eight (7.55%, 8/106) cases were ultimately upgraded to invasive carcinoma or ductal carcinoma in situ (DCIS). Among these eight cases, two cases of sclerosing adenosis were upgraded (one to DCIS and one to tubular carcinoma), two cases of complex sclerosing lesions were upgraded (one to DCIS and one to apocrine carcinoma), one case of adenosis was upgraded [to typical invasive lobular carcinoma (ILC)], one case of intraductal papilloma (IP) with atypical hyperplasia was upgraded (to microinvasive adenocarcinoma), one case of lobular atypical hyperplasia was upgraded (to ILC), and one case of atypical ductal epithelial hyperplasia was upgraded (to DCIS). The final pathological outcomes comprised 228 malignancies, the most common of which was invasive ductal carcinoma (IDC). The remaining cases with high-risk and benign findings formed the non-malignant disease group, in which the most common lesion was a radial scar or complex sclerosing lesion (Table 1).
Table 1
| Pathologic outcomes | N (%) |
|---|---|
| Malignancy (n=228) | |
| Invasive ductal carcinoma (13 cases were mixed with invasive micropapillary carcinoma components) | 149 (43.06) |
| Invasive lobular carcinoma with or without ductal components | 43 (12.43) |
| Ductal carcinoma in situ (8 cases of ductal carcinoma in situ with microinfiltration, and one case of mixed with lobular carcinoma in situ) | 20 (5.78) |
| Apocrine carcinoma | 13 (3.76) |
| Tubular carcinoma | 3 (0.87) |
| Non-malignancy (n=118) | |
| Radial scars or complex sclerosing lesions | 48 (13.87) |
| Adenosis or sclerosing adenosis | 22 (6.36) |
| Intraductal papilloma with or without atypical hyperplasia | 33 (9.54) |
| Fibroadenoma/fibroadenomatoid change with or without atypical hyperplasia | 8 (2.31) |
| Fibrocystic changes and ductal hyperplasia | 3 (0.87) |
| Myofibroblastoma | 1 (0.29) |
| Fibromatosis | 2 (0.58) |
| Ductal dilation with chronic inflammation | 1 (0.29) |
N, number of patients.
Of the patients, 16 requested surgical excision rather than undergoing the recommended imaging follow-up because of the anxiety associated with BI-RADS categorized as 4B or 4C, even though their biopsy results were non-malignant. Imaging follow-up was recommended for 102 patients with consistent non-malignant biopsy or surgical excision results. Ultimately, 17 patients did not undergo imaging follow-up at our institution, but the remaining 85 patients completed at least one US and/or mammography examination. The median follow-up period was 29 months (range, 24–76 months). Among the lesions, one AD case initially diagnosed as adenosis with lobular and ductal atypical hyperplasia showed an enlargement of the hypoechoic area on US after 26 months of follow-up, and the final surgical pathology outcome was DCIS mixed with lobular carcinoma in situ (low-grade). In another case, which was initially diagnosed as ductal epithelial atypical hyperplasia, new amorphous calcifications were observed on mammography after 19 months of follow-up, which was later confirmed as DCIS upon surgical excision. For the remaining 83 women (81.37%, 83/102), imaging follow-up revealed no progression.
Table 2 presents the clinical and imaging features and their association with malignant ADs. The mean age of patients diagnosed with malignancy and non-malignancy were 50.12±8.70 and 42.14±10.48 years, respectively, and this difference was statistically significant (t=7.525, P<0.001). The results also suggested that palpable AD on mammography was more likely to represent malignancy (141/169, 83.43%) than non-palpable AD (87/177, 49.15%) (P<0.001).
Table 2
| Multimodal features | Total | Pathological outcomes | P | |
|---|---|---|---|---|
| Malignancy (n=228) | Non-malignancy (n=118) | |||
| Clinical features | ||||
| Age (years) | 47.40±10.07 | 50.12±8.70 | 42.14±10.48 | <0.001* |
| Palpability | <0.001* | |||
| Palpable | 169 (48.84) | 141 (83.43) | 28 (16.57) | |
| Non-palpable | 177 (51.16) | 87 (49.15) | 90 (50.85) | |
| Mammographic features | 0.005* | |||
| Pure AD | 187 (54.05) | 111 (59.36) | 76 (40.64) | |
| Associated with other findings | 159 (45.95) | 117 (73.58) | 42 (26.42) | |
| Associated findings | 0.06 | |||
| Calcification | 70 (44.03) | 45 (64.29) | 25 (35.71) | |
| Asymmetry | 51 (32.08) | 41 (80.39) | 10 (19.61) | |
| Asymmetry and calcification | 38 (23.90) | 31 (81.58) | 7 (18.42) | |
| Ultrasonographic manifestations† | <0.001* | |||
| Non-mass-like hypoechoic area | 217 (62.90) | 158 (72.81) | 59 (27.19) | |
| Mass | 74 (21.45) | 65 (87.84) | 9 (12.16) | |
| Architectural distortion | 40 (11.59) | 3 (7.50) | 37 (92.50) | |
| Other abnormalities | 14 (4.06) | 2 (14.29) | 12 (85.71) | |
| Scores of space-occupying effect on US† | <0.001* | |||
| 1 | 58 (16.81) | 5 (8.62) | 53 (91.38) | |
| 2 | 76 (22.03) | 34 (44.74) | 42 (55.26) | |
| 3 | 211 (61.16) | 189 (89.57) | 22 (10.43) | |
Age is presented as the mean ± standard deviation, the other data are expressed as the count with the percentage in parentheses. *, statistically significant P values; †, data from one patient were unavailable. n, number of patients; AD, architectural distortion; US, ultrasonography.
Within this cohort, pure AD (Figure 2) was less likely to represent malignancy than AD associated with other mammographic findings (Figures 3,4) (59.36% vs. 73.58%, respectively, P=0.005) (Table 2). The ADs associated with both calcifications and asymmetry were more likely to indicate malignancy than the ADs associated with calcifications or asymmetry alone; however, this difference was not statistically significant (P=0.06). In the group of patients with ADs associated with calcifications, those with intermediate-concern calcifications showed a higher likelihood of malignancy (61/80, 76.25%) than those with typical benign calcifications (15/28, 53.57%), and the difference was statistically significant (P=0.02).



US examinations were performed in 344 patients with 346 AD lesions. Sonographic correlates were observed in 345 AD lesions (345/346, 99.71%), except for one negative case confirmed to be adenosis. The most common US correlate was non-mass-like hypoechoic area (217/345, 62.90%) (Figures 5,6), followed by mass (74/345, 21.45%) (Figure 7), AD (40/345, 11.59%) and other abnormalities (14/345, 4.06%). Among all the corresponding US manifestations, 63 (18.26%, 63/345) were detected by “second look” ultrasound, the majority of which (84.13%, 53/63) manifested as non-mass-like hypoechoic areas. Overall, in terms of the US correlates, the mammography-detected AD lesions that appeared as non-mass-like hypoechoic areas or masses on US were more likely to indicate malignancy than those that appeared as AD or other abnormalities on US, and the difference was statistically significant (P<0.001) (Table 2).



In addition to analyzing the sonographic features of the AD lesions, we also scored the performance of their occupying effect on US (Figures 5-7). The results showed that the more pronounced the space occupying effect on US for mammography-detected AD lesions, the more likely these lesions were malignant, and the difference was statistically significant (P<0.001) (Table 2).
We defined BI-RADS 4B, 4C, and 5 as correct malignancy diagnosis and BI-RADS 2, 3, and 4A as correct non-malignancy diagnosis, and used the histopathological outcomes from biopsy and/or surgical excision as the reference standard. The sensitivity, specificity, positive predictive value (PPV), and negative predictive value (NPV) of mammography were 93.0% (212/228), 37.29% (44/118), 74.13% (212/286), and 73.33% (44/60), respectively. The sensitivity, specificity, PPV, and NPV of US were 94.74% (216/228), 36.75% (43/117), 74.48% (216/290), and 78.18% (43/55), respectively. The sensitivity, specificity, PPV, and NPV of combined mammography and US were 95.61% (218/228), 58.97% (69/117), 81.95% (218/266), and 87.34% (69/79), respectively.
A ROC analysis was conducted to assess the diagnostic efficacy of mammography, US, and combined mammography and US in differentiating between malignant and benign mammographic ADs (Figure 8). The areas under the ROC curves (AUCs) of mammography, US, and combined mammography and US were 0.65 [95% confidence interval (CI): 0.60–0.70], 0.66 (95% CI: 0.61–0.71), and 0.77 (95% CI: 0.73–0.82), respectively. There was no significant difference in the diagnostic efficacy between mammography and US (Z=0.34, P=0.74). However, the performance of combined mammography and US in differentiating between malignant and non-malignant AD lesions was better than that of mammography or US alone (Z=4.74 and 5.64, respectively, P<0.001). Additionally, the PPV and NPV of the US correlates which manifested as non-mass-like hypoechoic area, mass, AD, and other abnormalities were as follows: 78.13% (150/192) and 68.00% (17/25); 89.04% (65/73) and 100% (1/1); 5.56% (1/18) and 90.91% (20/22); and 0% (0/7) and 71.43% (5/7), respectively.

There were 160 ADs with malignant outcomes and 82 ADs with nonmalignant outcomes in the training set, and there were 68 ADs with malignant outcomes and 36 ADs with nonmalignant outcomes in the validation set. Univariate and multivariate logistic regression analyses on the training and validation datasets were used to examine the ability of the clinical and imaging features in predicting the malignancy of mammographic AD lesions (Table 3). The features of age, clinical palpability, associated with other mammographic findings, the manifestations and score of space-occupying effect of US correlates were used to construct machine learning model to predict the pathological malignancy of mammographic ADs. The sensitivity, specificity and diagnostic accuracy of the XGBoost model based on clinical and imaging features in predicting the malignancy of ADs in the validation set were 66.46%, 94.23% and 78.9%, respectively, and the AUC was 0.886 (95% CI: 0.825–0.947) (Figure 9).
Table 3
| Factors | Training set | Validation set | |||||||
|---|---|---|---|---|---|---|---|---|---|
| Univariate analysis | Multivariate analysis | Univariate analysis | Multivariate analysis | ||||||
| P | OR (95% CI) | P | OR (95% CI) | P | OR (95% CI) | P | OR (95% CI) | ||
| Age | <0.001* | 1.099 (1.061–1.138) | <0.001 | 1.125 (1.072–1.181) | <0.001* | 1.107 (1.047–1.17) | <0.001 | 1.182 (1.079–1.295) | |
| Palpability | <0.001* | 10.477 (5.47–20.068) | – | – | <0.001* | 4.231 (1.737–10.303) | – | – | |
| With associated mammographic findings | 0.03* | 1.782 (1.03–3.085) | – | – | – | – | – | – | |
| Associated with asymmetry | 0.03* | 2.02 (1.036–3.941) | – | – | 0.005* | 6.395 (1.777–23.015) | – | – | |
| Associated with typical benign calcification | 0.38 | 0.434 (0.212–1.337) | – | – | – | – | – | – | |
| Associated with intermediate-concern calcification | 0.06 | 1.861 (1.013–3.561) | – | – | – | – | – | – | |
| Ultrasonographic manifestations† | <0.001* | 0.18 (0.101–0.321) | – | – | <0.001* | 0.154 (0.06–0.395) | – | – | |
| Score of space-occupying effect on US† | <0.001* | 9.756 (5.663–16.805) | – | – | <0.001* | 10.746 (4.603–25.088) | – | – | |
| 1 | – | 1 | – | – | – | 1 | – | – | |
| 2 | <0.001* | 8.743 (3.147–24.287) | <0.001 | 13.04 (3.21–52.972) | <0.001* | 7.729 (3.041–21.267) | <0.001 | 19.87 (2.415–163.47) | |
| 3 | <0.001* | 92.782 (33.556–256.541) | <0.001 | 146.465 (35.274–608.161) | <0.001* | 24.314 (20.51–156.421) | <0.001 | 337.597 (33.373–715.07) | |
*, statistically significant P values; †, data from one patient were unavailable. US, ultrasonography; OR, odds ratio; CI, confidence interval.

Discussion
The ADs observed on mammography may have a variety of causes, including both breast cancer and benign conditions. This poses a diagnostic challenge for breast radiologists and consequently a complex decision-making challenge for clinicians and patients. The present study aimed to provide a comprehensive assessment of detailed mammographic, ultrasonic, and clinical features to aid in predicting the malignancy of ADs detected on mammography. The insights from this study have the potential to enhance the confidence of breast radiologists in differentiating between benign and malignant AD lesions and could be instrumental in refining clinical management strategies.
Consistent with the findings of previous studies (3,4,6), in our study, the most common malignant pathology was IDC, while radial scars or complex sclerosing lesions were the most common non-malignant pathology. Notably, rare pathological outcomes were observed in our study, including 13 cases of apocrine carcinoma and three cases of tubular carcinomas. Additionally, we observed a substantial number of IP cases among non-malignant AD lesions. Specific histopathological features may explain the imaging presentations of these findings (8,24,25).
Previous studies have reported a wide range (from 10% to 83%) of PPVs for malignancy in cases of AD (4,5,7,9). This variation may be influenced by the case selection criteria and calculation methods. Spiculated masses that were obscured by dense breast tissue on 2D mammography may be classified as areas of AD (26). In our study, some cases of AD were detected without DBT images, which might have contributed to the higher PPV of the present study compared with studies exclusively involving DBT-detected AD lesions (3,9).
Our findings demonstrated that palpable AD lesions were more likely to be malignant than non-palpable AD lesions. This supported the findings of previous research that suggested that in cases of AD, patients with clinical symptoms faced a higher risk of malignancy than those without symptoms (4). Our study also found that patients with malignant AD lesions tended to be older than those with non-malignant lesions, which was consistent with the well-established association between breast cancer and advanced age.
Our study adopted a different approach compared with previous studies, as they primarily focused on comparing traditional 2D mammography and DBT (3,5,11-13). We concentrated on the scrupulous imaging manifestations of mammography and US, regardless of whether the AD was initially detected by 2D mammography or DBT, to evaluate features that could differentiate malignant AD from non-malignant lesions. Thus the results could enhance diagnostic confidence and accuracy for breast radiologist, particularly in institutions where DBT has not been commonly used. Our findings revealed that pure AD lesions were less likely to indicate malignancy than AD lesions associated with other mammographic findings, which was consistent with the findings of previous research (4,11). Furthermore, those ADs associated with intermediate-concern calcifications exhibited a higher likelihood of malignancy than those associated with typical benign calcifications. Moreover, it was important to note that more than half of the cases of AD associated with typical benign calcifications exhibited malignant histopathological findings following surgical excision.
Another notable issue in the literature was whether the presence or absence of corresponding abnormality on sonography is related to the likelihood of malignancy in cases of AD. Several studies have suggested that mammographic ADs without a US correlate have a low incidence of malignancy, regardless of whether it was initially detected by mammography or DBT (3,5). Our results showed that the detection rate of US correlate was remarkably high compared to other studies (3-5,13). On the one hand, this may be due to different criteria for case selection. This study focused on the detailed imaging and clinical manifestations of mammographic ADs, so those cases without ultrasound and pathological results in our unit were excluded. But those excluded cases may contain some ADs without US correlate. On the other hand, this inconsistency in the results could be attributed to our use of mammograms-guided “second look” ultrasound, which enhanced the detection of non-mass features that may be subtle on sonographic images. To the best of our knowledge, there is little literature analyzing the detailed sonographic manifestations that correspond to mammographic AD and their predictive value in malignancy diagnosis. Of the US correlates, 21.45% (74/345) presented as masses while the remaining 78.55% (271/345) presented as non-mass features. This result contradicted that of Bahl et al. (4) who reported that the most common sonographic feature which correlated with mammographic AD was hypoechoic mass (227/304, 74.7%), while the second most common feature was non-mass findings. In addition to different case selection criteria, this inconsistency in the results could be attributed to our use of targeted “second look” ultrasound, which enhanced the detection of subtle non-mass findings.
Non-mass features on US have not been formally incorporated into the current BI-RADS lexicon for ultrasound and are described using various terms in the literature (22,27). However, they are of great importance, as they are frequently encountered in practice in routine breast ultrasounds and can serve as imaging correlates for mammographic and MRI findings which may indicate a wide range of histopathologic etiologies, including both malignant and benign lesions (27). Consequently, non-mass features may be a crucial factor in the management of AD. Our results showed that AD cases with a non-mass-like hypoechoic area as the ultrasound correlate had a higher PPV, whereas those with structural distortions as the ultrasound correlate exhibited a higher NPV. A meticulous and thorough US evaluation was beneficial for distinguishing between benign and malignant mammographic ADs.
We conducted a score analysis for space-occupying effect of the US correlates and a correlation analysis between the score and pathological findings. The findings indicated that the more pronounced the space-occupying effect on US for mammographic AD lesions, the more likely these lesions were malignant, and the difference was statistically significant (P<0.001). Moreover, the logistic regression analysis revealed that a score of 3 on the US space-occupying effect scale was an independent risk factor for indicating malignant AD. To our knowledge, it has not been reported previously and calls for further investigation and validation.
Another important aspect to consider is that some AD cases with non-malignant pathology following biopsy may be upgraded to cancer after surgical excision (28,29). In our study, all eight upgraded cases exhibited non-mass-like hypoechoic areas on US with a space-occupying effect score of 2. In addition, during the follow-up period of our study, two cases underwent surgery due to the discovery of an enlarged non-mass-like hypoechoic area on US and new amorphous calcifications on mammography, and postoperative pathology confirmed malignancy. Therefore, both mammography and US examinations may be necessary for ADs with high-risk outcomes following biopsy, and during follow-up, especially for cases with high-risk lesions.
This study had several limitations. It was retrospective and included a relatively small number of lesions, which limits the generalizability of the results. The study was conducted at a single academic cancer institute and hospital with dedicated breast radiologists, which potentially introduced case selection bias and reader bias. We did not analyze the agreement among readers due to the relatively small number of readers involved. The majority of US examinations at our institution were handheld rather than ABVS. Despite the application of “second-look” ultrasound, the limitations and deficiencies of handheld ultrasound technology, such as equipment dependence and operator dependence, were unavoidable. Additionally, we did not analyze the MRI features of AD, which represented an avenue for further research. The relatively short follow-up period for non-malignant AD cases was another limitation that should be addressed in future studies.
Conclusions
In conclusion, our study revealed that palpable ADs and ADs associated with other abnormalities on mammography were more likely to indicate malignancy than non-palpable and pure AD lesions, respectively. The application of mammograms-guided “second-look” ultrasound could enhance the detection of US correlates, particularly non-mass-like features. The space-occupying effect score of the US correlates was helpful in differentiating between benign and malignant ADs, but further research was needed to confirm these results. The comprehensive analysis based on clinical and multimodal imaging features could be beneficial in improving the diagnostic and differential efficacy for AD lesions detected on mammography and instrumental in refining clinical management strategies for ADs.
Acknowledgments
The authors thank Dr. Mary Salvatore (Columbia University Irving Medical Center, New York, USA) for the critical comments and valuable advice on this study. All the authors would also like to express their gratitude to the breast pathologists at the Department of Breast Pathology and Research Laboratory, Tianjin Medical University Cancer Institute and Hospital for their professional assistance.
Funding: This study received funding from
Footnote
Reporting Checklist: The authors have completed the STARD reporting checklist. Available at https://gs.amegroups.com/article/view/10.21037/gs-24-110/rc
Data Sharing Statement: Available at https://gs.amegroups.com/article/view/10.21037/gs-24-110/dss
Peer Review File: Available at https://gs.amegroups.com/article/view/10.21037/gs-24-110/prf
Conflicts of Interest: All authors have completed the ICMJE uniform disclosure form (available at https://gs.amegroups.com/article/view/10.21037/gs-24-110/coif). The authors have no conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved. The study was conducted in accordance with the Declaration of Helsinki (as revised in 2013). The study was approved by the Ethics Committee of Tianjin Medical University Cancer Institute and Hospital (No. bc2021093) and individual consent for this retrospective analysis was waived.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Gaur S, Dialani V, Slanetz PJ, et al. Architectural distortion of the breast. AJR Am J Roentgenol 2013;201:W662-70. [Crossref] [PubMed]
- Magny SJ, Shikhman R, Keppke AL. Breast Imaging Reporting and Data System. In: StatPearls. Treasure Island (FL): StatPearls Publishing; 2024.
- Alshafeiy TI, Nguyen JV, Rochman CM, et al. Outcome of Architectural Distortion Detected Only at Breast Tomosynthesis versus 2D Mammography. Radiology 2018;288:38-46. [Crossref] [PubMed]
- Bahl M, Baker JA, Kinsey EN, et al. Architectural Distortion on Mammography: Correlation With Pathologic Outcomes and Predictors of Malignancy. AJR Am J Roentgenol 2015;205:1339-45. [Crossref] [PubMed]
- Bahl M, Lamb LR, Lehman CD. Pathologic Outcomes of Architectural Distortion on Digital 2D Versus Tomosynthesis Mammography. AJR Am J Roentgenol 2017;209:1162-7. [Crossref] [PubMed]
- Wang LC, Philip M, Bhole S, et al. Pathologic Outcomes in Single Versus Multiple Areas of Architectural Distortion on Digital Breast Tomosynthesis. AJR Am J Roentgenol 2023;220:50-62. [Crossref] [PubMed]
- Walcott-Sapp S, Garreau J, Johnson N, et al. Pathology results of architectural distortion on detected with digital breast tomosynthesis without definite sonographic correlate. Am J Surg 2019;217:857-61. [Crossref] [PubMed]
- Ambinder EB, Plotkin A, Euhus D, et al. Tomosynthesis-Guided Vacuum-Assisted Breast Biopsy of Architectural Distortion Without a Sonographic Correlate: A Retrospective Review. AJR Am J Roentgenol 2021;217:845-54. [Crossref] [PubMed]
- Partyka L, Lourenco AP, Mainiero MB. Detection of mammographically occult architectural distortion on digital breast tomosynthesis screening: initial clinical experience. AJR Am J Roentgenol 2014;203:216-22. [Crossref] [PubMed]
- Miner N, Meng K. Mammographic architectural distortion caused by cyst aspiration. Acta Radiol Open 2019;8:2058460119859353. [Crossref] [PubMed]
- Vijapura C, Yang L, Xiong J, et al. Imaging Features of Nonmalignant and Malignant Architectural Distortion Detected by Tomosynthesis. AJR Am J Roentgenol 2018;211:1397-404. [Crossref] [PubMed]
- Dibble EH, Lourenco AP, Baird GL, et al. Comparison of digital mammography and digital breast tomosynthesis in the detection of architectural distortion. Eur Radiol 2018;28:3-10. [Crossref] [PubMed]
- Ahmed SA, Samy M, Ali AM, et al. Architectural distortion outcome: digital breast tomosynthesis-detected versus digital mammography-detected. Radiol Med 2022;127:30-8. [Crossref] [PubMed]
- Romanucci G, Fornasa F, Caneva A, et al. Tomosynthesis-Detected Architectural Distortions: Correlations between Imaging Characteristics and Histopathologic Outcomes. J Imaging 2023;9:103. [Crossref] [PubMed]
- DiPrete O, Wei CJ, Phillips J, et al. Management of Mammographic Architectural Distortion Based on Contrast-enhanced MRI and US Correlation. J Breast Imaging 2023;5:425-35. [Crossref] [PubMed]
- Ferre R, Kuzmiak CM. Meta-analysis: Architectural distortion and breast MRI. Breast Dis 2022;41:205-14. [Crossref] [PubMed]
- Amitai Y, Scaranelo A, Menes TS, et al. Can breast MRI accurately exclude malignancy in mammographic architectural distortion? Eur Radiol 2020;30:2751-60. [Crossref] [PubMed]
- Mei H, Xu J, Yao G, et al. The diagnostic value of MRI for architectural distortion categorized as BI-RADS category 3-4 by mammography. Gland Surg 2020;9:1008-18. [Crossref] [PubMed]
- Goh Y, Quek ST, Pillay P, et al. Evaluation of architectural distortion with contrast-enhanced mammography. Clin Radiol 2024;79:163-9. [Crossref] [PubMed]
- Wang S, Wang Z, Li R, et al. Association between quantitative and qualitative image features of contrast-enhanced mammography and molecular subtypes of breast cancer. Quant Imaging Med Surg 2022;12:1270-80. [Crossref] [PubMed]
- Steyerova P, Burgetova A. Current imaging techniques and impact on diagnosis and survival —a narrative review. Ann Breast Surg 2022;6:25.
- Ito T, Ueno E, Endo T, et al. The Japan Society of Ultrasonics in Medicine guidelines on non-mass abnormalities of the breast. J Med Ultrason (2001) 2023;50:331-9. [Crossref] [PubMed]
- Fleuren LM, Klausch TLT, Zwager CL, et al. Machine learning for the prediction of sepsis: a systematic review and meta-analysis of diagnostic test accuracy. Intensive Care Med 2020;46:383-400. [Crossref] [PubMed]
- WHO Classification of Tumours Editorial Board. Breast tumours. 5th ed. Lyon, France: IARC; 2019.
- Zhu Y, Zhang S, Liu P, et al. Solitary intraductal papillomas of the breast: MRI features and differentiation from small invasive ductal carcinomas. AJR Am J Roentgenol 2012;199:936-42. [Crossref] [PubMed]
- Andersson I, Ikeda DM, Zackrisson S, et al. Breast tomosynthesis and digital mammography: a comparison of breast cancer visibility and BIRADS classification in a population of cancers with subtle mammographic findings. Eur Radiol 2008;18:2817-25. [Crossref] [PubMed]
- Choe J, Chikarmane SA, Giess CS. Nonmass Findings at Breast US: Definition, Classifications, and Differential Diagnosis. Radiographics 2020;40:326-35. [Crossref] [PubMed]
- Zhou J, Sun S, Lin L, et al. The value of imaging combined with clinicopathological features in the diagnosis of high-risk breast lesions. Gland Surg 2022;11:1323-32. [Crossref] [PubMed]
- Villa-Camacho JC, Bahl M. Management of Architectural Distortion on Digital Breast Tomosynthesis With Nonmalignant Pathology at Biopsy. AJR Am J Roentgenol 2022;219:46-54. [Crossref] [PubMed]


